Abstract
Brain metastasis are the most frequent central nervous system tumors that usually occur during systemic malignancy. However, they can be diagnosed before the primary cancer site. The number of brain and systemic metastases and the identification of the primary cancer can favor specific treatments and impacts survival estimates. During surgery, intraoperative identification of metastatic tissue may help the surgical strategy and postoperative adjuvant treatment. The study of endogenous fluorescence of the brain tissue has already demonstrated its capability to differentiate metastasis tissue from control and identify specific optical signatures. In this study, based on deep ultraviolet and near-infrared excitation, we investigated for the first time the potentiality of the endogenous fluorescence analysis to characterize brain metastasis origin and to highlight the heterogeneity of molecular components and properties according to the metastasis sub-types, This study gives thus a glimpse of the possibility to find the primary cancer site from the secondary tumor.
Similar content being viewed by others
Introduction
Brain tumors are usually classified according to their cellular types. Two main groups can be distinguished: primary tumors and secondary ones, namely metastasis. While primary tumors such as glioma and meningioma, develop within the central nervous system (CNS), metastasis derive from cancer cells that originate from a malignancy outside the CNS and migrate to the brain, principally via the bloodstream1,2. Contrary to a primary brain tumor, metastasis present either as a single tumor mass or as multiple tumors in different locations in the CNS3. The most common cancers that metastasize to the brain include lung cancer (50%), breast cancer (15–19%), and skin melanoma (10%), making up 70–80% of all brain metastases1. CNS metastatic tumors are 1.0 to 1.5 times more frequently diagnosed than primary brain tumors4, but the latter have a poorer prognosis with lower survival rates5. Some metastases can appear many years after discovering the originating primary cancer while others can appear even before detecting the originating primary cancer3. Usually, the therapeutic options for brain metastases involve a combination of surgery, radiosurgery, chemotherapy, or immunotherapy, targeting both the metastatic brain tumor and primary cancer. Therefore, the therapeutic options depend on the number, location, size, and severity of brain lesions and the primary cancer as well6. Hence, accurately identifying the primary cancer from which a brain metastasis develops is necessary information to guide the therapeutic options7.
Determining the origin of brain metastasis is not a new challenge. Solving this problem could be crucial in future translational research identifying diagnostic or therapeutic targets that could disrupt or revert the growth of brain metastasis7,8. Several studies tried to correlate the spatial distribution of metastases with the primary cancer location7,8,9. Schroeder et al. reported, based on a large panel of brain metastastatic tissue types (lung, skin, breast, gastrointestinal organ, genitourinary organ), a correlation between the metastasis originating organ and their location in the brain where the spatial distribution of brain metastases is significantly impacted by the primary organ site10. J.Neman, also using a large panel of metastasis subtypes (lung, skin, breast, colon and kidney), used predictive spatial modeling to show that the spatial distribution of brain metastasis is not uniform and varies depending on the primary cancer. He showed that certain regions of the brain are more prone to develop metastases than others8.
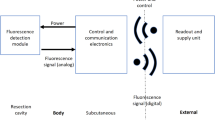
Following these studies, we attempted to identify the primary malignancy based on the metastasis location, using a new diagnostic technique. Our team has been developing a rapid tissue diagnosic tool for intraoperative use during brain tumor surgery, allowing a fast and reliable in vivo brain tissue diagnosis. This tool is based on autofluorescence measurements of several endogenous molecules using different contrasts based on quantitative and qualitative analysis. This tool consists of a multimodal endomicroscope based on two-photon excitation, coupled to a miniature handheld probe11, which is capable of detecting the endogenous fluorescence of several molecules within cells, such as Nicotinamide adenine dinucleotide (NADH), Flavins (FAD), Lipopigments and Porphyrins, as well as the second harmonic generation of collagen fibers and crosslinks12. The use of endogenous fluorophores such as NADH, FAD, and porphyrins to track tissue metabolism to differentiate between healthy, tumoral, and infiltrated tissues has gained significant momentum in the past two decades13,14,15,16,17. The fluorescence lifetime and the spectral emission of these fluorophores have been extensively studied in numerous tissue types, such as bladder, breast, and brain tissues16,18,19,20, and have proven to be effective indicators of tissue cancerization. Their metabolic ratios, such as redox and Optical Index (OI) ratios, can provide reliable information to differentiate healthy from cancer tissues15,18,21,22.
