Abstract
The oxidative balance score (OBS) serves as a comprehensive measure of exposures related to oxidative stress, considering both dietary antioxidants and lifestyle exposures. There is no evidence regarding the relation between OBS and postpartum depression (PPD). In this study, we aimed to determine the relationship between OBS during pregnancy and PPD. In this cohort study, 243 Iranian pregnant women were recruited using a convenience sampling method from 2022 to 2023. Dietary intakes were obtained using a validated food frequency questionnaire. OBS was separately constructed based on nutrients/lifestyle (NLOBS) and food groups/lifestyle (FLOBS) according to the previously proposed methods. PPD was diagnosed 4 to 6 weeks after delivery using the Edinburgh Postpartum Depression Scale. Cox proportional hazards regression was utilized to examine the relationship between OBS and PPD. Participants had a mean (SD) age of 30.9 ± 6.12 years. In total, 43 females were diagnosed with PPD. Findings revealed that, after controlling all confounders, subjects with the highest level of NLOBS, compared to the lowest, had a 69% lower risk of PPD (HR: 0.31; 95% CI: 0.12–0.83). Although a significant inverse relation was found between FLOBS and PPD in the crude model (HR: 0.43; 95% CI: 0.19–0.96); this association was not significant in fully adjusted model (HR: 0.53; 95% CI: 0.22–1.27). Considering subtypes of each score, inverse relations were significant for NOBS and LOBS, but not for FOBS. This study suggests that a higher OBS, particularly nutrient-based OBS, during pregnancy may be associated with a reduced risk of PPD. Further research is necessary to validate these findings.
Similar content being viewed by others
Introduction
The postpartum period poses a high susceptibility to mood disorders, such as depression and psychosis1. Postpartum depression (PPD) is a psychological condition marked by feelings of depression, anxiety, irritability, and other clinical symptoms that could emerge following childbirth, mostly around six weeks after delivery2. It has been shown that roughly 40% of the four million women who undergo childbirth annually encounter different types of postpartum mood disorders3. The occurrence of PPD in Asian nations varies between 3.5% and 63.3%4. According to a meta-analysis in 2013, the pooled prevalence of PPD in Iran was recorded at 25.3%5. If left untreated, PPD may progress to chronic depression or psychosis and can negatively affect children’s development, closely tied to maternal health 6,7.
The etiology of PPD involves the interplay of biological, psychological, and sociological factors including type of delivery, childbirth rank and unwanted pregnancy, all interacting with an individual woman’s unique risk profile4,8. As one of several causes, nutrition influences various associated factors such as hormones and immune responses, so it could potentially exert a significant influence on the development and progression of this illness9. For instance, it has been documented that the consumption of fruits and vegetables, milk and vitamin C is linked to a lower PPD risk10,11,12. Also, there is evidence that supplementation with selenium can act as an effective treatment for PPD13.
There is strong evidence that oxidative stress plays an important role in various health outcomes including cancer, neurodegenerative diseases, and most psychiatric problems14,15,16. The elevated risk of oxidative stress is particularly pronounced during pregnancy and even further escalates after delivery17. Furthermore, lower levels of antioxidants in plasma have been associated with the development of PPD1,18. Oxidative stress can cause brain changes, neuronal apoptosis, and inflammation, which contribute to depression 19,20,21. Dietary antioxidants may help protect against neural damage and mitochondrial dysfunction, potentially improving depressive symptoms22,23,24.
The oxidative balance score (OBS) proposed by Goodman is a comprehensive measure that combines antioxidant and pro-oxidants, both in diet and lifestyle25. The OBS underwent several adaptations, encompassing various nutrients, lifestyle factors, and, additionally establishing a food-based version26,27,28.
Two recent studies found a significant inverse association between OBS and depressive symptoms29,30. Despite the potential role of oxidative stress in development of PPD, no study has investigated the relation between OBS and PPD so far. Therefore, we sought to explore the relationship between both nutrient- and food-based OBS and risk of PPD in a sample of Iranian pregnant women. We hypothesized that a higher OBS may be related to a lower risk of PPD.
Methods and materials
Study population and design
This prospective cohort study was conducted among pregnant women residing in Tehran, Iran. The overarching aim of this cohort study was to investigate the relationship between diet and lifestyle during pregnancy and their impact on pregnancy complications, as well as postpartum and neonatal outcomes. To achieve this, comprehensive data were collected on maternal dietary intake, lifestyle factors, psychological status during and after pregnancy, and neonatal outcomes. Specifically, this study aimed to examine the relationship between OBS during pregnancy and the risk of PPD. Participants were recruited from outpatients visiting healthcare centers and Yas hospital affiliated with Tehran University of Medical Sciences between October 2022 and May 2023. Participants were recruited by a convenience sampling method. Among the pregnant women invited to participate in the study, 300 successfully completed the pregnancy questionnaires in their entirety and were subsequently enrolled. The following inclusion criteria were considered: (1) pregnant women in the third trimester (week 27 to 40) of pregnancy, (2) aged above 18 years old, and (3) had a singleton pregnancy. Exclusion criteria encompassed: (1) individuals with hepatitis, tumors (malignancies), severe infections, HIV/AIDS, and other autoimmune diseases, (2) those who had used specific medications (corticosteroids, immunosuppressive drugs, neurologic medications), (3) those with a history of mental disorders, specifically assessed through self-reported data. Participants were directly asked whether they had experienced any mental health issues before pregnancy, including a history of using psychiatric medications or seeking other mental health services, and (4) those reporting energy intakes outside the range of 800–4200 kcal/d 31. Ultimately, 243 participants were eligible to be included in the final analyses. All participants provided written informed consent forms. This study received approval from the School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences Research Ethics Committee (IR.TUMS.MEDICINE.REC.1401.845), following the principles of the Helsinki Declaration.
