Abstract
For the 30 million people living with HIV on antiretroviral therapy, routine viral load testing is recommended to monitor treatment effectiveness. However, only an estimated 77% of eligible people accessed viral load testing in 2022, due to barriers including the high costs of tests. Here we assessed implementation of pooled testing to increase viral load testing efficiency at a reference laboratory in Cameroon. Plasma specimens were tested in pools of three using the Abbott RealTime HIV-1 Viral Load assay. For pools with HIV-1 detected, each specimen was then tested and reported individually; for the negative pools, the pooled result was reported with no further testing. From July to December 2023, results for 12,396 specimens tested in pools were produced using 6,797 assays, or 0.55 assays per result, with 3.6% (449) reported as unsuppressed (> 1,000 copies/mL), enabling an additional 5,409 people (+ 80%) to have test results. When testing pools of three, the limit of detection per specimen increases from < 40 copies/mL to < 120 copies/mL, with only an estimated 0.01% of specimens with results of ≥ 1,001 copies/mL (unsuppressed) having results misclassified as suppressed. These results demonstrate that pooled testing can be an efficient and accurate approach to increase access to viral load monitoring.
Similar content being viewed by others
Introduction
The World Health Organization (WHO) recommends viral load testing as the preferred treatment monitoring approach for the more than 30 million people living with HIV who are on antiretroviral therapy (ART)1,2. Viral load testing is recommended to be conducted initially at 6 and 12 months after start of ART, and then annually. Viral load results are classified as undetectable (no viral load detected on the test used), suppressed (≤ 1,000copies/mL) or unsuppressed (> 1,000 copies/mL)3. The aim of antiretroviral therapy is to achieve and maintain viral suppression, to both improve health and reduce transmission3,4,5. The 2025 Global AIDS Targets aim for all people living with HIV and receiving ART to achieve viral suppression by 20256.
Approximately 25.6 million HIV viral load tests were performed for people on antiretroviral therapy, representing a coverage of 77% in 2022; approximately 1.4 million (~ 5%) of the total viral load tests performed were point-of-care tests, while the remainder were conventional tests, typically performed in reference laboratories7. From the Global Fund Public Price Reference Report, the average unit price for the most commonly used conventional HIV viral load test is > $16 per test8. The relatively high prices of viral load tests combined with limited funding of many HIV programs have contributed to fragmented availability of viral load testing in many settings9,10,11.
In 2023, the WHO issued updated policy guidance on the role of HIV viral suppression in improving health for individuals and reducing HIV transmission3. In these guidelines, increased access to viral load testing is emphasized as a key programmatic priority, and use of alternative tests or specimen types to enable scaling up access to testing is recommended3. This updated guidance is based on recent evidence showing that sample and assay combinations with higher limits of detection, such as point-of-care tests and dried blood spot samples, can be used to accurately classify whether a person living with HIV has achieved viral load suppression12,13. This guidance also highlights the inherently variable nature of PCR-based viral load testing; even when performed using a plasma specimen and a lab-based assay, the accepted variability of a viral load result of 1,000 copies/mL is from 500 to 2,000 copies/mL (+/- 0.3 log copies/mL)3,14,15,16.
One approach to increase access to HIV viral load testing is with pooled testing, which can be used to improve testing efficiency by reducing the number of viral load tests needed as compared to individual testing. In pooled, or group, testing as described by Dorfman, a pool of several specimens is first tested using a single assay17. For any pool with a positive result, each individual specimen from the pool is then re-tested and the individual result is reported; for pools with negative results, all individual specimens within that pool are reported as negative. An increased testing efficiency compared to individual testing can be achieved when the test positivity rate in the population is relatively low and when the pool size is chosen judiciously based on the positivity rate18. Pooled testing has been used to increase testing efficiency for the detection of HIV, TB and other diseases19,20,21,22,23,24,25.
Pooled testing for HIV viral load monitoring has also been described previously, with reports of increased efficiency and only minor decreases in testing accuracy due to specimen dilution with pooled testing26,27,28,29,30. Despite this evidence, pooled testing has not yet been widely evaluated or adopted for HIV viral load monitoring, perhaps because of a lack of standardized procedures and policy guidance. The recent uptake of pooled testing for SARS-CoV-2 viral detection during the COVID-19 pandemic indicates that routine specimen pooling for diagnostic testing is feasible and acceptable in some situations31,32,33,34,35,36. In the case of COVID-19, standardized procedures and recommendations were developed to facilitate the widespread scale-up of pooled testing to increase testing coverage37,38,39.
