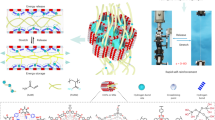
Abstract
Enhancing the stability of polymer gels under harsh conditions is vital for improving enhanced oil recovery (EOR) applications. This study introduces a novel gel synthesized using a MgO-HPAM nanocomposite and zirconium sulfate crosslinker (CL). The gel demonstrates exceptional durability, capable of maintaining its properties under high temperature and salinity, addressing long-standing challenges in EOR. The research involved several key experiments. Initial tests revealed that MgO does not influence gelation time; this is controlled by polymer and CL concentrations. The nanocomposite significantly improved the storage modulus, with increases of 72.47% and 84.52% at 250 and 500 ppm MgO, respectively. Syneresis studies showed that after 50 days, gels with 250 ppm nanocomposite retained 88% of their weight, unlike the nanocomposite-free gels which lost 75%. In core flooding experiments, nanocomposite gels demonstrated higher injection pressure and reduced permeability compared to nano-free gels. SEM imaging confirmed nanoparticle agglomeration in the sand face, enhancing the gel’s barrier properties. Over 50 days at 90 °C and 2000 psi, permeability reductions were observed at 58%, 98.1%, and 97.9% across the samples. These findings underscore the potential of this gel in advancing EOR techniques.
Similar content being viewed by others
Introduction
The oil and gas industry, as a cornerstone of global energy supply, is essential for economic development and meeting the energy demands of nations worldwide1,2,3. Beyond providing fossil fuels crucial for electricity generation, transportation, and industrial processes, this sector represents a major source of revenue for many countries. However, with dwindling easily accessible oil reserves, one pressing challenge is extending the life and production capacity of existing reservoirs4,5,6. Enhanced Oil Recovery (EOR) techniques have become increasingly central to addressing this challenge, particularly in mature reservoirs where production has naturally declined due to reduced reservoir pressure7,8,9,10. Through EOR, oil extraction from these depleted reservoirs is enhanced, proving critical as new resources become scarce, thus helping to boost global oil and gas reserves11,12,13,14.
Excess water production is a prevalent issue in mature reservoirs, posing significant operational challenges in oil extraction15,16. This phenomenon, stemming from decreased reservoir pressure, results in unwanted water fluid migration into oil production streams. The resultant excess water production degrades oil quality, escalates separation costs, and reduces oil output, thereby inflating operational expenses linked to water management17,18,19. Consequently, strategies to mitigate excess water production are vital for sustaining oil production efficiency, especially in low-yield reservoirs. Further exacerbating economic and environmental issues, increasing volumes of produced water necessitate intricate treatment and disposal operations20.
Numerous methods have been developed to tackle excess water production challenges. Among them, the injection of preformed particle gels emerges as a common approach. These gels, pre-manufactured and injected into reservoirs, restrict unwanted water flow and hinder its incorporation into oil wells, thereby facilitating enhanced oil recovery21,22,23.
Techniques such as polymer flooding24,25,26,27 and strategic injection and production management in oil fields are also utilized to curb excess water production. By incorporating polymers as fluid additives, these methods enhance the mobilization of petroleum fluids within reservoirs, thus effectively regulating reservoir pressure and mitigating water production. Moreover, in situ gel injection represents a sophisticated, advanced technique in managing excess water. This involves injecting reactive chemicals into the reservoir, where they undergo transformations into gels under specific reservoir conditions, serving as temporary barriers against water flow28,29,30,31,32.
In situ gel injection stands out as a cutting-edge, effective EOR technique for blocking unproductive fluid flow and enhancing oil extraction in underperforming reservoir zones. The method typically involves injecting a mixture containing polymers, crosslinking agents, and often nanoparticles into the reservoir. These components, responding to high-temperature and high-salinity environments, form gels that act as water flow barriers33,34. This technique holds significant potential for amplifying oil recovery, especially in mature or low-capacity reservoirs. Despite its advantages, in situ gel injection faces challenges such as short gelation times, which complicate gel delivery before reaching desired reservoir depths35. Moreover, gels may degrade rapidly in harsh reservoir conditions, particularly under high temperature, salinity, and pressure, which compounds issues regarding component adsorption on reservoir rocks that diminish gel efficiency36,37,38.