Dietary data
The dietary intake of participants was gathered through a valid and reliable semi-quantitative 168-item Food Frequency Questionnaire (FFQ) at baseline32,33. Participants were asked to provide details on the frequency and quantity of their consumption for each food item during the pregnancy. This face-to-face interview was conducted by a well-trained and experienced researcher. The FFQ comprised a list of food items, each with standard serving sizes, allowing participants to report frequency of consumption on a daily, weekly, monthly, or yearly basis. Considering the specified portion size and consumption frequency for each food item, all foods were calculated on a daily scale and then converted to grams per day using household measures34. Nutrient values, including energy and other nutritional components for each subject, were computed using Nutritionist 4 software (First Databank, Hearst Corp., San Bruno, CA, USA), which had been customized for Iranian foods. The questionnaire was completed during the third trimester of pregnancy and was used to assess the usual dietary intake of participants throughout pregnancy.
Anthropometry measurements
Height was measured in a normal standing position, and without shoes, using a non-elastic tape measure attached to the wall. To acquire the pre-pregnancy weight, we first checked for any existing records for each pregnant woman. If a record was available, we extracted the pre-pregnancy weight from it. In the absence of a record, we relied on self-reported pre-pregnancy weight. If self-reporting was unavailable, we utilized the weight recorded during the first three months of pregnancy in the existing record. Body mass index (BMI) before pregnancy was calculated by dividing weight (in kilograms) by the square of height (in meters) using the formula.
By subtracting the weight at childbirth from the pre-pregnancy weight, we determined gestational weight gain. According to the guidelines in 2009, gestational weight gain was categorized into three groups based on the pre-pregnancy BMI: insufficient (below the recommended range), adequate (within the recommended range), or excessive (above the recommended range)35. The recommended gestational weight gain is 12.5–18, 11.5–16, and 7–11.5 kg for women with underweight, normal weight, and overweight at the beginning of pregnancy, respectively. Weight retention was calculated by considering the difference between mother’s weight at 4–6 weeks after delivery and her pre-pregnancy weight.
Physical activity
For assessing the physical activity of pregnant women in the last trimester of pregnancy, we used the Pregnancy Physical Activity Questionnaire (PPAQ), a standard questionnaire developed by Chasan-Taber et al.36. Its validity and reliability have been assessed among the Iranian population37. This questionnaire encompasses 32 items divided into four sets of questions pertaining to activities: at home (15 questions), community (3 questions), workplace activity (5 questions), sports and entertainment (7 questions). Also, there is a section available for adding additional activities that are not included in the questionnaire list (2 questions). The intensity of physical activity is determined based on Metabolic Equivalent of Task (MET). Total score for physical activity was calculated as MET-hours/week. Finally, we classified participants based on MET-hours/week scores into tertiles, indicating three groups of low, moderate, and high physical activity levels.
OBS
We utilized two types of OBS in this study, which involved calculating Nutrient-Lifestyle OBS (NLOBS) and Food-Lifestyle OBS (FLOBS). The oxidative status in body attributed to dietary intakes is generally determined by the interaction between anti- and pro-oxidant foods and nutrients, underscores the importance of developing OBS as a proxy to indicate the overall oxidative status38. All dietary, lifestyle, and food elements were chosen based on their potential antioxidant and pro-oxidant properties, taking into account their influence on the individual’s oxidative/antioxidant status. The justification for incorporating them into these OBSs has been outlined in a review discussing the applications and methodologies of OBSs39.
Modified NLBOS
Goodman’s proposed OBS is a comprehensive measure that integrates both dietary and lifestyle-related antioxidants and pro-oxidants25. For our study, we tailored this OBS to better fit our study population, resulting in a modified version. Specifically, we added dietary antioxidants such as folate, vitamin D, fiber, zinc, and calcium, and included physical activity as a non-dietary antioxidant component. We excluded aspirin and NSAIDs from the OBS due to their contraindications during pregnancy. Additionally, we included obesity as a non-dietary pro-oxidant by considering pre-pregnancy BMI and excessive gestational weight gain. Following Mayne and colleagues’ recommendations, we replaced saturated fatty acids (SFA) with polyunsaturated fatty acids (PUFA) in the score, since saturated fats are less susceptible to lipid peroxidation due to the absence of carbon–carbon double bonds40. We also accounted for secondhand smoke exposure alongside maternal smoking. These modifications were based on adaptations seen in other OBS implementations and were specifically tailored to our study population. This modified OBS uniquely includes factors such as passive smoking, pre-pregnancy obesity, and excessive gestational weight gain, which have not been incorporated in previously published OBS versions.
In this modified NLOBS, designed for pregnancy, a total of 19 oxidative and antioxidant components from both dietary and non-dietary sources were selected. These components were divided into four groups: (1) Dietary antioxidants (obtained from diet and supplements) including vitamin C, folate, beta-carotene, lycopene, beta-cryptoxanthin, lutein and zeaxanthin, vitamin E, vitamin D, selenium, fiber, zinc, and calcium; (2) Dietary prooxidants (derived from diet and supplements) including iron and PUFA; (3) Non-dietary antioxidants including physical activity; (4) Non-dietary prooxidants encompass smoking, passive smoking, pre-pregnancy obesity, and excessive weight gain during pregnancy.
For the nutrients of NLOBS, we first adjusted each nutrient for energy intake based on residual method41. Then each nutrient was divided into tertiles. For dietary antioxidants, a score of 0, 1, and 2 was assigned to tertiles of intake, respectively. A reverse scoring system was applied for dietary prooxidants. For physical activity, subjects were categorized into tertiles and similar scoring system was applied as we did for dietary antioxidants. A predetermined scoring system was considered for non-dietary prooxidants. For current, previous, and non- smokers a score of 0, 1, and 2 was allocated, respectively. For being a current passive smoker, a score of 0 was assigned, for past exposure, a score of 1, and for no exposure, a score of 2 was given. For excessive weight gain during pregnancy, a score of 0 was assigned, and for sufficient or insufficient weight gain, a score of 2 was given. Pre-pregnancy BMI was categorized as follows: a BMI of less than 25 kg/m2 received 2 points, 25–29.9 kg/m2 received 1 point, and a BMI of 30 kg/m2 or higher received 0 points. Finally, scores from each component were summed to calculate the total NLOBS. A higher score implies the dominance of antioxidants over prooxidants. Participants received a score range from 0 to 38. Detailed description of scoring system is provided in Supplementary Table 1.