Cameroon is a lower middle income country with a population of 28 million people and an HIV prevalence of 2.7% among adults40. As of 2021, approximately 390,000 people living with HIV had been placed on ART, and the viral load testing coverage was 62%41. Disruptions in the availability of viral load tests have negatively impacted viral load testing coverage, which is similar to the situation in other settings9. Ensuring access to routine viral load testing is especially important for the most vulnerable populations, including adolescents and children, who have lower viral suppression rates than adults in Cameroon and globally42,43. Increasing access to routine viral load testing in this setting is also critical to ensure early detection and appropriate care for people with treatment failure44.
In this work, we evaluated the performance of pooled testing for routine HIV viral load monitoring to increase viral load testing access among people living with HIV on ART in Cameroon. We assessed the efficiency, accuracy, precision, and misclassification of pooled HIV viral load testing to detect treatment failure as compared to individual testing,
Methods
Setting and design
This was an analysis of the efficiency, accuracy (agreement), precision and misclassification of pooled testing conducted using the Abbott RealTime HIV-1 Viral Load assay (Illinois, USA). The evaluation was conducted in the Northwest region of Cameroon, where more than 41,000 people living with HIV are on ART. Laboratory testing was performed at the Tuberculosis Reference Laboratory Bamenda, which is accredited in accordance with the recognized International Standard ISO 15189:2022 (SANAS Accredited Medical Laboratory, No. M0593).
This evaluation was conducted together with the regional arm of the National AIDS Control Committee. This study was approved by the Regional Ethics Committee for Human Health Research in the North-west Region (2023/05/17/CERSH-NW on 31 May 2023) and the Institutional Review Board of the University of Bamenda, with waiver of informed consent. All methods were carried out in accordance with approved guidelines. All testing data was de-identified prior to analysis.
Clinical specimens
The reference laboratory received specimens for HIV viral load testing from 149 HIV care and treatment centers in the Northwest region. Pooling was conducted only on specimens referred for routine antiretroviral monitoring among people 19 years and older. Specimens referred from people < 19 years old or for evaluation of potential treatment failure or for other non-routine testing were tested individually; the proportion of adult specimens tested for routine viral load among all specimens received from adults for viral load testing was approximately 96%. Only plasma specimens with a volume of at least 1.2mL were included in pools to ensure that if the pool tested positive there would be sufficient material left for individual re-testing. Testing was performed in batches of 96 or 48 tests, from plasma collected and stored at 2–8◦C for testing within 7 days, or from plasma collected and frozen for transport and storage prior to testing. We aimed to include approximately 12,000 routine specimens in the analysis; this is approximately the number of specimens expected to be received over a 6-month period at the reference laboratory.
Pool size determination
Historical data were used to determine the optimal pool size for testing. In 2022, of the specimens received for routine monitoring of antiretroviral therapy at the reference laboratory, 4.7% (1,019/21,833) had HIV viral load results of > 1,000 copies/mL, so were classified as unsuppressed, while in total 15.7% (3,425/21,833) had results of > 40 copies/mL (Table S1). Based on the positivity rate of 15.7% of specimens with any HIV-1 detected ≥ 40 copies/mL, the theoretical optimal pool size for detection of HIV-1 was 317 .
Creation of pools for testing
To create pools of 3 specimens, 270µL of each individual plasma specimen was pipetted and added to the reaction tube, so that the total combined plasma volume for testing was 810µL; this is above the minimum sample volume of 0.8mL for the 0.6mL assay application as recommended by the manufacturer. The leftover plasma for each individual specimen was then stored in the refrigerator; if the pool tested positive for HIV-1 RNA, this remaining plasma from each of the three individual samples (with volume ≥ 900µL) was tested separately for result determination.
Method validation
Initially, all specimens with a pool result of any HIV-1 detected were subsequently re-tested individually. The results of the pooled result and individual results were compared and the proportion of unsuppressed specimens (> 1,000 copies/mL) that would be misclassified as suppressed was determined for each potential pooled result cut-off (< 40 copies/mL, 40–120 copies/mL, 121–200 copies/mL and 201-1,000 copies/mL). This validation was used to choose the most appropriate cut-off for individual re-testing of pooled specimens during subsequent pooled testing for routine viral load testing, using the Abbott RealTime HIV-1 assay.
HIV viral load testing- pooled and individual specimens
After creating the pools of 3, the resulting pooled plasma was run on the Abbott RealTime HIV-1 assay on the automated m2000 system following the manufacturer’s instructions. During routine testing (following method validation), for specimen pools with a result of HIV-1 not detected or detected < 40 copies/mL, all specimens from the pool were then reported as HIV-1 not detected or detected below the limit of detection of the assay. For specimen pools with any result of HIV-1 detected ≥ 40 copies/mL, each specimen was re-tested individually, and the individual result was reported. During data analysis, results from specimens with both pooled and individual results with HIV-1 detected were compared to assess the viral load result shift due to dilution from pooled testing and to estimate the variance in results.