To counter these challenges, researchers have explored several methods. Utilizing robust polymers capable of withstanding harsh conditions enhances gel stability, facilitating efficient gel injection. Stronger crosslinking agents further stabilize gel structures against environmental degradation. Integrating nanoparticles, especially inorganic variants like nanoparticles, can significantly augment the performance of polymer gels by improving their physical and chemical properties and preventing rapid degradation39,40,41.
nanoparticles, characterized by high thermal stability and resistance to harsh chemical and pressure conditions, are particularly promising for enhancing polymer gel formulations42,43. The improvement of gel properties by nanoparticles is primarily attributed to their ability to enhance the gel’s structural integrity and resistance to harsh environmental conditions44,45,46. Nanoparticles function at a nanometric scale, allowing them to interact intimately with the polymer matrix, which enhances crosslinking density and overall network structure. This interaction leads to increased mechanical strength and thermal stability, as the nanoparticles serve as additional crosslinking agents or reinforce existing crosslinks within the polymer chains. Furthermore, nanoparticles’ high surface area facilitates improved distribution within the gel, effectively imparting superior viscoelastic properties. They help mitigate syneresis, the process wherein a gel shrinks due to the expulsion of liquid, by fortifying the network and preventing polymer chain aggregation. In high salinity and temperature environments, the presence of nanoparticles reduces degradation rates of the polymer matrix, thereby maintaining the gel’s functionality over extended periods. Consequently, incorporating nanoparticles not only improves the gel’s ability to block or divert water in enhanced oil recovery processes but also ensures stability and effectiveness under extreme reservoir conditions47,48.
These properties render nanoparticles ideal for applications in high-temperature, saline environments where they bolster gel strength and stability. Nonetheless, comprehensive laboratory studies on the optimization and effects of MgO nanocomposite gels for EOR remain scarcely explored, underscoring the need for further investigation into their potential benefits within the oil industry.
This study systematically synthesizes MgO-HPAM nanocomposites with suitable crosslinking agents. It evaluates their properties through experiments such as bottle testing, syneresis, and core flooding. The study investigates how varying MgO concentrations influence gelation time, rheological properties, and gel stability under extreme salinity and temperature. These experiments aim to identify optimal conditions for deploying nanocomposite gels in EOR initiatives and assess gel interactions with reservoir porous media. Collectively, this research aspires to establish the most effective formulations for employing MgO-HPAM nanocomposite gels in real-world reservoir conditions.
Experimental section
Materials
HPAM polymer
Hydrolyzed polyacrylamide (HPAM) polymer is one of the most widely used polymers in enhanced oil recovery (EOR) processes. It is suitable for forming stable gels due to its excellent mechanical properties and high stability under extreme conditions, including harsh salinity and high-temperature environments. This polymer contains amide groups in its structure, contributing to its chemical resistance. Additionally, the presence of hydroxyl groups enables it to interact with other materials, especially CLs, facilitating the formation of gels with high viscosity and structural integrity in adverse conditions.
The present study used HPAM with an average molecular weight of 5–8 million Daltons and a hydrolysis degree of approximately 25–30%. The polymer was purchased from Sigma-Aldrich in a research-grade purity suitable for gel synthesis. Its properties make it particularly effective in forming strong crosslinked gels capable of resisting extreme salinity and temperature conditions. When combined with MgO nanoparticles, HPAM increased the gel’s stability and performance, thus improving the resilience of the gel under harsh conditions.
Zirconium sulfate
Zirconium-based CLs are widely utilized in gelation processes because they form strong bonds with polymers, specifically with amide groups in HPAM. These crosslinkers create a robust and interconnected gel structure, thus enhancing the stability and resistance of the gel to environmental challenges such as high salinity, elevated temperatures, and significant pressure variations.
This research employed zirconium sulfate (Zr(SO4)2·4 H2O) as a CL to improve the gel’s mechanical properties and reduce syneresis, particularly in high-temperature and high-pressure environments. The compound was obtained from Merck, with a reported purity of 99%, ensuring consistency and reliability during the synthesis process. Its interactions with HPAM polymers ensured the formation of a highly stable gel suitable for field applications in EOR.
MgO nanoparticles
Due to their excellent physical and chemical properties, magnesium oxide (MgO) nanoparticles have garnered significant interest in various industries, including EOR. These properties include high specific surface area, remarkable thermal stability, and surface catalytic activity. At the nanoscale, MgO exhibits enhanced interactions with polymers and crosslinkers, significantly improving the mechanical strength and stability of the resulting gel.
This study utilized MgO nanoparticles with an average particle size of less than 50 nm to synthesize a nanocomposite gel. The nanoparticles were purchased from Sigma-Aldric, with a reported purity of 98%. Their ability to form strong interactions with HPAM polymer and zirconium sulfate CL was critical for improving the hydrogel’s performance in high salinity and temperature conditions. Furthermore, due to their high surface area, these nanoparticles significantly enhanced the gel structure’s stability, making them ideal for use in challenging reservoir environments.
Experiments
Bottle test
In this test, gelation time and gel strength are evaluated visually. This experiment is such that the desired mixture for making the gel is first prepared. For this step, the required amount of polymer is added to distilled water and stirred for 24 h with a rod mixer until fully hydrated. Then, the remaining additives, including CL, are added, and the resulting mixture is poured into a bottle. By placing this bottle at the test temperature, which is 95 °C, the gel evaluation test begins. At specific time intervals, the gel bottle is inverted, and the changes in the appearance of the composition in it are coded according to Table 1.