Modified FLOBS
The calculation of FLOBS was introduced by Hernández-Ruiz and colleagues28. This new OBS score, is designed to account for the nutritional effects through foods. In general, 12 food components were included: seven antioxidant-rich foods (vegetables, fruits and fruit juices, legumes and nuts, olive oil, fish, whole grains, coffee and tea) and five prooxidant foods (meat and meat products, biscuits and pastries, fats and oils—except for olive oil, snacks and sauces, refined grains and their derivatives). While Hernández-Ruiz et al. incorporated "excess energy intake relative to total energy expenditure" as part of the FLOBS lifestyle domain, we decided to exclude this factor from our calculation. Instead, we incorporated "adequate, inadequate, and excessive weight gain during pregnancy". Gestational weight gain reflects the appropriateness of total energy intake (whether low or high), and is a more relevant measure for this specific group. Similar items as NLOBS were considered for FLOBS lifestyle domain (physical activity, smoking, passive smoking, pre-pregnancy obesity, and excessive weight gain during pregnancy). Therefore, a total of 17 components were included in this index.
For constructing the food domain, dietary components of FLOBS were first adjusted for energy according to residual method. Each dietary component of FLOBS was then categorized into quintiles. For antioxidants, scores ranging from 1 to 5 were assigned for the first to fifth categories, while for prooxidants, scores were reversed. To account for the varying antioxidant or pro-oxidant potential of different dietary components, we assigned weights to these components. The weights were based on their antioxidant or pro-oxidant capacity as measured in laboratory assessments of Total Antioxidant Capacity (TAC). For antioxidant components with moderate antioxidant potential, such as legumes and nuts, olive oil, fish, biscuits and pastries, snacks and sauces, and grains and their derivatives, we halved the scores. This means that a score of 1 was reduced to 0.5, a score of 2 was reduced to 1, and so on, with a score of 5 being reduced to 2.5. For the lifestyle domain, we applied the same approach as we did for NLOBS since the components are similar. Finally, FLOBS was computed by summing up the scores, ranging from 13.5 to 67.5. Detailed description of scoring system is provided in Supplementary Table 2.
PPD
PPD was assessed 4–6 weeks after delivery. To examine PPD, we used Edinburgh Postpartum Depression Scale (EPDS) developed by Cox et al.42. This scale consists of ten self-report questions, each rated from 0 to 3. Total score in calculated by summing scores of these 10 questions ranging from 0 to 30. Higher scores reflect more severe symptoms of depression43. Considering a threshold of 10 on EPDS for PPD, a sensitivity ranging from 84 to 100%, and a specificity ranging from 82 to 84% was reported previously44. Earlier, the validity and reliability of this questionnaire have been assessed in Iranian population45,46. The questionnaire was completed through an interview-based method. A score of 13 and above indicates clinical depression in the Iranian version46. In this study, we considered a score of ≥ 13 as PPD incidence.
Assessment of other variables
Required information such as age, level of education, occupation, satisfaction with pregnancy, illness or hospitalization during pregnancy, number of childbirths, duration of marital life, supplementation (type and brand), smoking status (including current smoker, previous smoker and passive smoker) were collected through a pre-structured demographic questionnaire at baseline of study. Also, infant illness at birth, infant gender, premature birth, type of delivery, infant feeding (breast milk, formula, or both), infant hospitalization, and other information were collected through another questionnaire after delivery. For assessing the quality and quantity of sleep over the past month, the Pittsburgh Sleep Quality Index (PSQI) questionnaire was utilized47. Its validity and reliability have been previously assessed in Iran48. The 19-item questionnaire is scored on a four-point scale ranging from 0 to 3, with the total score spanning from 0 to 21. Higher scores indicate poorer sleep quality. This questionnaire finally describes an individual’s sleep status in seven aspects, including mental sleep quality, sleep onset latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medications, and daytime dysfunction. A total score below 6 was considered indicative of sufficient sleep quality. PSQI was completed in the third trimester of pregnancy. The socioeconomic status (SES) of the participants was evaluated using household income, education levels of both parents, and the occupations of both mother and father. For each component, individuals received a specific score based on their responses, and then, the sum of all components’ scores was considered as score of SES.
Statistical analysis
First of all, individuals were categorized into tertiles of NLOBS and FLOBS. Quantitative variables are presented as mean ± standard deviation (SD), while qualitative variables are expressed as frequency and percentage. Subsequently, one-way analysis of variance (ANOVA) and Chi-square test were employed to examine the difference of continuous and categorical variables, respectively, across tertiles of NLOBS and FLOBS. Dietary intakes were examined across tertiles of NLOBS and FLOBS using analysis of covariance (ANCOVA). For energy and macronutrients, age was adjusted. For other dietary variables age and energy intake were adjusted. The relationship between NLOBS/ FLOBS and PPD was assessed using Cox proportional hazards models in both crude and multivariable-adjusted models to estimate hazard ratio (HR) and its corresponding 95% confidence intervals (CI). In the first model, maternal age (continuous) and maternal energy intake (continuous) were adjusted. Further adjustment was made for duration of marriage (continuous), pregnancy satisfaction (planned/ unplanned pregnancy), parity (primiparous/ multiparous), occurrence of maternal diseases (gestational diabetes, pregnancy-induced hypertension, and thyroid disorders), history of abortion (yes/ no), and SES (low, moderate, high) in the second model. Additionally, infant gender (girl/ boy), infant feeding (breastfeeding/ formula feeding/ both), mode of delivery (cesarean/ natural), preterm birth (yes/ no), infant illness at birth (yes/ no), and neonatal hospitalization (yes/ no) were controlled in the third model. Sleep quality was considered an important confounder in previous studies49. However, as all the participants in this study had a good sleep quality, we did not consider that in our analyses. Trend tests were computed across quintiles, treating the tertiles of NLOBS and FLOBS as an ordinal variable. Separate analyses were carried out for examining the relation of each domain of NLOBS and FLOBS with PPD. All analyses were conducted using SPSS version 22 (IBM Corp, Armonk, NY, USA). Statistical significance was determined for P-values below 0.05.