Data extraction and Preparation
Specimens from pools with positive results were entered into a database and matched to the individual specimen results using the unique specimen number assigned by the laboratory. All results from viral load testing data were also entered into the routine laboratory database; results from specimens with positive pool and/or individual testing results were compared and verified. Only results of tests with positive or negative results were retained for analysis; the proportion of pooled and individual assays with invalid results was determined and used to estimate testing efficiency. All analyzed data are included as supplemental material (Supplemental Dataset S1).
Statistical analysis
For pooled and individual testing, the number of results reported were summarized by result classification (unsuppressed, suppressed-detectable and suppressed-undetectable)3, and by quantitative result category within the classifications. The number of overall pools and specimens tested, the number of pools and specimens that tested positive, and the total number of assays performed were summarized.
The observed efficiency was determined by dividing the number of assays used by the number of specimens with test results. The percentage of assays saved due to pooled testing as compared to individual testing was estimated by dividing the number of tests used for pooled testing by the number of assays that would have been needed to test all specimens individually, including additional assays for anticipated invalid results.
The theoretical difference in log copies/mL for a pool of three as compared to an individual result is log10(1/3) or -0.48 log copies/mL. Two method comparison approaches, the Bland-Altman method45,46 and Passing-Bablok regression47, were conducted to compare the difference in viral load measurements for specimens tested both in pools of three and individually to this theoretical difference. These analyses were conducted for specimens with results over the range 500 copies/mL to 5,000,000 copies/mL, which is the range over which the manufacturer reports that the assay was designed to achieve an inter-assay standard deviation of less than or equal to 0.25 copies/mL48, Viral load values were log-transformed prior to these analyses due to the non-normal distribution.
The Bland-Altman analysis was also used to assess the standard deviation in results for pooled testing with this assay. The variation in results is attributable to assay components as well as to the procedure of pooling.
The probability that a specimen with 1,001 copies/mL would be misclassified as suppressed following the pooled testing approach, depending on the value of the pool result for individual re-testing, was estimated using the standard deviation of the bias determined from the Bland-Altman analysis and assuming a normal distribution of results.
All analyses were performed in R version 4.4.1; the method comparison analyses were performed using the ‘mcr’ package49.
Results
Initial pooled testing and method validation to determine cut-off for individual re-testing
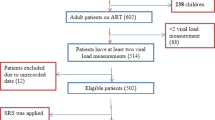
From June 24 to July 2, 2023, all specimens from pooled results with HIV-1 detected, including those with detectable results lower than the quantification limit of < 40copies/mL, were individually retested (Fig. 1), with results as shown in Table 1. Of the initial 447 specimens re-tested, 207 (46%) were re-tested following a pooled test result of < 40 copies/mL and 93 (21%) were re-tested following a pooled test result of 41–200 copies/mL; among these, none (0/290) had an individual result classified as unsuppressed. Among the 42 specimens in pools with results of 200-1,000 copies/mL, 5 (12%) had results of > 1,000 copies/mL, classified as unsuppressed, when tested individually. Based on these results, the decision was taken that subsequent individual re-testing would be performed only on pools with results of ≥ 40copies/mL, while specimens in pools with results of < 40copies/mL would be reported as being tested in pools of 3, with pool results of HIV-1 not detected or detected below the limit of detection of the assay. This approach was adopted to increase testing efficiency while minimizing the chance for misclassification of unsuppressed results (> 1,000 copies/mL).
Schematic of pooled testing. Specimens received for routine HIV viral load monitoring were pooled in groups of three and tested on the Abbot HIV-1 Real-Time Viral load assay. For pools with HIV-1 not detected, each individual specimen was reported as HIV-1 not detected or detected below the limit of detection of the assay, and no further testing was performed. For pools with HIV-1 detected, each individual specimen was tested individually, and the individual result was reported. *During method validation, all specimens in pools with any HIV-detected (including < 40 copies/mL) were re-tested individually; after method validation, only specimens in pools with ≥ 40 copies/mL were re-tested individually.