This method of examining the gel conditions over time is known as the Sydansk method. In which gelation time and gel strength are evaluated visually. Based on this method, this study evaluated 24 gel samples with different compositions to determine the appropriate range of polymer and CL concentrations for optimal gel formation. A gelation time of less than 8 h causes the composition not to be pumpable and creates operational problems. Also, if this parameter is more than 24 h, the gel strength is not suitable, and the gel composition in a porous medium is uncontrollable due to high adsorption on the surface of the rocks.
Gel strength measurement
The Gel Strength Measurement test is crucial for evaluating the strength and stiffness of gels under various conditions, especially in the fields of petroleum engineering and other chemical industries where gels are used as material transport systems or flow modifiers. This test determines gels’ elastic and viscous properties under mechanical stress. We can analyze a gel’s elastic and viscoelastic properties by measuring the Storage Modulus and Loss Modulus. The storage modulus represents the ability of a gel to store energy and respond elastically, while the loss modulus represents the amount of energy lost due to instability or viscosity within the gel structure. These measurements are important in determining how a gel behaves under external stresses and also in evaluating the performance of a gel in processes such as EOR.
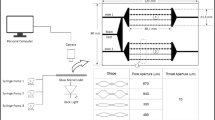
A Rheometric Scientific (ARES G2 Rheometer) was used to perform the gel strength measurement test presented in Fig. 1. This device is one of the most accurate laboratory instruments for measuring materials’ rheological properties and can simultaneously measure the storage and loss modulus. In this test, gel samples previously evaluated as suitable for the bottle test were subjected to different temperature and time conditions.
The viscometer can accurately record the changes in viscosity and elasticity of the gels during the gelatinization process. The samples are subjected to a specific compression or tension during the test, and data on the storage and loss modulus are collected. These measurements are continuously performed with changes in environmental conditions to obtain the best possible assessment of the strength and properties of the gel.
Syneresis experiment
The Syneresis Measurement experiment is essential in evaluating gels’ stability and resistance to extreme environmental conditions, especially in high salinity, temperature, and pressure. This test is specifically designed to investigate the stability of gels in environments that may cause physical changes in their polymer network structure. One of the most important phenomena that occurs under these conditions is the phenomenon of syneresis, in which water separates from the gel structure and causes a decrease in the gel volume. This phenomenon can significantly negatively affect the performance of gels, especially in applications such as enhanced oil recovery (EOR). Therefore, the syneresis test is recognized as a key tool for evaluating the stability of gels against salinity, high temperature, and high-pressure conditions.
This test uses previously evaluated gels suitable for bottle and gel strength measurements. To perform the test, 10 g of each gel sample is cut and placed in water with a salinity of 250,000 ppm (the composition is presented in Table 2). This high salinity is typically chosen to simulate conditions where highly saline solutions are present, such as subsurface environments in oil wells. The gel samples are then stored at 95 °C for 60 days. The 95 °C temperature is intended to simulate the high-temperature conditions in industrial processes and oil wells. This long storage period is designed to investigate the changes and stability of the gels under these challenging conditions.
During these 60 days, the gel samples are regularly measured for weight loss. This weight loss indicates the syneresis process and the separation of water from the polymer structure of the gel. If the gels show a significant weight loss during this period, it means that the gel structure is not stable enough and has reduced its water-holding capacity against high salinity and temperature conditions. This is particularly concerning for applications such as EOR, where gels increase permeability and control flow. Excessive weight reduction of gels can reduce their effectiveness in controlling flow and directing fluids to specific areas in oil reservoirs.
Synthesizing nanocomposite gel
In the synthesis process of nanocomposite gels, MgO nanoparticles are first dispersed using an ultrasonic device for one hour. This step is crucial because nanoparticles, due to their small size and tendency to aggregate in the environment, must be uniformly dispersed in the solution to maximize their efficiency in the following steps. The use of ultrasonic waves causes changes in pressure and tension in liquids, transforming the nanoparticles from an aggregated state to a dispersed state. After proper dispersion of the nanoparticles, the next step is to add the polymer in the optimal amount. The selected polymer must be able to form a polymer network with desirable mechanical and chemical properties to ensure the stability of the final gel. In the third step, a zirconium sulfate CL is added to the combination of nanoparticles and polymer. This CL is responsible for creating crosslinks in the polymer network. Adding a CL causes the polymer molecules to connect and form a three-dimensional and resistant network, giving the gel more excellent mechanical properties and stability.
Gel performance evaluation in porous media
The gel performance evaluation test in porous media is a key step in evaluating the effect of gel injection in reducing the permeability of reservoir rocks and enhancing oil recovery (EOR). First, the cores are carefully prepared to provide the desired test conditions. After preparation, the cores are saturated with brine, and their initial permeability is measured. This step is performed to determine the natural permeability of the cores and is the basis for calculating the gel performance indices.
Then, the gel composition obtained from the previous steps is injected into the cores using the core flooding device presented in Fig. 2, and the injection pressure is recorded. This step is important for understanding how the gel behaves in the porous pores and can provide key information about the injection process and gel formation under reservoir conditions.