In parallel to the endomicroscope development, an extensive optical tissue database is under construction in collaboration with GHU Paris psychiatric & neurosciences hospital center. This tissue database aims to characterize all types of brain tumors through multimodal autofluorescence analysis to establish a specific optical signature for each cancer type. The database, covering NIR to Deep UV excitation measurements to access a large panel of endogenous fluorescent molecules, is coupled to the endomicroscope providing the ability to establish real-time, precise information about the tissue’s nature.
In the context of this database construction, we have demonstrated the ability to discriminate brain tumors from healthy tissues using multimodal and multiscale autofluorescence analysis16,20,21,22,23. Specifically, our multimodal optical tool demonstrated significant differences in optical signatures between healthy tissues and meningiomas (grade I and II)23and of low- and high-grade gliomas22. In addition, the discrimination between primary (gliomas) and secondary tumors (metastasis) was highlighted in two previous studies21,24. However, in these past studies, metastasis samples were assigned to a single group without considering their systemic origin.
In this proof-of-concept study, we are splitting the global metastasis group into several sub-groups according to the organ of the primary tumor, intending to identify differences between these sub-groups, despite the limited statistic of the samples. The aim here is to evaluate the capacity of multimodal autofluorescence analysis to identify the primary organ of brain metastasis while discriminating brain metastasis sub-types from control healthy CNS tissues. This study could improve the ability of our endomicroscope to analyze metastatic tissue, facilitate a precise diagnosis, and establish a more adapted therapeutic strategy, opening then a way on how to find the primary cancer site from the secondary tumor analysis during the surgery.
To this end, we performed multiscale spectral acquisitions in the Deep Ultraviolet (DUV) domain using 275 nm as excitation wavelength to measure tyrosine, tryptophane, NADH and collagen fluorescence, as well as in the Near Infra-Red (NIR) domain, using 810 nm and 890 nm excitation wavelengths, to quantify the contributions of collagen, porphyins, FAD, NADH and lipopigments. In addition, Fluorescence Lifetime imaging (FLIM) acquisitions were performed to track and analyze the variation of free and protein-bound components of FAD between metastasis and control brain tissue, i.e. surgical specimen from non-tumoral epilepsy surgery.
Results
Samples
The sample cohorts used for DUV and NIR measurements are summarized in Table 1. Metastasis classification of each sample was based on the anatomical pathologist’s diagnosis and control samples come from post-epilepsy surgery and have been diagnosed as healthy cortex tissues.
Deep UV spectral study
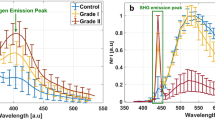
Figure 1.a shows the mean fluorescence spectra of the healthy control and pooled metastatic tissue samples, normalized to the overall peak fluorescence intensity under 275 nm excitation. Figure 1.b shows the spectra of control brain tissue, skin, lung, endocrine system, breast and colon metastasis.
The fluorescence is dominated by tryptophan, while a slight difference could be noticed in the collagen emission peak at 410 nm between the control and global metastasis group in Fig. 1a. However, Fig. 1b shows that this difference could be more prominent with a strong collagen peak in some metastasis types (endocrine system, colon). Indeed, this difference could be lower or even at the same level as the control sample in other metastasis types (lung, breast, skin). This heterogeneity of difference in collagen levels in metastasis types could be illustrated qualitatively through the tryptophan and collagen fluorescence images of a control sample shown in Fig. 1e, a lung metastasis sample shown in Fig. 1d, and an endocrine system metastasis sample shown in Fig. 1c. The collagen emission appears in green in these images, while tryptophan emission is presented in red. Yellow colors represent the superimposition of the two emissions. In control samples, the collagen emission is almost not apparent, and images are dominated by tryptophan. At the same time, it is slightly higher and visual in lung metastasis sample images, and endocrine system metastasis images present an even stronger collagen emission-associated emission.
Comparison of normalized deep UV fluorescence spectra acquired using 275 nm excitation wavelength for Control and global Metastasis group (a) and for Control and each organ of origin metastasis group (b). Tryptophan (red) + Collagen (green) Fluorescence images of an endocrine system metastasis sample (c), lung metastasis sample (d) and a control sample (e), where the scale white line represents a 100 μm length.
Looking into the signal composition, the individual integral proportion, which represents the relative contributions of tyrosine, tryptophan, and collagen, are presented in Fig. 2. A comparison of each fluorophore between the control and the global metastasis group is shown in Fig. 2.a for tyrosine, Fig. 2.b for tryptophan, and Fig. 2.c for collagen. The global metastasis group presents a significant increase in the relative contribution of each fluorophore contribution versus the control group, with the calculated p-values all being lower than 0.05. Such an increase in the fluorophore contributions is in line with our previous study24 and confirms the ability of the deep UV fluorescence to quantitatively discriminate a metastasis sample from control tissues.