Pooled testing results and testing efficiency
From July 16, 2023, to December 31, 2023, 12,396 plasma specimens collected for routine HIV viral load monitoring from people living with HIV were tested in 4,132 pools of three using the Abbott HIV-1 assay (Table 2). Among 2,442 specimens tested individually from 814 pools with HIV-1 detected (≥ 40 copies/mL), there were 1,168 (9.4% of the total 12,396) individual specimens with a result of HIV-1 detected (including 719 with < 1,000 copies/mL and 449 with > 1,000 copies/mL) and 1,274 specimens with results of HIV-1 not detected. Among the 814 positive pools, 446 (55%) had a single positive individual result, 271 (33%) had 2 positive individual results, and 60 (7.4%) had three positive results. There were 37 pools (4.5%) with HIV-1 detected ≥ 40 copies/mL that had no individual positive results; among these 37 pools, the median pool result was 51 copies/mL (range 40–220 copies/mL, IQR 46–60 copies/mL). All 9,964 (91.6%) specimens in pools with results of HIV-1 not detected or HIV-1 detected < 40copies/mL were reported as tested in pools of three with HIV-1 not detected or detected below the limit of detection of the assay. Among the assays tested, 3.4% had invalid results; pools with invalid results were excluded from the summary statistics, while the invalid rate was used to determine the number of assays used and testing efficiency.
When all individual specimens in pools with results ≥ 40 copies/mL were re-tested, 6,782 assays were used, and the number of assays used per result delivered was 0.55. With this approach, pooled testing in pools of three saved an estimated 45% of cartridges as compared to using a single assay per specimen.
In the hypothetical scenarios, where individual re-testing would be performed only for pools with results of ≥ 120 copies/mL or ≥ 200 copies/mL (Table 2), then the number of assays needed to test the 12,396 specimens would reduce to 5,950 and 5,786 assays, respectively. In these scenarios, the number of assays saved would have been 52% and 53% respectively.
Assay performance for pools of three versus individual testing
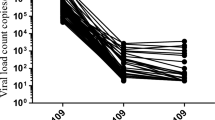
For the pools of three on the Bland-Altman analysis (Fig. 2), the mean bias between the pooled and individual cycle threshold values was − 0.51 log copies/mL (95% limits of agreement, -1.01 to -0.01). The standard deviation of the bias was 0.25 log copies/mL. From the Passing-Bablok regression, the intercept was − 0.37 (95% CI, -0.54 to -0.21) log copies/mL, with a slope of 0.97 (95% CI, 0.93 to 1.01, Figure S1); for an individual result of 3 log copies/mL, the pooled result difference is -0.46 log copies/mL. Results from both Bland-Altman and Passing-Bablok comparison methods are similar to the theoretical shift for pools of three (-0.48 log copies/mL).
Bland-Altman plot for specimens tested both in pools of three and individually on the Abbott HIV-1 RealTime assay. The solid red line indicates the mean bias and the dashed red lines indicate the 95% limits of agreement (LOA). Only specimens in pools of three with one positive and two negative results and with pool and individual results in the range from 500 to 5,000,000 copies/mL were included (214 specimens). The mean bias between the pooled and individual viral load was − 0.51 log copies/mL (95% limits of agreement, -1.01 to -0.01), which is similar to the theoretical value of -0.48 log copies/mL (or log10(1/3) for a pool of 3). The standard deviation of the bias is 0.25 log copies/mL.
Effect of pooled testing in pools of three on limit of detection and probability of misclassification
In the implemented scenario using a pooled cut-off of ≥ 40 copies/mL for individual re-testing, the estimated detectable viral load for an individual specimen in a pool of three is 120 copies/mL, with a 95% confidence interval of 39–371 copies/mL (Table 3). The probability that an individual specimen with an HIV-1 RNA concentration of ≥ 1,001 copies/mL would be misclassified as suppressed (< 1,000 copies/mL) is 0.01% in this scenario. The probability of misclassification for a specimen with concentration of 1,001copies/mL increases to 1.9% and 9.3% for hypothetical pooled result cut-offs of ≥ 120 and ≥ 200 copies/mL, respectively.
Discussion
Our results show that the pooled testing approach used here for routine HIV-1 RNA viral load monitoring was efficient, enabling an increase of + 80% (+ 5,409) in the number of people that were tested, with only a minor reduction in test accuracy to differentiate unsuppressed from suppressed viral load results as compared with individual testing. Plasma specimens from 12,396 people were tested using 6,797 assays, or 0.55 assays per person with a viral load result. With pools of 3, the limit of detection for the Abbott HIV-1 RealTime assay increases from < 40 copies/mL for testing an individual specimen to < 120 copies/mL for a specimen in a pool of 3. For pools with results of ≥ 40 copies/mL, all three specimens in the pool were re-tested individually, and the probability that an individual specimen with HIV-1 RNA concentration of ≥ 1,001 copies/mL (unsuppressed) would be misclassified as suppressed using this approach is estimated as just 0.01%. The good accuracy of this approach is clinically important because it means that people having unsuppressed viral load, whose treatment may be failing, can be accurately identified and followed up for appropriate care.