Next, the cores, now containing the gel, are held for 7 days at 95 °C and 2000 psi to complete the gel crosslinking and stabilization process. After this period, brine is injected into the core once again, and the new permeability of the cores is calculated. The resistance factor (RF) is calculated from the ratio of the initial permeability to the permeability at this stage and indicates the effect of the gel on reducing the permeability of the core. In the final step, the cores are placed in a saltwater environment for 60 days under the same conditions (95 °C and 2000 psi) to measure the stability of the gel and its long-term effect on permeability. After this period, the cores are flooded with brine, and the permeability is measured again. The residual resistance factor (RRF) is calculated by the ratio of initial permeability to permeability at this stage. RRF indicates the ability of the gel to maintain permeability reduction and resistance to washing over time. This critical parameter provides us with information about the long-term performance and stability of the gel, which is essential for designing and optimizing EOR designs and operational decisions.
Results and discussion
Bottle test results
This detailed analysis of the bottle test results aims to investigate and analyze the gelation time and the effect of different concentrations of CL and polymer. In these experiments, different amounts of CL and polymer have been added to the gel composition to evaluate how these materials affect the speed and quality of gel formation. A detailed analysis of these experiments will help clarify the design and selection of optimal compositions for enhanced oil recovery (EOR) processes.
All compositions and results obtained from the bottle test using Sydansk codes are presented in Table 3. First, the results showed that increasing the CL concentration to 0.3 wt% reduces the gelation time. This is consistent with the basic principles of gel chemistry, as CLs act as crosslinking agents in the polymer structure. Adding a CL links the polymer molecules to form a three-dimensional network, allowing the material to gel more quickly. Reducing gelation time can be very useful in rapid gel formation processes. This is especially important in the EOR field, which requires precise control of the gelation time to block the hydraulic channels and create a stable oil flow. However, after 0.3 wt%, increasing the CL concentration beyond this value increases the gelation time. This increase in time is most likely due to the phenomenon of over-crosslinking. Crosslinks are formed inefficiently and disorderly when the CL concentration is too high, which leads to a slow crosslinking and gelation process. This phenomenon usually occurs when excess CLs create bonds that increase the unnecessary concentration and complicate the polymer structure. This situation not only increases the gelation time but can also negatively affect the stability and final efficiency of the gel.
Therefore, it can be concluded from the experimental results that the optimal CL concentration is 0.3 wt%, which both minimizes the gelation time and prevents the over-crosslinking phenomenon. This concentration can be effectively used in industrial processes where there is a need for rapid and stable gel formation under different operating conditions.
The effect of polymer concentration on gelation time was investigated, and the results showed a notable dependence of gelation behavior on the polymer concentration. At concentrations below 1 wt%, the gelation time exceeded 24 h, which is unsuitable for many industrial applications, particularly enhanced oil recovery (EOR) processes that require gelation times within a practical range. This extended gelation time at low concentrations occurs because fewer polymer chains are present, leading to prolonged network formation and delayed gelation.
Conversely, polymer concentrations above 1.5 wt% resulted in very short gelation times—less than 8 h. While rapid gelation might seem advantageous, it can lead to the formation of unstable and uncontrollable gels, which are undesirable in many practical scenarios. A very short gelation time can cause the gel to settle in porous media prematurely, potentially impairing its uniform distribution and reducing effectiveness.
Considering these factors, the 1 to 1.5 wt% polymer concentration range was identified as the most suitable for this application. This range strikes a balance by maintaining gelation times that are neither too long nor too short, ensuring optimal distribution and stability in the porous medium. Furthermore, within this range, a polymer concentration of 1.5 wt% was selected for this study to take advantage of its ability to enhance gel strength and mechanical properties while still achieving a practical gelation time. This optimal composition supports the development of high-stability and performance gels tailored to the demands of EOR processes.
The gelation mechanism between zirconium sulfate (Zr(SO4)2·4 H2O) and hydrolyzed polyacrylamide (HPAM) occurs through the formation of coordination bonds between the zirconium ions (Zr⁴⁺) and the amide groups (-CONH2) on the polymer chains. As highly charged cations, Zirconium ions exhibit a strong affinity for electron-rich sites such as the oxygen atom in the carbonyl group (C=O) and the nitrogen atom in the amide group.
Upon dissolving in water, zirconium sulfate dissociates to release zirconium ions and associated sulfate ions. The zirconium ions interact with the available amide groups along the HPAM polymer backbone, forming stable coordination bonds. These bonds orchestrate the crosslinking of multiple HPAM chains, creating a three-dimensional interconnected gel structure. The hydrolysis degree of HPAM facilitates the overall process, which ensures sufficient availability of reactive sites on the polymer backbone for interaction with zirconium ions.
The crosslinking network significantly enhances the gel’s viscoelastic properties, thermal stability, and resistance to syneresis, especially in high-temperature and high-salinity conditions typical of enhanced oil recovery (EOR) applications.