Results
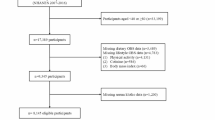
Figure 1 depicts the flowchart of study participants. Among the pregnant women invited, 300 completed the baseline questionnaires. Among them, 22 individuals were excluded due to a history of pre-pregnancy mental disorder (n = 12) and reporting energy intake outside the range of 800–4200 kcal/d (n = 10). Therefore, 278 pregnant women were eligible and had sufficient data for inclusion. Of them, 35 failed to complete the follow-up due to: (1) stillbirth or infant death (n = 6), and (2) not completing EPDS at weeks 4–6 after delivery (n = 29). Ultimately, 243 participants were included in the final analyses, yielding a response rate of 87.4%.
In total, 243 participants were included with a mean age of 30.9 ± 6.12 years. Pre-pregnancy BMI had a mean of 26.1 ± 4.42 kg/m2, and the average weight gain during pregnancy was 12.7 ± 5.63 kg. The mean duration of follow-up was 9 weeks and 43 PPD cases (17.7%) were diagnosed. Baseline characteristics of subjects across tertiles of NLOBS and FLOBS are provided in Table 1. Individuals in the highest tertile of NLOBS were older, and were more likely to have normal gestational weight gain compared to those in the lowest tertile. Furthermore, individuals in the last tertile of NLOBS demonstrated higher levels of physical activity compared to the first tertile. No further significant differences were found in terms of other variables across NLOBS tertiles. In terms of FLOBS, mean age and marriage duration of individuals with the last tertile was higher than those in the first tertile. Also, pre-delivery weight was lower in subjects with the highest FLOBS than those in the reference group. Additionally, participants in the third tertile were more likely to have normal pre-pregnancy weight and BMI status, be multiparous, and non-smoker compared to the first tertile. Also, they were less likely to be passive smokers and to have abnormal gestational weight gain. Moreover, individuals in top tertile of FLOBS had higher physical activity level compared to the first category. Other characteristics showed no significant differences across tertiles of FLOBS.
Dietary intakes of participants across tertiles of NLOBS and FLOBS are reported in Table 2. Individuals with the highest score of NLOBS had higher consumption of protein, vitamin A, riboflavin, vitamin B6, vitamin B12, magnesium, potassium, vitamin C, folate, beta-carotene, lycopene, beta-cryptoxanthin, lutein and zeaxanthin, vitamin D, zinc, fiber, calcium, iron, meats, vegetables, fruits, and legumes/ nuts, as well as lower intakes of refined grains and fats and oils-except olive oil. However, no significant difference was found in terms of other dietary variables. On the other hand, participants in the highest tertile of FLOBS reported higher intakes of carbohydrate, vitamin A, vitamin B6, magnesium, potassium, vitamin C, folate, beta-carotene, lycopene, lutein and zeaxanthin, vitamin D, fiber, calcium, iron, fish, whole grains, vegetables, fruits, legumes/ nuts, and tea/ coffee. Conversely, they reported lower intakes of energy, total fat, MUFA (monounsaturated fatty acids), PUFA, refined grains, and fats and oils-except olive oil. No other significant difference in dietary intakes was seen across FLOBS tertiles.
The incidence of PPD across tertiles NLOBS and FLOBS is illustrated in Fig. 2. As shown, the incidence of PPD is significantly decreasing among NLOBS tertiles (26.2%, 17.5%, 8.9%; P = 0.01). Similarly, a decreasing trend was observed in the incidence of PPD across tertiles of FLOBS (24.0%, 20.5%, 10.0%; P = 0.04).
Crude and multivariable-adjusted hazard ratio (95% CI) for the relation between NLOBS and its dietary sub-domain with PPD is demonstrated in Table 3. The highest tertile of NLOBS was significantly related to risk of PPD in both crude (HR: 0.34; 95% CI: 0.14–0.81) and fully-adjusted (HR: 0.32; 95% CI: 0.12–0.84) models, compared to the lowest tertile. Considering dietary domain of NLOBS, participants in the highest tertile had 68% lower risk of PPD compared to those in the lowest tertile after adjusting all confounders (HR: 0.32; 95% CI: 0.11–0.90).
Table 4 provides crude and multivariable-adjusted hazard ratio (95% CI) for the relation between FLOBS and its dietary sub-domain with PPD. A significant inverse association was observed between the last tertile of FLOBS and risk of PPD in the crude model (HRT3 vs. T1: 0.43; 95% CI: 0.19–0.96). However, after adjusting all of the confounders, this association did not remain significant (HRT3 vs. T1: 0.53; 95% CI: 0.22–1.27). Similarly, no significant relation was found between the dietary sub-domain of FLOBS and PPD in the crude (HRT3 vs. T1: 0.65; 95%CI: 0.31–1.35) and maximally-adjusted (HRT3 vs. T1: 0.85; 95% CI: 0.35–2.02) models.
The association between lifestyle sub-domain of NLOBS and FLOBS and risk of PPD is shown in Table 5 Individuals in the third tertile of LOBS (lifestyle domain of NLOBS and FLOBS) had significantly 64% lower risk of PPD compared to those in the first tertile (HR: 0.36; 95% CI: 0.17–0.77). After considering all of the confounders, this inverse association remained significant as well (HR: 0.41; 95% CI: 0.17–0.96).