Previous studies of pooled testing for HIV viral load monitoring have also reported increased testing efficiency, starting with Smith et al. in 200927. This study described the use of both array and Dorfman pooling (in minipools) for 150 specimens and reported that > 50% of tests could be saved, with minimal decrease in accuracy. Subsequently, several studies have also reported increases in testing efficiency with minimal decrease in accuracy, using various approaches for pooled testing on plasma and dried blood spot (DBS) samples26,28,30. To our knowledge, this is the first report of larger scale implementation of pooled testing for HIV viral load monitoring. Concern about the impact of pooling on test accuracy, combined with a lack of standardized guidance, may have contributed to reluctance to scale up this approach. However, following the extensive adoption of pooled testing by many countries to extend reagent availability for SARS-CoV-2 testing during the COVID-19 pandemic, there may now be an opportunity to use these lessons learned to adopt pooled testing for other scenarios where testing is limited by reagent availability or cost. Uptake of pooled testing during COVID-19 was facilitated by guidance on how to implement and monitor pooled testing for PCR testing, such as that provided by the U.S. Food and Drug Administration37. Similar guidance could facilitate widespread adoption of pooled testing for HIV viral load monitoring.
The efficiency of pooled testing depends on both the rate of positivity in the population tested and on the pool size chosen17. We tested specimens in pools of three and then tested individually all specimens in pools with ≥ 40 copies/mL, and 9.4% of these individually tested specimens (1,168/12,396) had a result of any HIV-1 detected, with 0.55 assays used per specimen with result. Additional gains in efficiency would be possible by choosing a higher pooled result cut-off for individual re-testing, as shown in Table 2. Larger pool size could also be used to increase efficiency; at positivity rates < 12%, pools of 4 become somewhat more efficient than pools of three18. We used a pre-selection step to identify specimens with higher probability of unsuppressed viral load results for individual testing, including specimens collected from people to be evaluated for possible treatment failure and among people < 19 years. Additional refinement of the pre-selection process could also be used to further increase testing efficiency.
The measured standard deviation in viral load results obtained with this approach was 0.25 log copies/mL (Fig. 2), which is the same as what the manufacturer reports as the target inter-assay standard deviation48. Variability in quantitative polymerase chain reaction testing generally is attributable to variations in chemical efficiencies (of the enzymes, primer and/or template) and variable equipment factors including fluorescence detection, temperature and reagent volume50,51. The similarity between the measured standard deviation in the results reported here and that of the assay suggests that the procedure used to pool three plasma specimens prior to testing does not contribute to significant additional variability to the viral load result; this is as expected, since the only process difference between individual and pooled testing is whether 800µL of one or 270µL of each of three specimens are pipetted into the testing tube.
While the probability of misclassification from unsuppressed to suppressed viral load is low with this approach, the use of pooled testing will mean that some specimens from people living with HIV, mostly with very low levels of HIV-1 RNA (< 200 copies/mL) but also some with levels of 200-1,000 copies/mL, will be classified as undetectable rather than suppressed but detectable. For clinical management, viral load results between 200 and 1,000 copies/mL have been shown to be associated with the development of virological failure with older ART regimens, although there is a lack of information about how viral load levels of 200-1,000 copies/mL are associated with treatment failure on newer ART regimens52. In the population tested here prior to the start of pooled testing, 97.6% (20,305/20,814, Table S1) of specimens tested individually and with suppressed viral load (< 1,000 copies/mL) had results of < 200 copies/mL, which is similar to previous reports53,54. When all testing was performed individually (prior to the start of pooled testing), 21.3% of all specimens tested (Table S1) had a result of < 120 copies/mL, and many of these will be instead reported as not detected with pooled testing. The clinical implication is that, with pooled testing, a proportion of people with suppressed but detectable viral load concentrations will miss out on enhanced adherence counseling and/or follow-up viral load testing, which is recommended by national and WHO guidelines3.
Due to the inadequate coverage of viral load testing in many settings, the WHO has recommended that HIV programs consider the use of alternative specimens and testing approaches, including those with higher limits of detection, to ensure all people living with HIV can access viral load testing. These approaches include the use of dried blood spot (DBS) specimens instead of plasma, to enable the collection and transportation of specimens at room temperature; some of the DBS approaches have a sensitivity of > 95% as compared to plasma to detect viral load failure at 1,000 copies/mL12. Another alternative approach is to use point-of-care or near point-of-care testing, such as the Cepheid Xpert HIV-1 viral load plasma assay, which also has a limit of detection of 40 copies/mL13. In comparison to these techniques, the pooled testing approach described here offers greater testing efficiency with a similar limit of detection, enabling more people with unsuppressed viral load results to be accurately classified. However, the pooled testing described here was performed at a reference laboratory with plasma specimens rather than closer to the point of need or with easier to collect (e.g. DBS) specimens. The use of pooled testing on point-of-care or near point-of-care PCR-based platforms, as used for diagnostic testing for COVID-19 and TB21,22, could further expand access to routine HIV viral load monitoring. Another widely used conventional system for viral load testing, in Cameroon and elsewhere, is the Roche COBAS TaqMan HIV-1, with a limit of detection of 20 copies/mL; this platform could also be evaluated for pooled testing of HIV-1 viral load monitoring and may be expected to have similar performance for pooled testing as the Abbott platform described here. To decide whether to implement pooled testing, local factors influencing the coverage of viral load monitoring in different settings will contribute to the selection of appropriate testing approaches. Pooled testing may be especially advantageous in settings with established reference laboratories and specimen transport networks but with limited resources, where the high cost of a viral load test is a barrier to wider scale-up of viral load monitoring.