Nanocomposite testing results
In this study, five gel samples were synthesized using the synthesis method, one of which was without nanoparticles, and the other four samples contained nanocomposites with different concentrations of nanoparticles. The nanoparticles used were MgO and were added to the gels at concentrations ranging from 100 to 1000 parts per million (ppm). The main objective of this part of the study was to investigate the effect of nanoparticles on the gelation time and the gels’ mechanical and chemical properties.
The results of the initial experiments, presented in Table 4, disclosed that the concentration of nanoparticles did not affect the gelation time. The gelation time for all samples was 10 h, indicating that the nanoparticles themselves did not play a significant role in accelerating or delaying the gelation process. However, the effect of nanoparticles on other gel properties, especially mechanical strength, was very significant.
Gel strength data obtained from loss and storage modulus measurements showed that MgO nanoparticles caused a significant increase in the storage modulus of the gels. At 250 and 500 ppm concentrations, an increase in the storage modulus of 72.47% and 84.52% was observed, respectively. This increase represents a significant improvement in the gel’s ability to maintain its structure under mechanical stress, which could be important in applications such as enhanced oil recovery, where strong gels that can be uniformly distributed in porous media are required.
According to Fig. 3, increasing the concentration of nanoparticles also improved the gel’s resistance to syneresis. The data presented in Table 5 showed that after 50 days, the nano-free gel had lost 75% of its weight. In contrast, the gel containing 250 ppm of MgO nanocomposite lost only 12% of its weight. This significant difference in weight loss of the gels suggests that the nanoparticles can help maintain stability and reduce fluid loss by reinforcing the gel’s polymer network. Consequently, these properties make nanocomposite gels an attractive option for applications that require long-term durability and stability under variable environmental conditions.
The MgO nanoparticles, due to their high surface area and multifunctional surface chemistry, can form strong physical and chemical interactions with the amide groups in HPAM. These interactions potentially enhance crosslinking efficiency, facilitating a denser and more robust gel network. The nanoparticles act as additional crosslinking points, further reinforcing the polymer chains and enhancing their ability to retain water even under challenging thermal and saline conditions. Furthermore, MgO’s inherent thermal stability and surface catalytic activity may contribute by promoting tighter polymer coils within the gel structure, thereby reducing the tendency for syneresis and increasing the longevity of the gel under extreme reservoir conditions. This comprehensive framework of interactions underscores the role of MgO nanoparticles in significantly bolstering the mechanical and thermal stability of the polymer gel, as evidenced in Table 5.
In general, nanoparticles at high concentrations can contribute to the polymer network components and impart unique mechanical and chemical properties to gels, which could lead to broader industrial applications for these gels. This includes improving resistance to extreme environmental conditions such as high temperature and pressure and resistance to chemical changes. Further studies in this area can help better understand nanoparticle action mechanisms and optimize nanocomposite gels’ production conditions.
Gel core flooding
In this part of the study, the core flooding experiment was conducted to evaluate the performance of nanocomposite gels and compare them with gels without nanoparticles in a porous medium. All cores were identical, with a length of 11 cm, a diameter of 3.81 cm, and a gas porosity of 23.1%. This experiment, a standard method in evaluating the properties of gels in the oil and gas industries, is specifically designed to investigate how gels prevent flow and reduce permeability in oil reservoirs. This study tested three types of gels: nano-free gel, 250 ppm nanocomposite gel, and 500 ppm nanocomposite gel. The data obtained from the core flooding experiment of these three gel samples are presented in Table 6.
In the table above, initial permeability stands for the permeability measure by brine injection in the first phase, the injection pressure is the maximum injection pressure for gel injection, post permeability is related to the brine injection after gelation, and syneresis permeability is related to the permeability of the core after exposure to harsh condition. One of the outstanding results of this experiment was the significant increase in injection pressure of the nanocomposite gels compared to the nano-free gels. This difference in injection pressure was explicitly related to the nanocomposite composition. More specifically, the injection pressure for nano-free gels, 250 ppm nanocomposite gel, and 500 ppm nanocomposite gel was measured to be 810 psi, 1020 psi, and 1850 psi, respectively. This increase in pressure indicates an increase in the adhesion and viscosity of the gels due to the presence of nanoparticles. The presence of nanoparticles in gels, especially at higher concentrations, can increase the adhesion between polymer molecules and reduce the flow of the gel inside the porous medium. This can be especially useful in cases where we need to prevent water flow and prevent water from contacting specific areas in oil reservoirs. However, one of the challenges of this study was that at higher concentrations of nanoparticles, the accumulation of nanoparticles on the sand surface was observed, which can prevent the gel from completely penetrating the porous medium. This phenomenon was confirmed by SEM images, which showed that nanoparticles tend to accumulate on the sand surface, which may reduce the gel’s effectiveness in reducing permeability in deeper areas of the porous medium. SEM images taken from formed gel at the interface of cores are illustrated in Fig. 4.