Discussion
In this cohort study, we investigated the association between NLOBS and FLOBS during pregnancy and PPD risk. Our results demonstrated that individuals in the highest tertile of NLOBS had a lower risk of developing PPD compared to those in the lowest tertile. This association was independent of potential confounding variables. Similar findings were observed in the case of its nutrients and lifestyle sub-domains. In contrast, although we found a significant inverse relation between FLOBS and risk of PPD, this relation disappeared after adjusting for confounders. Similarly, no significant relation was found between its dietary sub-domain and PPD in both crude and adjusted models. Our findings suggest that oxidative balance during pregnancy could have a pivotal role in the pathogenesis of PPD.
OBS is a tool for assessing individuals’ antioxidant status by assigning scores to antioxidant and pro-oxidant components of diet and lifestyle factors38. In this study, we examined OBS at two levels of nutrient and food. While a significant negative relation was observed between NLOBS and its sub-domains and PPD, we failed to detect any significant relation between FLOBS and PPD risk. Our findings highlight the importance of adhering to a diet rich in antioxidants and adopting a healthy lifestyle during pregnancy to prevent PPD. Due to the relatively high incidence of PPD and its related consequences, it is advisable that future recommendations consider this aspect of diet and lifestyle in order to control PPD.
According to this study, we conclude that NLOBS, particularly its dietary sub-domain, could broaden our insights toward diet-disease relations better than FLOBS. In fact, nutrients are globally consumed and do not vary geographically, whereas, food consumption might differ based on geographical regions and cultures. Therefore, analyzing nutrient patterns enables a more proportional comparison of dietary consumption across nations and regions. Additionally, unlike foods, nutrients can provide insights regarding non-consumers. Overall, analyzing nutrient patterns allows for a more accurate understanding of the relationship between diet and disease and, by integrating measurements related to dietary metabolomics, it provides a more comprehensive understanding of the nutritional effects on health 50,51.
Numerous studies have examined the relationship between dietary patterns during pregnancy and postpartum depression. One study indicated that a healthy dietary pattern during pregnancy, characterized by vegetables, fruits, legumes, nuts, dairy products, fish, and olive oil, may protect against PPD symptoms52. These findings align with our results, demonstrating that a higher NOBS in the diet is associated with a reduced incidence of PPD. However , another study found no association between the consumption of vegetables, fruits, and fish during pregnancy and depression at 12 months postpartum53. Perhaps this discrepancy could be attributed to the non-representative sample of that study, where all participants had a high SES, and on the other hand, significantly lower likelihood of PPD is associated with higher income and education levels54.
Our study demonstrated that a higher OBS during pregnancy is associated with a reduced risk of PPD, consistent with the findings of Alamolhoda et al., who showed an association between serum total antioxidant capacity and PPD55. Furthermore, Ranjbaran et al. revealed the involvement of antioxidant systems in the occurrence of PPD56. Other studies have also investigated the significance of antioxidant systems in the development of depression in non-pregnant individuals. Milajerdi et al. demonstrated a significant inverse relationship between dietary total antioxidant capacity and depression in Iranian adults57. The findings of a systematic review concluded that consuming a diet rich in antioxidants is inversely associated with depression, as indicated by higher scores of dietary total antioxidant capacity58. Additionally, two recent studies found a strong inverse relationship between OBS and depression30, particularly in women29.
Numerous studies have investigated the relationship between maternal lifestyle during pregnancy and PPD. Ding et al. suggested that promoting healthy lifestyle behaviors during pregnancy, such as reducing sedentary time, improving sleep quality, and increasing dietary diversity, may effectively reduce the incidence of PPD59. A cohort study demonstrated that women engaging in moderate to vigorous physical activity for more than 150 min per week in bouts of over 10 min were significantly less likely to experience PPD compared to those with no moderate-intensity physical activity60. Conversely, another study indicated that physical activity during pregnancy increases the risk of PPD61. Another research has shown an association between poor sleep quality in mothers and PPD62; although all of our study participants had good sleep quality. In a study conducted among Iranian women, pre-pregnancy obesity was found to be associated with PPD63. Additionally, a meta-analysis study showed a significant association between gestational weight gain and the risk of PPD64. Smoking or exposure to secondhand smoke during pregnancy may also be associated with PPD risk65,66. Given the above-mentioned studies, having a healthy lifestyle during pregnancy may likely contribute to maintaining oxidative balance in the body and could have beneficial effects on the mental health of mothers.
The mechanism by which OBS affects the risk of depression is not well understood, but oxidative stress likely plays a vital role in this relationship. Oxidative stress refers to the imbalance between the production of ROS (Reactive Oxygen Species), or free radicals, and the antioxidant defense system38. Various modifiable factors, such as diet and smoking, can influence the oxidative stress process67. The brain is more susceptible to damage from increased ROS than any other organ in the body, and increased ROS production and decreased antioxidant defense are responsible for altering brain structure19. Moreover, studies have shown that high levels of active oxygen and nitrogen species, as a result of oxidative-antioxidant imbalance, may lead to increased DNA damage68, ultimately resulting in apoptosis and cell death20. Additionally, there is evidence suggesting that neuronal apoptosis is involved in neurodegeneration, a characteristic of depression69. On the other hand, neuroinflammation resulting from oxidative stress is a significant risk factor for psychiatric disorders, including depression21.
We highlight several strengths of our study. Firstly, it is the first to explore the relationship between OBS and PPD. Additionally, the utilization of valid and reliable questionnaires for assessing dietary intake, sleep, physical activity, and PPD enhances the robustness of our findings. Furthermore, adjusting for potential confounders in our analysis allows for a more thorough examination of the independent association between OBS and PPD; nonetheless, residual confounding must be considered when interpreting the results. Moreover, the cohort design of our study adds strength to our findings. However, our study also faces several limitations. Firstly, employing a FFQ to assess dietary intake may introduce recall and reporting biases. Secondly, dietary information was collected only in the third trimester, and data from the first and second trimesters were not evaluated. Also, lifestyle behaviors were assessed only once during pregnancy, despite their dynamic nature. Thirdly, PPD was not diagnosed through clinical evaluation by psychiatrists; instead, it relied on a self-reported questionnaire. Fourthly, in cases where maternal records were unavailable, anthropometric data for the mother was self-reported, potentially introducing measurement errors. Finally, our study population might not be a good representative of all Iranian females, therefore further studies are required among other Iranian populations. Studies are also needed in other nations in this regard.