However, looking at the comparisons between the metastasis sub-types, shown in Fig. 2d for tyrosine, Fig. 2.e for tryptophan, and Fig. 2.f for collagen, one observes some apparent differences, albeit a large variability. Whereas most metastasis types can be discriminated from control, this is not the case when attempting to discriminate between metastasis types. Colon and endocrine system metastasis are characterized by high collagen and low tyrosine and tryptophan contributions compared to other metastasis types and can be distinguished from one another based on the collagen fluorescence intensity. On the contrary, breast origin has a low collagen emission and the highest tyrosine signal. Skin and lung origins are differentiated based on their collagen emission contributions. Figure 2.g, h, and i display, in inverse log-scale, the p-values histograms of the t-test used to quantify the differences of metastasis fluorophore emissions, respectively for tyrosine, tryptophan, and collagen, according to their original organs, with their 95% confidence level (CL) and the corresponding values are reported in Table 2. As a result of all the 1 by 1 comparisons, almost every origin can be separated from at least one another, with a 95% CL and a p-value < 0.05.
(a, b, c) Box plots comparing relative intensity of Tyrosine, Tryptophan and Collagen molecules for Control and Metastasis groups. (d, e, f) and for Control and metastasis origin organ groups. (g, h, i) 3D histograms, presented in inverse log-scale, of the calculated P-values through a t-test for all 1 by 1 group (Control, Skin, Lung, Endocrine system, Breast and Colon metastasis) comparing (95% C.L. and tail = 2), respectively for the Tyrosine, Tryptophan and Collagen molecules.
Near Infra-Red spectral study
810 nm excitation
The spectral fluorescence results acquired using 810 nm as the excitation wavelength are displayed in Fig. 3. Normalized mean fluorescence spectra of control vs. global metastasis groups are shown in Fig. 3.a while Fig. 3.b represents the fluorescence spectra of the control and each of the mestastases subtypes.
The fluorescence distribution slightly differs between control and global metastasis group in the NADH emission region around 460 nm.
In the same way, the spectral differences for each fluorophore contribution between the metastasis subtypes are more heterogeneous. For example, normalized mean spectra of breast metastasis are red-shifted in comparison to the control, corresponding to an increase of the porphyrin emission. On the other side, endocrine system are blue-shifted in comparison to the control, corresponding to an increase of the NADH emission.
Box plots of redox and optical index (OI) ratios comparing control vs. global metastasis group are presented in Fig. 3.c and f respectively, while Fig. 3.d and g show these ratios comparing control with metastasis sub-types. The redox ratio is higher in mestastases than in control, while the OI ratio is lower for metastases. However these differences did not reach significance (p-values = 0.64 and 0.58, respectively). As expected, such differences are not similar when looking at the metastasis sub-types in Fig. 4.d and g, presenting the redox and OI ratios, respectively. For the redox ratio, only skin and breast metastasis follow the same trend as for the global metastasis group, observed in Fig. 3.c, with significant difference (p-values < 0.05, 95% C.L.).
In contrast, the endocrine system and lung derived metastasis show an opposite trend. Similarly, for the OI ratio, skin and breast metastasis follow the same trend observed in Fig. 4.f between the control and global metastasis groups, while the endocrine system and lung metastasis present with the opposite trend. Figure 3.e and h display the p-values calculated for a t-test comparing the redox and the OI ratios for all combinations of 2 tissue types, respectively. One can notice that only lung metastasis can not be differentiated from the control sample.
890 nm excitation
Normalized mean fluorescence intensity spectra acquired under 890 nm excitation are shown on Fig. 4.a and b, respectively for control and global metastasis groups and for control, skin, breast and endocrine system metastasis subgroups. The corresponding fluorophore contribution histograms are presented as well in Fig. 4.c and d. In addition to NADH, FAD, Lipopigments and Porphyrin fluorescence emissions, 890 nm excitation enables the detection of SHG signal at 445 nm.