Another key indicator in evaluating the performance of gels is permeability reduction. In core flooding tests, the permeability of samples was evaluated before and after gel injection. The measured permeability reduction values for nano-free gels, 250 ppm nanocomposite, and 500 ppm nanocomposite were 97.54%, 99.12%, and 98.34%, respectively. These values indicate the high effectiveness of these gels in reducing permeability and preventing fluid movement in the porous medium.
This permeability reduction indicates that the gels effectively block the space between sand particles, thereby reducing fluid flow (usually salt water). Gels are beneficial when we need to prevent flooding or water breakthroughs. In these situations, gels can effectively prevent water from passing through the reservoir and help increase oil recovery. Another aspect investigated in this study was the stability of the gels under extreme environmental conditions. After 50 days at 90 °C, high salinity, and 2000 psi, the results showed that the gels still maintained their ability to reduce permeability, especially the nanocomposite gels. Under these conditions, the permeability reduction for the nano-free gel, 250 ppm nanocomposite gel, and 500 ppm nanocomposite gel was 58%, 98.1%, and 97.9%, respectively. These results show that nanocomposite gels with higher concentrations of nanoparticles (250 and 500 ppm) are much more stable than gels without nanoparticles under high temperature, pressure, and salinity conditions.
This stability can be essential in industrial and oil environments that face extreme changes in temperature and pressure. In these conditions, there is a need for polymeric materials that can maintain their properties and continue to perform optimally even over time and under harsh environmental conditions. For this reason, using nanocomposite gels can be very effective, especially in the oil and gas industries, which require long-term durability and stability.
The presence of nanoparticles in the composition of the gels can help strengthen the polymer structure of the gels. Nanoparticles can act as reinforcement points and effectively strengthen the polymer network of the gel. This network reinforcement increases the adhesion and viscosity of the gel, which can lead to an increase in injection pressure and a decrease in permeability in the porous medium. These effects are especially clearly visible at higher concentrations of nanoparticles. However, one of the common problems in the use of nanoparticles is their aggregation on different surfaces, which can prevent the gel from completely penetrating the micropores of the porous medium.
Nanoparticles, especially MgO, can help create crosslinks between polymer molecules, thus creating a network with high strength and excellent resistance to extreme environmental conditions. Also, these nanoparticles can reduce the possibility of syneresis (loss of liquid from the gel), making the gel more stable against temperature and pressure changes.
The results of this study show that the addition of nanoparticles to polymer gels can significantly improve the performance of gels in reducing permeability and increasing injection pressure. While nanocomposite gels with higher concentrations of nanoparticles increase the injection pressure, this also means better performance in porous media that require flow inhibition. Also, these gels are more stable to extreme environmental conditions (high temperature, pressure, and high salinity) than gels without nanoparticles and can effectively prevent water breakthroughs and other related problems.
Conclusion
This study aimed to enhance the stability of polymer gels under harsh conditions by utilizing MgO-HPAM nanocomposite and zirconium sulfate coupling agent. The key findings are:
-
1.
Gelation Time:
-
The bottle test indicated that MgO nanoparticles did not affect gelation time, primarily influenced by polymer concentration and the coupling agent.
-
-
2.
Rheological Properties:
-
Loss and storage moduli measurements revealed that the nanocomposite improved these properties significantly.
-
Optimal concentrations were determined to be 1.5 wt% for the polymer and 0.3 wt% for the coupling agent.
-
MgO nanoparticles at 250 ppm and 500 ppm increased the storage modulus by 72.47% and 84.52%, reinforcing gel strength and toughness in harsh environments.
-
-
3.
Syneresis Studies:
-
After 50 days, gels without nanoparticles lost up to 75% of their weight, whereas gels with 250 ppm of nanocomposite lost only 12%, demonstrating superior stability.
-
-
4.
Core Flooding Experiments:
-
Nanocomposite gels showed increased injection pressure, with values of 810 psi for nano-free, 1020 psi for 250 ppm, and 1850 psi for 500 ppm gels.
-
SEM analysis revealed nanoparticle aggregation on the sand surface, which affected complete gel penetration.
-
Permeability reduction was 97.54% for nano-free gel and improved to 99.12% for 250 ppm and 98.34% for 500 ppm gels after initial testing, with sustained reductions of 58%, 98.1%, and 97.9% after 50 days under high temperature, salinity, and pressure.
-
-
5.
Overall Implications:
-
The study demonstrated significant improvements in nanocomposite gel stability and performance, making them suitable for EOR applications under severe conditions.
-
Challenges such as nanoparticle aggregation need addressing to optimize gel penetration and performance.
-
Data availability
Data will be available at the academic request of the corresponding author.
References
Sun, H. et al. Innovations and applications of the thermal recovery techniques for heavy oil. Energy Geosci. 5(4), 100332 (2024).
Wu, H. et al. Limosilactobacillus regulating microbial communities to overcome the hydrolysis bottleneck with efficient one-step co-production of H2 and CH4. Adv. Sci. 11(43), 2406119 (2024).