In conclusion, the present study suggests that a higher OBS during pregnancy, particularly based on nutrients, is possibly associated with a reduced risk of PPD. Effective nutritional counseling during pregnancy to promote a diet rich in antioxidants and a healthy lifestyle may offer the best benefits for maternal mental health, subsequently contributing to the well-being and health of the newborn. Ultimately, this study included a population of pregnant women from Tehran province in Iran, and caution should be exercised when generalizing the findings to the entire pregnant population. Indeed, further studies with larger sample sizes are needed to confirm our findings.
Availability of data and materials
Data described in the manuscript will be made available upon request pending. For data requests, please contact Razieh Tabaeifard (raziehtabaeifard@gmail.com).
Abbreviations
- ANCOVA:
-
Analysis of covariance
- ANOVA:
-
Analysis of variance
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- DNA:
-
Deoxyribonucleic acid
- EPDS:
-
Edinburgh postpartum depression scale
- FFQ:
-
Food frequency questionnaire
- HIV:
-
Human immunodeficiency virus
- AIDS:
-
Acquired immunodeficiency syndrome
- HR:
-
Hazard ratio
- MET:
-
Metabolic equivalent of task
- MUFA:
-
Monounsaturated fatty acids
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- OBS:
-
Oxidative balance score
- NLOBS:
-
Nutrient lifestyle oxidative balance score
- FLOBS:
-
Food lifestyle oxidative balance score
- LOBS:
-
Lifestyle oxidative balance score
- NOBS:
-
Nutrient oxidative balance score
- FOBS:
-
Food oxidative balance score
- PPAQ:
-
Pregnancy physical activity questionnaire
- PSQI:
-
Pittsburgh sleep quality index
- PPD:
-
Postpartum depression
- PUFA:
-
Polyunsaturated fatty acids
- ROS:
-
Reactive oxygen species
- SES:
-
Socioeconomic status
- TAC:
-
Total antioxidant capacity
References
Alamolhoda, S. H., Kariman, N. & Mirabi, P. Relationship between oxidative stress concentration and postpartum depression: A cohort study. Iran. J. Psychiatry Behav. Sci. 14, e84188. https://doi.org/10.5812/ijpbs.84188 (2020).
Jiang, D. Dietary factors in the prevention and management of postpartum depression: A literature review. J. Food Nutr. Res. 10, 123–143 (2022).
O’Connor, E., Rossom, R. C., Henninger, M., Groom, H. C. & Burda, B. U. Primary care screening for and treatment of depression in pregnant and postpartum women: Evidence report and systematic review for the US preventive services task force. JAMA 315, 388–406. https://doi.org/10.1001/jama.2015.18948 (2016).
Klainin, P. & Arthur, D. G. Postpartum depression in Asian cultures: A literature review. Int. J. Nurs. Stud. 46, 1355–1373. https://doi.org/10.1016/j.ijnurstu.2009.02.012 (2009).
Veisani, Y., Delpisheh, A., Sayehmiri, K. & Rezaeian, S. Trends of postpartum depression in Iran: A systematic review and meta-analysis. Depress. Res. Treat. 2013, 291029. https://doi.org/10.1155/2013/291029 (2013).
Fisher, S. D. et al. Factors associated with onset timing, symptoms, and severity of depression identified in the postpartum period. J. Affect. Disord. 203, 111–120. https://doi.org/10.1016/j.jad.2016.05.063 (2016).
Slomian, J., Honvo, G., Emonts, P., Reginster, J.-Y. & Bruyère, O. Consequences of maternal postpartum depression: A systematic review of maternal and infant outcomes. Women’s Health 15, 1745506519844044 (2019).
Bahadoran, P., Shokrani, F. S., Ehsanpour, S. & Abedi, A. A meta-analysis on studies about obstetric risk factors of postpartum depression in Iran within 1995–2005. (2008).
Leung, B. M. & Kaplan, B. J. Perinatal depression: Prevalence, risks, and the nutrition link–a review of the literature. J. Am. Diet. Assoc. 109, 1566–1575. https://doi.org/10.1016/j.jada.2009.06.368 (2009).
Miyake, Y. et al. Milk intake during pregnancy is inversely associated with the risk of postpartum depressive symptoms in Japan: The Kyushu Okinawa Maternal and Child Health Study. Nutr. Res. 36, 907–913. https://doi.org/10.1016/j.nutres.2016.06.001 (2016).
Azizi, M., Taghavi, S.-A., Asemi, Z., Dadkhahtehrani, T. & Bazarganipour, F. Association between dietary intake in each trimester during pregnancy and postpartum depression and hypochondriasis. (2020).
Apostolaki, I. et al. Dietary patterns during pregnancy and the risk of postpartum depression: the mother–child ‘Rhea’ cohort in Crete, Greece. Public Health Nutr. 14, 1663–1670. https://doi.org/10.1017/S1368980010003629 (2011).
Mokhber, N. et al. Effect of supplementation with selenium on postpartum depression: a randomized double-blind placebo-controlled trial. J. Matern. Fetal. Neonatal. Med. 24, 104–108. https://doi.org/10.3109/14767058.2010.482598 (2011).
Aggarwal, J. et al. Oxidative stress in breast cancer. Perspect. Recent Adv. Med. Res. 4, 69–76. https://doi.org/10.9734/bpi/pramr/v4/4047B (2023).
Olufunmilayo, E. O., Gerke-Duncan, M. B. & Holsinger, R. M. D. Oxidative stress and antioxidants in neurodegenerative disorders. Antioxidants 12, 517 (2023).
Lu, Z. et al. Oxidative stress and psychiatric disorders: Evidence from the bidirectional Mendelian randomization study. Antioxidants 11, 1386 (2022).