No difference between control and global metastasis groups can be observed in Fig. 4.a. However the spectra of metastasis sub-types show more differences. Firstly, breast and endocrine system tissue types do not generate SHG Moreover the endocrine system metastasis indicates a decrease of porphyrins, which can thus be used to discriminate this subgroup from breast and lung tissue at 90% C.L. with a p-value < 0.10 (Fig. 4.g). The histograms show that FAD contribution represents 45% of the signal for endocrine system subgroup whereas it is less than 35% for control, skin and lung subgroups. The corresponding discrimination is then possible at 90% C.L. with a p-value < 0.10 (Fig. 4.e). Finally, lipopigments contribution represents less than 30% of the fluorescence signal and enables to differentiate the lung sub-group from the skin and breast ones at 90% C.L. (Fig. 4.f).
(a, b) Comparison of 810 nm excitation fluorescence intensity spectra respectively for Control and Metastasis groups and for Control and each metastasis origin organ groups (c, f) Box plots comparing respectively Redox ratio and Optical Index ratio of Control vs. global metastasis group. (d, g) and for Control and metastasis sub-groups. (e, h) 3D histograms, presented in inverse log-scale, of the calculated P-values through a t-test for all 1 by 1 groups (Control, Skin, Lung, Endocrine system, Breast metastasis) comparing (95% C.L. and tail = 2), respectively for Redox ratio and Optical Index ratio.
(a, b) Comparison of 890 nm excitation fluorescence intensity spectra respectively for Control and Metastasis groups and for Control and each metastasis origin organ groups. (c, d) Histograms of molecular contributions respectively for Control and Metastasis groups and for Control and each metastasis origin organ groups. (e, f, g) 3D histograms, presented in inverse log-scale, of the calculated P-values through a t-test for all 1 by1 group (Control, Skin, Lung, Endocrine system, Breast metastasis) comparisons (90% C.L. and tail = 2), respectively for the FAD, Lipopigments and Porphyrin molecules.
Fluorescence lifetime study
In addition to the fluorescence spectral measurements, the multimodal microscope enables fluorescence lifetime measurements. As an illustration, the phasor FLIM histograms of NADH acquired at 810 nm for control and skin metastasis group are displayed respectively on Fig. 5.a and Fig. 5.b. Those obtained for FAD at 890 nm are plotted in Fig. 6.a and Fig. 6.b respectively. Differences are visible both at 810 nm and 890 nm excitations, long and short lifetimes are shorter for skin subgroups (1.7 ns, 0.20 ns under 810 nm excitation ; 1.8 ns, 0.29 ns under 890 nm) than for control group (2.2 ns, 0.55 ns under 810 nm excitation ; 2.3 ns, 0.66 ns under 890 nm).
Long Lifetime Intensity Fraction (LLIF) distributions of NADH under 810 nm excitation and FAD under 890 nm excitation for global and each metastasis subgroup are respectively plotted in Fig. 5.c and 6.c. Due to the large spread of the distributions, we cannot quantify the differences between the metastasis subgroups. For 810 nm excitation, NADH LLIF of endocrine system and breast subgroups tend to be smaller than others. For FAD under 890 nm excitation, control and skin groups have respectively a LLIF median value of 0.66 and 0.5.
Discussion and conclusion
The aim of this study was to investigate the ability of multimodal autofluorescence analysis to discriminate brain metastasis according to the site of the primary malignancy. It was already successful to discriminate healthy control tissues from cancerous ones16,24,25, as well as for the classification of cancerous tissues according to their tumor grade22,23. However using autofluorescence to determine the organ of origin of a brain metastasis sample has not been presented in the literature to the best of our knowledge.
Previously, metastastatic tissue samples were considered as one cancer type and were differentiated from primary brain tumors.
Deep UV excitation of each metastasis sub-type highlighted the different fluorescence contributions of three primary molecules, tyrosine, tryptophan and collagen crosslinks. Tryptophan residues are widely used as a fluorescent marker in studying cellular mechanisms that regulate cell growth and proliferation, its autofluorescence signal and its derived metabolic ratios have been proven as an optical biomarker to discriminate cancer from healthy tissue24,26. On the other hand, collagen has multiple roles in different tissue compartments of the central nervous system and its fluorescence emission is considered as an excellent marker for tissue vascularization27.
Regarding the obtained results, and as expected, the global metastases group was discriminated significantly with the relative contribution of the three excited fluorophores increasing in this group. This result is in line with a past study where tryptophan/collagen ratio was found higher in the metastasis group24. Furthermore, all the metastasis sub-types show the same trend as for the global group but with a high intra-group heterogeneity, in particular for the collagen contribution. Colon metastasis presented indeed the largest proportion of collagen compared to other sub-types we studied and is thus well discriminated from breast, lung and skin metastasis.