This evaluation had several limitations. We implemented testing in pools of three for all specimens received for routine viral load monitoring, and we did not assess other characteristics of the people whose specimens were referred for viral load testing; additional characteristics, including treatment history, regimen, demographic information, and potential correlation of treatment failure, for example from specimens tested in groups from the same region or health facility55,56,57,58 could be useful to further refine criteria to inform the use of pool testing and pool size. In addition, we did not evaluate the overall cost-effectiveness of pooled testing in this work; however, we report that 45% of assays were saved using this approach. Due to these savings, the assay cost per specimen with result was approximately $9.28, as compared to the average unit cost of $16.88 for this assay as reported on the Global Fund Public Price Reference Report8.
In summary, the results reported here indicate that pooled testing is a promising approach to extend reagent availability and ensure more people living with HIV on ART can access accurate routine viral load testing. The pooling approach described here can be readily integrated in reference laboratories currently performing HIV viral load testing on individual specimens, with the same expected significant increase in testing efficiency at similar positivity rates. Expanding access to viral load monitoring will help more people living with HIV achieve and maintain viral suppression, to reduce further transmission of the infection and improve health.
Data availability
All analyzed data are included in the Supplementary Information files.
References
World Health Organization. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring; recommendations for a public health approach. https://iris.who.int/bitstream/handle/10665/342899/9789240031593-eng.pdf?sequence=1 (2021).
UNAIDS. The urgency of now: AIDS at a crossroads. https://www.unaids.org/en/resources/documents/2024/global-aids-update-2024 (2024).
World Health Organization. The role of HIV viral suppression in improving individual health and reducing transmission: policy brief. https://www.who.int/publications/i/item/9789240055179 (2023) ISBN 978-92-4-005517-9.
World Health Organization (WHO). HIV prevention, infant diagnosis, antiretroviral initiation and monitoring. https://www.who.int/publications/i/item/9789240022232 (2021).
World Health Organization (WHO). HIV Molecular Diagnostics Toolkit to Improve Access to Viral Load Testing and Infant Diagnosis. (2019).
UNAIDS, Global & Geneva AIDS Strategy 2021–2026 - End Inequalities. End AIDS. https://www.unaids.org/sites/default/files/media_asset/global-AIDS-strategy-2021-2026_en.pdf (2021).
Clinton Health Access Initiative (CHAI). HIV Market Report 2023. https://www.clintonhealthaccess.org/report/2023-hiv-market-report-the-state-of-hiv-market-in-low-and-middle-income-countries/, https://doi.org/10.1021/ie50276a029 (2023)
The Global Fund to Fight AIDS Tuberculosis and Malaria. Price Reference Report. Price & Quality Reporting database https://www.theglobalfund.org/en/sourcing-management/price-quality-reporting/ (2024).
Pham, M. D., Nguyen, H. V., Anderson, D., Crowe, S. & Luchters, S. Viral load monitoring for people living with HIV in the era of test and treat: progress made and challenges ahead – a systematic review. BMC Public. Health. 22, 1–23 (2022).
Resch, S., Ryckman, T. & Hecht, R. Funding AIDS programmes in the era of shared responsibility: an analysis of domestic spending in 12 low-income and middle-income countries. Lancet Glob Heal. 3, e52–e61 (2015).
Roberts, T., Cohn, J., Bonner, K. & Hargreaves, S. Scale-up of routine viral load testing in resource-poor settings: current and future implementation challenges. Clin. Infect. Dis. 62, 1043–1048 (2016).
Vojnov, L. et al. The performance of using dried blood spot specimens for HIV-1 viral load testing: A systematic review and Meta-Analysis. PLoS Med. 19 (2022).
Sacks, J. A. et al. Performance of cepheid Xpert HIV-1 viral load plasma assay to accurately detect treatment failure. AIDS 33, 1881–1889 (2019).