Hirose, A., Terauchi, M., Odai, T., Kato, K. & Miyasaka, N. Depressive symptoms at postpartum are associated with those at the second trimester of pregnancy and the antioxidant activity immediately after delivery. J. Psychosom. Obstet. Gynaecol. https://doi.org/10.1080/0167482x.2019.1709817 (2020).
Bhatt, S., Nagappa, A. N. & Patil, C. R. Role of oxidative stress in depression. Drug Discov. Today 25, 1270–1276. https://doi.org/10.1016/j.drudis.2020.05.001 (2020).
Bhatt, S., Nagappa, A. N. & Patil, C. R. Role of oxidative stress in depression. Drug Discov. Today 25, 1270–1276 (2020).
Bakunina, N., Pariante, C. M. & Zunszain, P. A. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 144, 365–373 (2015).
Ng, F., Berk, M., Dean, O. & Bush, A. I. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 11, 851–876 (2008).
Leonard, B. & Maes, M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav. Rev. 36, 764–785 (2012).
Rezin, G. T., Amboni, G., Zugno, A. I., Quevedo, J. & Streck, E. L. Mitochondrial dysfunction and psychiatric disorders. Neurochem. Res. 34, 1021–1029 (2009).
Maciejczyk, M. et al. Oxidative stress, mitochondrial abnormalities and antioxidant defense in Ataxia-telangiectasia, Bloom syndrome and Nijmegen breakage syndrome. Redox Biol. 11, 375–383 (2017).
Goodman, M., Bostick, R. M., Dash, C., Flanders, W. D. & Mandel, J. S. Hypothesis: Oxidative stress score as a combined measure of pro-oxidant and antioxidant exposures. Ann. Epidemiol. 17, 394–399. https://doi.org/10.1016/j.annepidem.2007.01.034 (2007).
Goodman, M. et al. Combined measure of pro- and anti-oxidant exposures in relation to prostate cancer and colorectal adenoma risk: An update. Ann. Epidemiol. 20, 955–957. https://doi.org/10.1016/j.annepidem.2010.08.011 (2010).
Kong, S. Y. et al. Oxidative balance score as predictor of all-cause, cancer, and noncancer mortality in a biracial US cohort. Ann. Epidemiol. 25, 256-262.e251. https://doi.org/10.1016/j.annepidem.2015.01.004 (2015).
Hernández-Ruiz, Á. et al. Oxidative Balance Scores (OBSs) Integrating Nutrient, Food and Lifestyle Dimensions: Development of the NutrientL-OBS and FoodL-OBS. Antioxidants (Basel) 11, 300. https://doi.org/10.3390/antiox11020300 (2022).
Liu, X. et al. Association between depression and oxidative balance score: National Health and Nutrition Examination Survey (NHANES) 2005–2018. J. Affect. Disorders 337, 57–65 (2023).
Li, H. et al. Oxidative balance scores and depressive symptoms: Mediating effects of oxidative stress and inflammatory factors. J. Affect. Disorders 334, 205–212 (2023).
Willett, W. Nutritional epidemiology (Oxford University Press, 2012).
Mirmiran, P., Esfahani, F. H., Mehrabi, Y., Hedayati, M. & Azizi, F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 13, 654–662 (2010).
Esfahani, F. H., Asghari, G., Mirmiran, P. & Azizi, F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran Lipid and Glucose Study. J. Epidemiol. 20, 150–158 (2010).
Ghafarpour, M., et al. The manual for household measures, cooking yields factors and edible portion of food (Keshavarzi Press, 1999).
Institute of, M. & National Research Council Committee to Reexamine, I. O. M. P. W. G. in Weight Gain During Pregnancy: Reexamining the Guidelines (eds K. M. Rasmussen & A. L. Yaktine) (National Academies Press (US) Copyright © 2009, National Academy of Sciences., 2009).
Chasan-Taber, L. et al. Development and validation of a Pregnancy Physical Activity Questionnaire. Med. Sci. Sports Exerc. 36, 1750–1760. https://doi.org/10.1249/01.mss.0000142303.49306.0d (2004).
Fathnezhad Kazemi, A., Hajian, S. & Sharifi, N. The psychometric properties of the Persian version of the pregnancy physical activity questionnaire. Int. J. Women’s Health Reprod. Sci. 7, 54–60. https://doi.org/10.15296/ijwhr.2019.09 (2018).
Hernández-Ruiz, Á. et al. Oxidative balance scores (OBSs) integrating nutrient, food and lifestyle dimensions: development of the NutrientL-OBS and FoodL-OBS. Antioxidants 11, 300 (2022).
Hernández-Ruiz, Á. et al. A review of a priori defined oxidative balance scores relative to their components and impact on health outcomes. Nutrients 11, 774. https://doi.org/10.3390/nu11040774 (2019).
Mayne, S. T., Wright, M. E. & Albanes, D. Re: Hypothesis: oxidative stress score as a combined measure of pro-oxidant and antioxidant exposures. Ann. Epidemiol. 17, 930. https://doi.org/10.1016/j.annepidem.2007.06.012 (2007) (author reply 931).
Willett, W. & Stampfer, M. J. Total energy intake: Implications for epidemiologic analyses. Am. J. Epidemiol. 124, 17–27 (1986).
Cox, J. L., Holden, J. M. & Sagovsky, R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 150, 782–786. https://doi.org/10.1192/bjp.150.6.782 (1987).
Nourollahpour Shiadeh, M. et al. The correlation between Toxoplasma gondii infection and prenatal depression in pregnant women. Eur. J. Clin. Microbiol. Infect. Dis. 35, 1829–1835. https://doi.org/10.1007/s10096-016-2734-5 (2016).
Nierop, A., Bratsikas, A., Zimmermann, R. & Ehlert, U. Are stress-induced cortisol changes during pregnancy associated with postpartum depressive symptoms?. Psychosom. Med. 68, 931–937. https://doi.org/10.1097/01.psy.0000244385.93141.3b (2006).