The spectral results acquired at 810 nm excitation showed that metastasis fluorescence emission has a similar spectral shape as control samples but with a slightly lower NADH levels (Fig. 3.a). However, not all the metastasis sub-types showed the same trend to that for the global group. In addition, the extracted molecular ratios at 810 nm, the redox and the OI ratio, led to the same conclusion. The redox and the OI ratios were similar between the global metastasis group and the control (Fig. 3. c and f), but most of the sub-types could be differentiated from the control group (skin and breast cancer subtypes have significantly different ratios, Fig. 3.d and g) and from one to another as well. Endocrine system and lung metastasis are particularly well discriminated from skin and breast tissue.
For the NIR excitation domain at 890 nm excitation, the spectral results showed that the fluorescence emissions of metastases have a similar spectral shape as that of control samples. However, some of the metastasis sub-types did not show the same trend as for the global group, especially when looking at SHG and porphyrin levels. In particular, SHG is not present in the emission spectra of endocrine system and breast cancer subgroups while a lower porphyrin contribution differentiated this latter subgroup from that of the endocrine system one.
Considering both DUV and NIR excitation domain results, it was shown that the endocrine system group presented significant collagen fluorescence compared to control, while it had no SHG signal which would occur normally within collagen fibers. This could suggest that a malignant endocrine tumor could be characterized by a large production of collagen with a high level of ultrastructural disorder, not compatible with SHG detection, as reported by D. Tokarz et al. in their studies relative to SHG microscopy for pathological thyroid tissue characterization28,29.
The final modality, fluorescence lifetime phasor analysis, gave clues on the variation of free and protein-bound components of FAD and NADH. This approach was used previously to discriminate and to specify meningioma grades23and low- and high-grade glioma as well22. Following the variation of the two lifetime components of FAD between control and tumor tissues permitted tracking changes in the metabolic state of tissue at the molecular scale, due to its role in tissue metabolism. In a past study, the FAD LLIF distribution was shown to be lower in the global metastasis group25, due to an increase of protein-bound FAD, and a decrease of free FAD levels, resulting in higher LLIF values. Indeed, the results obtained for NADH and FAD LLIF at 810 nm and 890 nm in metastasis sub-types, gave a similar trend but with degrees depending of the sub-type. The breast metastasis showed a strong shift toward lower LLIF values, indicating a strong increase of protein-bound FAD, while the lung metastasis showed LLIF distribution similar to the control. This observation strengthens the conclusion, established from spectral results, that the metastasis subtypes do not have similar metabolic properties, with some types having an opposite trend compared to the pooled metastases group.
Overall, this work is a proof-of-concept study which demonstrates the ability of multimodal autofluorescence, an already validated technique, to discriminate brain metastasis samples according to their primary systemic malignancy. As expected, and in line with our previously published studies, we were able to significantly discriminate, through spectral analysis, the control samples from the global metastasis. It was also possible to observe inter-subtype differences, especially in the DUV domain and NIR domain. At 810 nm wavelength excitation, the difference trend could be specific and linked to the metastasis subtype. At 890 nm wavelength excitation the metastases subtypes were differentiated by their SHG signal. Indeed, this study shows that the molecular composition of metastasis that have migrated to the brain differ according to their origin and emphasizes the potential of autofluorescence analysis to discriminate brain metastases. Further investigation to continue characterizing metastases subtypes based on their multiscale autofluorescence is warranted with a larger sample size.
Materials and methods
Samples cohort
Samples were obtained, directly after the brain tumor resection in the neurosurgery department of GHU Paris psychiatric & neurosciences hospital center in Paris, after approval of the GHU Paris – University Paris Descartes Review Board (CPP Ile de France 3, S.C.3227). Informed written informed consent was obtained from all patients that participated in this study while all measurements were carried out in accordance with the guidelines and regulations of this approval.
Each sample was sent directly to our laboratory (Orsay-France) in a normal saline solution for NIR multiphoton microscope analysis. Samples were fixed immediately post measurements and returned to the hospital.
For DUV measurements at the DISCO Beamline located at Synchrotron SOLEIL (Saint-Aubin, France), fresh samples stored at −80 °C were sent from the hospital to our laboratory and raised to − 18 °C before cutting. The samples were cut into 10 μm slices by cryostat (CM 1950, Leica Microsystems), fixed in pure ethanol (100 °C), and stored at 4 °C.
The cohort statistic is presented in Table 1. For DUV spectrum measurements, several (3 to 6) regions of interest (ROI) have been considered for each sample, one ROI representing the average spectrum of 300 to 1600 spectra. Heterogeneity of those numbers are mainly due to the differences of the sizes of the samples. For NIR measurements the ROI are composed of a 3 × 3 images mosaic.