Feng, C. et al. Prediction of vitrinite reflectance of shale oil reservoirs using nuclear magnetic resonance and conventional log data. Fuel 339, 127422 (2023).
Dashtaki, S. R. M. et al. Evaluation the role of natural surfactants from Tanacetum and Tarragon plants in EOR applications. J. Mol. Liq. 361, 119576 (2022).
Chen, X. et al. A review of seismic resilience of shield tunnels. Tunn. Undergr. Space Technol. 136, 105075 (2023).
Lu, X. et al. Study on precise fuel injection under multiple injections of high pressure common rail system based on deep learning. Energy 307, 132784 (2024).
Ramirez, F., Greaves, R. & Montilva, J. Experience using microbubbles-aphron drilling fluid in Mature Reservoirs of Lake Maracaibo. In International Symposium and Exhibition on Formation Damage Control SPE-73710-MS (2002).
Yu, Z. et al. Effects of hydrogen addition on ignition characteristics and engine performance of ammonia-hydrogen blended fuel: A kinetic analysis. Int. J. Hydrog. Energy 87, 722–735 (2024).
Aghdam, S. K. Y. et al. Thermodynamic modeling of saponin adsorption behavior on sandstone rocks: An experimental study. Arab. J. Sci. Eng. 48(7), 9461–9476 (2023).
Aghdam, S. K. Y., Kazemi, A. & Ahmadi, M. A laboratory study of a novel bio-based nonionic surfactant to mitigate clay swelling. Petroleum 7(2), 178–187 (2021).
Amrouche, F. et al. A novel hybrid enhanced oil recovery technique to enhance oil production from oil-wet carbonate reservoirs by combining electrical heating with nanofluid flooding. Mater. Today Sustain. 27, 100915 (2024).
Aghdam, S. K. Y., Kazemi, A. & Ahmadi, M. Theoretical and experimental study of fine migration during Low-Salinity water flooding: Effect of Brine composition on interparticle forces. SPE Reservoir Eval. Eng. 26, 1–16 (2022).
Aghdam, S. K. Y., Kazemi, A. & Ahmadi, M. Studying the effect of various surfactants on the possibility and intensity of fine migration during low-salinity water flooding in clay-rich sandstones. Results Eng. 18, 101149 (2023).
Aghdam, S. K. Y. et al. Thermodynamic modeling of saponin adsorption behavior on sandstone rocks: An experimental study. Arab. J. Sci. Eng. (2022).
Sharma, P. & Kudapa, V. K. Study on the effect of cross-linked gel polymer on water shutoff in oil wellbores. Mater. Today Proc. 48, 1103–1106 (2022).
Al-Muntasheri, G. A. et al. Water shut-off with polymer gels in a high temperature horizontal gas well: A success story. In SPE Improved Oil Recovery Symposium SPE-129848-MS (2010).
Zeinijahromi, A. & Bedrikovetsky, P. A new method for water shut-off using induced formation damage. In SPE International Conference and Exhibition on Formation Damage Control D021S010R003 (2016).
Kazemi, A., Khezerloo-ye Aghdam, S. & Ahmadi, M. Theoretical and experimental investigation of the impact of oil functional groups on the performance of smart water in clay-rich sandstones. Sci. Rep. 14(1), 20172 (2024).
Khezerloo-ye Aghdam, S., Kazemi, A. & Ahmadi, M. Studying the effect of surfactant assisted low-salinity water flooding on clay-rich sandstones. Petroleum, 10(2), 306–318 (2023).
Dai, C. et al. A study on environment-friendly polymer gel for water shut-off treatments in low-temperature reservoirs. J. Appl. Polym. Sci. 131(8), 401-454 (2014).
Wang, L. et al. Mechanically robust re-crosslinkable polymeric hydrogels for water management of void space conduits containing reservoirs. Chem. Eng. J. 317, 952–960 (2017).
Leng, J., Wei, M. & Bai, B. Review of transport mechanisms and numerical simulation studies of preformed particle gel for conformance control. J. Petrol. Sci. Eng. 206, 109051 (2021).
Han, J. et al. Synergistic effect of poly (ADP-ribose) polymerase (PARP) inhibitor with chemotherapy on CXorf67-elevated posterior fossa group A ependymoma. Chin. Med. J. 137(22), 2770–2772 (2024).
Yuming, W. et al. The polymer flooding technique applied at high water cut stage in Daqing Oilfield. In North Africa Technical Conference and Exhibition SPE-164595-MS (2013).
Wassmuth, F. R. et al. Polymer flood application to improve heavy oil recovery at East Bodo. J. Can. Pet. Technol. 48(02), 55–61 (2009).
Yang, F. et al. High concentration polymer flooding is successful. In SPE Asia Pacific Oil and Gas Conference and Exhibition (2004).