Cobb, B. R., Vaks, J. E., Do, T. & Vilchez, R. A. Evolution in the sensitivity of quantitative HIV-1 viral load tests. J. Clin. Virol. 52, 77–82 (2011).
Jennings, C. et al. Cross-platform analysis of HIV-1 RNA data generated by a multicenter assay validation study with wide geographic representation. J. Clin. Microbiol. 50, 2737–2747 (2012).
Lelie, N. & van Drimmelen, H. Accuracy of quantitative HIV-1 RNA test methods at 1000 copies/ml and the potential impact of differences in assay calibration on therapy monitoring of patients. J. Med. Virol. 92, 3246–3253 (2020).
Dorfman, R. The detection of defective members of large populations. Ann. Math. Stat. 14, 436–440 (1943).
Bilder, C. R. A Shiny App for Pooled Testing. https://bilder.shinyapps.io/PooledTesting/
Westreich, D. J., Hudgens, M. G., Fiscus, S. A. & Pilcher, C. D. Optimizing screening for acute human immunodeficiency virus infection with pooled nucleic acid amplification tests. J. Clin. Microbiol. 46, 1785–1792 (2008).
Schalkwyk, C., Van, Maritz, J., Zyl, G. U., Van, Preiser, W. & Welte, A. Pooled PCR testing of dried blood spots for infant HIV diagnosis is cost efficient and accurate. BMC Infect. Dis. 19, (2019).
Vuchas, C. et al. Implementation of large – scale pooled testing to increase rapid molecular diagnostic test coverage for tuberculosis: a retrospective evaluation. Sci. Rep. 13, 1–10 (2023).
Cuevas, L. E. et al. Systematic review of pooling sputum as an efficient method for Xpert MTB/RIF tuberculosis testing during COVID-19 pandemic. Emerg. Infect. Dis. 27, 719–727 (2021).
Merav, L. et al. Implementation of pooled saliva tests for universal screening of cCMV infection. Nat. Med. 30, 1111–1117 (2024).
Waters, A. et al. Incidence of congenital cytomegalovirus infection in Ireland: implications for screening and diagnosis. 59, 156–160 (2014).
Xu, Y. et al. The diagnostic accuracy of pooled testing from multiple individuals for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae: a systematic review. Int. J. Infect. Dis. 118, 183–193 (2022).
Zyl, G. U. et al. Pooling strategies to reduce the cost of HIV-1 RNA load monitoring in a Resource-Limited setting. Clin. Infect. Dis. 52, 264–270 (2011).
Smith, D. M. et al. The use of pooled viral load testing to identify antiretroviral treatment failure. AIDS 23, 2151–2158 (2009).
May, S. J., Gamst, A., Haubrich, R., Benson, C. & Smith, D. M. Pooled nucleic acid testing to identify antiretroviral treatment failure during HIV infection. J. Acquir. Immune Defic. Syndr. 53, 194–201 (2011).
Pannus, P. et al. Pooled HIV-1 viral load testing using dried blood spots to reduce the cost of monitoring antiretroviral treatment in a resource-limited setting. J. Acquir. Immune Defic. Syndr. 64, 134–137 (2013).
Preiser, W. & van Zyl, G. U. Pooled testing: A tool to increase efficiency of infant HIV diagnosis and virological monitoring. Afr. J. Lab. Med. 9, a1035 (2020).
Verdun, C. M., Fuchs, T., Harar, P., Elbrächter, D. & Fischer, D. S. Group testing for SARS-CoV-2 allows for up to 10-Fold efficiency increase across realistic scenarios and testing strategies. Front. Public. Heal. 9, 583377 (2021).
Daniel, E. A. et al. Pooled testing strategies for SARS-CoV-2 diagnosis: A comprehensive review. Diagn. Microbiol. Infect. Dis. 101, 115432 (2020).
Barak, N. et al. Lessons from applied large-scale pooling of 133,816 SARS-CoV-2 RT-PCR tests. Sci. Transl Med. 13, 1–8 (2021).
Cabrera Alvargonzalez, J. J. et al. Assessment of the effective sensitivity of SARS-CoV-2 sample pooling based on a large-scale screening experience: retrospective analysis. JMIR Public. Heal Surveill. 10, e54503 (2024).
Bilder, C. R., Tebbs, J. M. & Mcmahan, C. S. Discussion on is group testing ready for prime-time in disease identification. Stat. Med. 3881–3886. https://doi.org/10.1002/sim.8988 (2021).
Abdalhamid, B. et al. Assessment of specimen pooling to conserve SARS CoV-2 testing resources. Am. J. Clin. Pathol. 153, 715–718 (2020).