NIR measurements setup
The NIR measurements setup consists of a confocal multimodal multiphoton benchtop microscope (TCS SP8 MP, Leica Microsystems, Wetzlar, Germany) that can perform the following acquisitions: (1) Two-photon fluorescence imaging, (2) SHG (second harmonic generation) imaging, (3) spectral imaging acquisitions, and (4) Fluorescence Lifetime Imaging (FLIM). This microscope is coupled with a FLIM module (PicoHarp 300, PicoQuant GmbH, Berlin, Germany) that uses Time-Correlated Single Photon Counting (TCSPC) technique for FLIM acquisitions.
This work used two excitation wavelengths: 810 nm and 890 nm. In imaging mode, our microscope configuration permits using 810 nm to detect NADH and FAD signals and 890 nm to detect SHG and FAD signals. The obtained NIR images were visualized and merged through the open source-image-processing software FIJI30. The spectrally-resolved fluorescence intensities were detected by a hybrid detector placed in the confocal head of the microscope.
In spectral mode, and using 810 nm as excitation wavelength, the detected signal corresponds to the fluorescence signal of NADH (free + bound), FAD, Lipopigments, and porphyrins (I and II). At 890 nm excitation the SHG signal and the fluorescence of FAD, Lipopigments, and Porphyrins (I and II) are detected.
More details about this microscope and its configuration were previously reported16,23,24.
DUV measurements setup
DUV measurements were performed at the DISCO beamline setup at Synchrotron SOLEIL, where spectral measurements were carried out using the 275 nm excitation wavelength and detected through a micro-spectrofluorimeter (Olympus IX71). Several Regions Of Interest (ROI) measuring 160 × 160 μm with a 4 μm pixel resolution were obtained from each sample. The spectrum in each pixel corresponds to the fluorescence signal emission of the four excited molecules: Tyrosine (Tyr), tryptophan (Tryp), collagen (Coll) and NADH.
Subsequently, wide-field microscopy (Zeiss Axio-observer Z-1) using 275 nm excitation was collected sequentially for two band-pass filters (323–357 nm and 408–438 nm, Semrock, USA) and detected by a CCD camera (Pixis BUV, Princeton Instrument, USA). Thus a large mosaic of the entire sample section for tryptophan and collagen was obtained. The DUV wide-field obtained images were merged using the open source-image-processing software FIJI30.
Spectral analysis
Spectral DUV data was extracted and in-house developed Matlab-based software at the DISCO beamline already described in a previously published paper24was applied. The FIJI software permitted the extraction of NIR Spectral images, and both image sets were combined as per our previous published works20.
A spectral fitting process extracts the emission contribution of each of the 4 fluorophores using both the DUV and NIR spectral data by representing the emission spectrum of each fluorophore by a Gaussian fit with the maximum emission wavelength and the spectral bandwidth as listed in Table 3. These Gaussian parameters were chosen based on our previous experiments and works22,23and a literature review31.
The spectral fitting process provides each fluorophore’s emission Integral contribution (Int) to the total spectra. The parameter (integral proportion) is the ratio of a fluorophore’s emission curve to the integral under the total fitted spectrum curve. By rationing the different calculated integral proportions, we were able to define the different molecular ratios mentioned in this paper following Eqs. 1 and 2 below:
FLIM and LLIF extraction
FLIM acquisition were performed and then extracted through the Symphotime software (PicoQuant, GmbH, Berlin, Germany) that controls the TCSPC module, coupled to the multiphoton NIR microscope. FLIM images were then processed using FIJI sofwatre and a home-developed Matlab algorithm. The algorithm utilized the phasor approach32to extract the Long Lifetime intensity Fraction (LLIF, alpha parameter) distribution for each tissue type. For each FLIM image, the size was reduced from 512 × 512 pixels to 16 × 16 pixels. Thus each new pixel represents the sum of 1024 pixels from the original image. The fluorescence decay curves of each new pixel were added together to obtain one global decay curve I(t). Afterwards, each global decay curve was represented by a unique vector in a graphical plot having its unique location, called phasor (or count) in the phasor plot32,33,34. Every global decay curve I(t) was converted in a Cartesian plot into two coordinates using the following equations :
The “i” index corresponds to the new global pixel in the raw image and the two coordinates of each phasor count were represented by Si(ω) and Gi(ω). The laser repetition angular frequency is represented by \(\:{\upomega\:}\) and it is related to the sampling period\(\:\:\left(\text{T}\text{s}\right)\) and to the signal length (\(\:\text{L})\) via the following equation :
The phasor counts of all the samples belonging to the same tissue type were regrouped to plot the global phasor histogram. The local maximum of the global histogram was localized and a fitting line (polynomial fitting) was drawn where the two intersections points between the phasor circle segment and this fitting line are related to the two fluorescence FAD components lifetime values32,34(and similarly for the fluorescence lifetime components of NADH). Then, a projection on the line of its phasor counts was performed to calculate the LLIF histogram of each molecule23,32.