Wang, J. et al. Synergistic utilization, critical mechanisms, and environmental suitability of bauxite residue (red mud) based multi-solid wastes cementitious materials and special concrete. J. Environ. Manag. 361, 121255 (2024).
Yin, H. et al. In situ crosslinked weak gels with ultralong and tunable gelation times for improving oil recovery. Chem. Eng. J. 432, 134350 (2022).
Yadav, U. S. & Mahto, V. Situ gelation study of organically crosslinked polymer gel system for profile modification jobs. Arab. J. Sci. Eng. 39(6), 5229–5235 (2014).
Amir, Z., Said, I. M. & Jan, B. M. Situ organically cross-linked polymer gel for high-temperature reservoir conformance control: A review. Polym. Adv. Technol. 30(1), 13–39 (2019).
Garg, A. et al. In-situ gel: A smart carrier for drug delivery. Int. J. Pharm. 652, 123819 (2024).
Wang, H. et al. Preparation of bamboo charcoal-reinforced polyamide 6 composites modified with diverse additives: Synergy and interface improvement. Ind. Crops Prod. 222, 119851 (2024).
Suriyaamporn, P. et al. Optimization of in situ gel-forming chlorhexidine-encapsulated polymeric nanoparticles using design of experiment for periodontitis. AAPS PharmSciTech 24(6), 161 (2023).
Wang, L. et al. A mesh-based physics-informed neural network for linear elastic problems in solid mechanics. Int. J. Numer. Methods Eng. 125(9), e7444 (2024).
Zhong, S. et al. Double-modal locomotion of a hydrogel ultra-soft magnetic miniature robot with switchable forms. Cyborg. Bionic Syst. 5, 0077 (2024).
Singh, R. & Mahto, V. Study of the polymer concentration and polymer/crosslinker ratio effect on gelation time of a novel grafted polymer gel for water shutoff using a central composite design method. Polym. Adv. Technol. 27(2), 204–212 (2016).
Salehi, M. B. et al. Study of salinity and pH effects on gelation time of a polymer gel using central composite design method. J. Macromol. Sci. Part. B 51(3), 438–451 (2012).
Hakiki, F. & Arifurrahman, F. Cross-linked and responsive polymer: Gelation model and review. J. Ind. Eng. Chem. 119, 532–549 (2023).
Kunitz, M., Syneresis and swelling of gelatin. J. Gen. Physiol. 12(2), 289–312 (1928).
Panja, S., Dietrich, B. & Adams, D. J. Controlling syneresis of hydrogels using organic salts. Angew. Chem. Int. Ed. 61(4), e202115021 (2022).
Liu, D. et al. Tough, transparent, and slippery PVA hydrogel led by syneresis. Small 19(14), 2206819 (2023).
Mandal, M., Prasanth Kumar, R. & Ojha, K. Impact assessment of polymer, cross-linker, and nanoparticles on gelation kinetics and properties of silica nanocomposite hydrogel for water shut-off treatment in harsh reservoir conditions. J. Mol. Liq. 411, 125746 (2024).
Li, W. et al. Sampled-Data asynchronous fuzzy output feedback control for active suspension systems in restricted frequency domain. IEEE/CAA J. Autom. Sin. 8(5), 1052–1066 (2021).
Zhuang, B. et al. Plasmon-enhanced NIR-II photoluminescence of rare-earth oxide nanoprobes for high-sensitivity optical temperature sensing. Opt. Lett. 49(22), 6489–6492 (2024).
Wu, H. et al. pH-sensitive phosphorescence in penicillamine-coated Au22 nanoclusters: Theoretical and experimental insights. Chem. Eng. J. 495, 153608 (2024).
Xu, Y., et al. Robust non-fragile finite frequency H∞ control for uncertain active suspensionsystems with time-delay using T-S fuzzy approach. J. Franklin Inst.358(8), 4209-4238 (2021).
Kumar, A. P. et al. Nanoscale particles for polymer degradation and stabilization—Trends and future perspectives. Prog. Polym. Sci. 34(6), 479–515 (2009).
Allen, N. S. et al. Degradation and stabilisation of polymers and coatings: nano versus pigmentary Titania particles. Polym. Degrad. Stab. 85(3), 927–946 (2004).
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
Author Contribution as below Z.S.G. Supervision, Experimental. A.H.A. Investigation, Writing. M.S.R. Writing, Analysis. G.C.S. Experimental. H.R.S. Theory, Resources. D.S. Investigation. S.J. Experimental, Writing. P.N.B. Experimental, Writing. M.S.M. Experimental, Writing. A.S.M. Experimental, Writing. U.K.R. Supervision. N.S.A. Methodology. A.B.* (corresponding author) Writing, Experimental.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ghnim, Z.S., Adhab, A.H., Reddy, M.S. et al. Crosslinking a nanocomposite by zirconium sulfate to synthesize a high-durable new-generation polymer gel. Sci Rep 15, 11471 (2025). https://doi.org/10.1038/s41598-025-92382-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-92382-1