U.S. Food and Drug Administration (FDA). Template for Developers of Molecular Diagnostic Tests. vol. 1, 1–60 https://www.fda.gov/media/135900/download (2021).
LabCorp. Labcorp’s COVID-19 RT-PCR, Test, E. U. A. & Summary LabCorp 1–33 https://www.fda.gov/media/136151/download (2022).
Hong, K. H. et al. Update of guidelines for laboratory diagnosis of COVID-19 in Korea. Ann. Lab. Med. 42, 391–397 (2022).
Institut National de la Statistique (INS). et ICF. Enquête Démographique et de Santé du Cameroun 2018. https://dhsprogram.com/publications/publication-FR360-DHS-Final-Reports.cfm (2020).
National AIDS Control Committee. Annual Report of the HIV Response, Cameroon. http://cnls.cm (2021).
Fokam, J. et al. Evaluation of viral suppression in paediatric populations: implications for the transition to Dolutegravir-Based regimens in Cameroon: the CIPHER-ADOLA study. Biomedicines 12, 2083 (2024).
Fokam, J. et al. Viral suppression in the era of transition to dolutegravir-based therapy in Cameroon. Med. (Baltim). 102, (2023).
Fokam, J. et al. Pre-treatment drug resistance and HIV-1 genetic diversity in the rural and urban settings of Northwest-Cameroon. PLoS One. 15, 1–14 (2020).
Bland, J. M. & Altman, D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. I, 307–310 (1986).
Bland, J. M. & Altman, D. G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 2802, 135–160 (1999).
Passing, H. & Bablok, W. A. New biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, part I. J. Clin. Chem. Clin. Biochem. 21, 709–720 (1983).
Abbott Abbott RealTime HIV-1 Assay Package Insert. https://www.molecular.abbott/us/en/products/infectious-disease/realtime-hiv-1-viral-load
R Core Team. R: A Language and Environment for Statistical Computing. https://www.r-project.org
Svec, D., Tichopad, A., Novosadova, V., Pfaffl, M. W. & Kubista, M. How good is a PCR efficiency estimate: recommendations for precise and robust qPCR efficiency assessments. Biomol. Detect. Quantif. 3, 9–16 (2015).
Bustin, S. A. et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622 (2009).
Ryscavage, P., Kelly, S., Li, J. Z., Harrigan, R., Taiwo, B. & P. & Significance and clinical management of persistent low-level viremia and very-low-level viremia in HIV-1-infected patients. Antimicrob. Agents Chemother. 58, 3585–3598 (2014).
Broyles, L. N., Luo, R., Boeras, D. & Vojnov, L. The risk of sexual transmission of HIV in individuals with low-level HIV viraemia: a systematic review. Lancet 402, 464–471 (2023).
Chang, G. C. et al. Population-based HIV impact assessments and viral load results: implications for u = u. Top. Antivir Med. 28 (1), 409–410 (2020).
Basso, L. J., Salinas, V., Sauré, D., Thraves, C. & Yankovic, N. The effect of correlation and false negatives in pool testing strategies for COVID-19. Health Care Manag Sci. 25, 146–165 (2022).
Comess, S., Wang, H., Holmes, S. & Donnat, C. Statistical modeling for practical pooled testing during the COVID-19 pandemic. Stat. Sci. 37, 229–250 (2022).
Saraiva, G. Q. Pool testing with Dilution effects and heterogeneous priors. Health Care Manag Sci. 26, 651–672 (2023).
Aprahamian, H., Bish, D. R. & Bish, K. Optimal Risk-Based group testing. Manage. Sci. 65, 4365–4384 (2019).
Acknowledgements
We appreciate the contributions of Che Ita Bi, Nsimen Anna, Kidio Gisele, Anim Faith and the entire laboratory team for their contributions to HIV viral load testing and results delivery. We thank Jacob Creswell for helpful feedback on the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: G.E.F.T., C.V., M.S. Data curation: U.N.S. Investigation and Methodology: U.N.S., C.V. Formal Analysis: N.N.M., M.S.; Project Administration and Supervision: G.E.F.T., G.A., V.K. Visualization: C.V., C.M., M.S., G.E.F.T., Writing- original draft: G.E.F.T., M.S. Writing- review and editing: G.E.F.T., C.V., N.N.M., U.N.S., C.M., A.F.Z.M., S.C.B., R.D., J.F., M.S., M.S. All authors read and agreed on the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fosah Tayong, G.E., Vuchas, C., Mbuh, N.N. et al. Implementation of pooled testing to increase access to routine viral load monitoring for people living with HIV on antiretroviral therapy. Sci Rep 15, 14713 (2025). https://doi.org/10.1038/s41598-025-92709-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-92709-y