Data availability
The datasets generated and analyzed in this work are not publicly available due to their confidentiality, as part of these data were acquired from human patient samples following the approval of the GHU Paris Psychiatrie et Neuroscience Hospital – University Paris Descartes Review Board (CPP Ile de France 3, S.C.3227), but they could be made available from the corresponding author on reasonable request.
References
Nayak, L., Lee, E. Q. & Wen, P. Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 14, 48–54 (2012).
Buckner, J. et al. (ed, C.) Central nervous system tumors. Mayo Clin. Proc. 821271–1286 (2007).
Brain metastases - Symptoms and causes. Mayo Clinic https://www.mayoclinic.org/diseases-conditions/brain-metastases/symptoms-causes/syc-20350136
Patchell, R. A. The management of brain metastases. Cancer Treat. Rev. 29, 533–540 (2003).
Hall, W. A., Djalilian, H. R., Nussbaum, E. S. & Cho, K. H. Long-term survival with metastatic cancer to the brain. Med. Oncol. Northwood Lond. Engl. 17, 279–286 (2000).
Tan, A. C., Heimberger, A. B., Menzies, A. M., Pavlakis, N. & Khasraw, M. Immune checkpoint inhibitors for brain metastases. Curr. Oncol. Rep. 19, 38 (2017).
Mahmoodifar, S. et al. A quantitative characterization of the Spatial distribution of brain metastases from breast cancer and respective molecular subtypes. J. Neurooncol. 160, 241–251 (2022).
Neman, J. et al. Use of predictive Spatial modeling to reveal that primary cancers have distinct central nervous system topography patterns of brain metastasis. J. Neurosurg. 136, 88–96 (2022).
Hengel, K. et al. Attributes of brain metastases from breast and lung cancer. Int. J. Clin. Oncol. 18, 396–401 (2013).
Schroeder, T. et al. Mapping distribution of brain metastases: does the primary tumor matter? J. Neurooncol. 147, 229–235 (2020).
Mehidine, H. et al. A customized two photon fluorescence imaging probe based on 2D scanning MEMS mirror including electrothermal two-Level-Ladder dual S-Shaped actuators. Micromachines 11, 704 (2020).
Mehidine, H., Devaux, B. & Varlet, P. Abi Haidar, D. Comparative study between a customized bimodal endoscope and a benchtop microscope for quantitative tissue diagnosis. Front. Oncol. 12, 881331 (2022).
Croce, A. C. & Bottiroli, G. Autofluorescence spectroscopy and imaging: a tool for biomedical research and diagnosis. Eur. J. Histochem. 58, (2014).
Toms, S. A. et al. Intraoperative optical spectroscopy identifies infiltrating glioma margins with high sensitivity. Oper. Neurosurg. 57, 382–391 (2005).
Papayan, G., Petrishchev, N. & Galagudza, M. Autofluorescence spectroscopy for NADH and flavoproteins redox state monitoring in the isolated rat heart subjected to ischemia-reperfusion. Photodiagnosis Photodyn Ther. 11, 400–408 (2014).
Zanello, M. et al. Multimodal optical analysis discriminates freshly extracted human sample of gliomas, metastases and meningiomas from their appropriate controls. Sci. Rep. 7, 41724 (2017).
Haidar, D. A., Leh, B., Zanello, M. & Siebert, R. Spectral and lifetime domain measurements of rat brain tumors. 15 (2015).
Palmer, S. et al. Optical redox ratio and endogenous porphyrins in the detection of urinary bladder cancer: A patient biopsy analysis. J. Biophotonics 12 (2016).
Ostrander, J. H. et al. Optical redox ratio differentiates breast Cancer cell lines based on Estrogen receptor status. Cancer Res. 70, 4759–4766 (2010).
Poulon, F. et al. Optical properties, spectral, and lifetime measurements of central nervous system tumors in humans. Sci. Rep. 7, 13995 (2017).
Poulon, F. et al. Real-time brain tumor imaging with endogenous fluorophores: a diagnosis proof-of-concept study on fresh human samples. Sci. Rep. 8, 14888 (2018).
Mehidine, H. et al. Optical signatures derived from deep UV to NIR excitation discriminates healthy samples from low and high grades glioma. Sci. Rep. 9, 8786 (2019).
Mehidine, H. et al. Molecular changes tracking through multiscale fluorescence microscopy differentiate meningioma grades and non-tumoral brain tissues. Sci. Rep. 11, 3816 (2021).
Poulon, F. et al. Multimodal analysis of central nervous system tumor tissue endogenous fluorescence with multiscale excitation. Front. Phys. 6, 109 (2018).
Sibai, M. et al. Comparison of optically-derived biomarkers in healthy and brain tumor tissue under one‐ and two‐photon excitation. J. Biophotonics 12, (2019).
Jamme, F. et al. Deep UV autofluorescence microscopy for cell biology and tissue histology. Biol. Cell. 105, 277–288 (2013).
Gregorio, I., Braghetta, P., Bonaldo, P. & Cescon, M. Collagen VI in healthy and diseased nervous system. Dis. Model. Mech. 11, dmm032946 (2018).
Tokarz, D. et al. Ultrastructural features of collagen in thyroid carcinoma tissue observed by polarization second harmonic generation microscopy. Biomed. Opt. Express. 6, 3475 (2015).
Tokarz, D. et al. Characterization of pathological thyroid tissue using polarization-sensitive second harmonic generation microscopy. Lab. Invest. 100, 1280–1287 (2020).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 9, 676–682 (2012).
Chorvatova, M. & Chorvat, D. A. Tissue fluorophores and their spectroscopic characteristics. in Fluorescence Lifetime Spectrosc. Imaging (2014).
Digman, M. A., Caiolfa, V. R., Zamai, M. & Gratton, E. The phasor approach to fluorescence lifetime imaging analysis. Biophys. J. 94, L14–L16 (2008).
Clayton, A. H. A., Hanley, Q. S. & Verveer, P. J. Graphical representation and multicomponent analysis of single-frequency fluorescence lifetime imaging microscopy data. J. Microsc. 213, 1–5 (2004).
Redford, G. I. & Clegg, R. M. Polar plot representation for Frequency-Domain analysis of fluorescence lifetimes. J. Fluoresc. 15, 805–815 (2005).
Acknowledgements
The authors thank Prof. Pascale Varlet for her contribution in providing pathological data, and the Delegation for Clinical Research of the GHU Paris – Psychiatrie and Neurosciences and the Neurosciences Biobank (BB-0033-00026) for their support.
Funding
This work is financially supported by ITMO Cancer AVIESAN (Alliance Nationale pour les Sciences de la Vie et de la Santé, National Alliance for Life Sciences and Health) within the framework of the Cancer Plan for MEVO and IMOP projects, by CNRS with “Défi instrumental” grant, by ligue nationale contre le cancer (LNCC) and the Institut National de Physique Nucléaire et de Physique des Particules (IN2P3). Part of this work was done at the PIMPA Platform (The confocal-mutlitphoton fluorescence microscope platform) partly funded by the French program“Investissement d’Avenir” run by the “Agence Nationale pour la Recherche” (grant “Infrastructure d’avenir en Biologie Santé – ANR – 11-INBS-0006”).
Author information
Authors and Affiliations
Contributions
C.R. analysed the data and participated on the writing of the manuscript. D.A.H., H.M. and M.S participated to the collect of the data and worked on the manuscript writing. B.D. provided samples and participated in the manuscript writing. F.J and M.R provided support and tools for data acquisition on the DISCO Beamline of Synchrotron SOLEIL. We acknowledge SOLEIL for provision of synchrotron radiation facilities (proposals numbers 20160206;20160978; 20191511; 20231478; 20241766) and we would like to thank F.J. and M.R. for assistance in using DISCO beamline. D.A.H .designed the research project, the protocols for experiments, supervised the work and participated in the manuscript writing. Equally important, D.A.H. provided necessary funds for the experiments. All authors reviewed the manuscript and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rimbault, C., Devaux, B., Mehidine, H. et al. Proof of concept study on differentiating metastatic brain samples by their originating organ using multimodal autofluorescence spectroscopy. Sci Rep 15, 11476 (2025). https://doi.org/10.1038/s41598-025-92366-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-92366-1