Mazhari, S. & Nakhaee, N. Validation of the Edinburgh Postnatal Depression Scale in an Iranian sample. Arch. Womens Ment. Health 10, 293–297. https://doi.org/10.1007/s00737-007-0204-x (2007).
Montazeri, A., Torkan, B. & Omidvari, S. The Edinburgh Postnatal Depression Scale (EPDS): Translation and validation study of the Iranian version. BMC Psychiatry 7, 11. https://doi.org/10.1186/1471-244x-7-11 (2007).
Buysse, D. J., Reynolds, C. F. 3rd., Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213. https://doi.org/10.1016/0165-1781(89)90047-4 (1989).
Farrahi Moghaddam, J., Nakhaee, N., Sheibani, V., Garrusi, B. & Amirkafi, A. Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P). Sleep Breath 16, 79–82. https://doi.org/10.1007/s11325-010-0478-5 (2012).
Maghami, M. et al. Sleep disorders during pregnancy and postpartum depression: A systematic review and meta-analysis. Int. J. Dev. Neurosci. 81, 469–478 (2021).
Akbarzade, Z. et al. Association of nutrient patterns with metabolic syndrome and its components in Iranian adults. Clin. Nutr. Res. 9, 318 (2020).
Rouhani, P. et al. Association between patterns of nutrient intake and circulating vitamin D with sleep status among Iranian adults. Sci. Rep. 13, 15318 (2023).
Chatzi, L. et al. Dietary patterns during pregnancy and the risk of postpartum depression: The mother–child ‘Rhea’cohort in Crete, Greece. Public Health Nutr. 14, 1663–1670 (2011).
Nathanson, R., Hill, B., Skouteris, H. & Bailey, C. Antenatal diet and postpartum depressive symptoms: A prospective study. Midwifery 62, 69–76 (2018).
Milgrom, J. et al. Antenatal risk factors for postnatal depression: a large prospective study. J. Affect. Disorders 108, 147–157 (2008).
Alamolhoda, S. H., Kariman, N. & Mirabi, P. Relationship between oxidative stress concentration and postpartum depression: A cohort study. Iran. J. Psychiatry Behav. Sci. https://doi.org/10.5812/ijpbs.84188 (2020).
Ranjbaran, M., Sadeghipour Roudsari, H. R., Nikseresht, S. & Etebary, S. Antioxidant status and endocannabinoid concentration in postpartum depressive women. Tehran Univ. Med. J. 72, 773–779 (2015).
Milajerdi, A., Keshteli, A. H., Afshar, H., Esmaillzadeh, A. & Adibi, P. Dietary total antioxidant capacity in relation to depression and anxiety in Iranian adults. Nutrition 65, 85–90 (2019).
Pereira, G. A., da Silva, A., Hermsdorff, H. H. M., Moreira, A. P. B. & de Aguiar, A. S. Association of dietary total antioxidant capacity with depression, anxiety, and sleep disorders: A systematic review of observational studies. J. Clin. Transl. Res. 7, 631 (2021).
Ding, Y., Li, G. & Wang, Z. Correlation of lifestyle behaviors during pregnancy with postpartum depression status of puerpera in the rural areas of South China. Front. Public Health 11, 1304226 (2023).
Shakeel, N. et al. Physical activity in pregnancy and postpartum depressive symptoms in a multiethnic cohort. J. Affect. Disorders 236, 93–100 (2018).
Susukida, R. et al. Association of prenatal psychological distress and postpartum depression with varying physical activity intensity: Japan Environment and Children’s Study (JECS). Sci. Rep. 10, 6390 (2020).
Okun, M. L., Mancuso, R. A., Hobel, C. J., Schetter, C. D. & Coussons-Read, M. Poor sleep quality increases symptoms of depression and anxiety in postpartum women. J. Behav. Med. 41, 703–710 (2018).
Salehi-Pourmehr, H., Mohammad-Alizadeh, S., Jafarilar-Agdam, N., Rafiee, S. & Farshbaf-Khalili, A. The association between pre-pregnancy obesity and screening results of depression for all trimesters of pregnancy, postpartum and 1 year after birth: A cohort study. J. Perinatal Med. 46, 87–95 (2018).
Qiu, X., Zhang, S. & Yan, J. Gestational weight gain and risk of postpartum depression: A meta-analysis of observational studies. Psychiatry Res. 310, 114448 (2022).
Chen, H.-L., Cai, J.-Y., Zha, M.-L. & Shen, W.-Q. Prenatal smoking and postpartum depression: A meta-analysis. J. Psychosomatic Obstetr. Gynecol. 40, 97–105 (2019).
Song, C. et al. Passive smoking and postpartum depression among Chinese women: A prospective cohort study in Tianjin, China. Women Health 59, 281–293 (2019).
Poljsak, B., Šuput, D. & Milisav, I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013, 1–11 (2013).
Maes, M., Galecki, P., Chang, Y. S. & Berk, M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro) degenerative processes in that illness. Progr. Neuro-Psychopharmacol. Biol. Psychiatry 35, 676–692 (2011).
Stockmeier, C. A. et al. Cellular changes in the postmortem hippocampus in major depression. Biol. Psychiatry 56, 640–650 (2004).
Funding
The financial support for conception, design, data analysis and manuscript drafting comes from Tehran University of Medical Sciences, Tehran, Iran (IR.TUMS.MEDICINE.REC.1401.845).
Author information
Authors and Affiliations
Contributions
LA, SH, and RT designed the study. RT, MMN, MK and NO carried out interviews, extracted data from the medical records and imported the data for analysis. LA and RT performed the statistical analyses. RT and MKD drafted the manuscript. LA and SH read and commented on the manuscript. LA supervised the study. The final manuscript was approved by all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study received approval from the School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences Research Ethics Committee (IR.TUMS.MEDICINE.REC.1401.845), following the principles of the Helsinki Declaration.
Consent for publication
All authors have read and approved the final manuscript. Informed consent was obtained from all individual participants included in the study for the publication of their data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tabaeifard, R., Hashempour, S., Karim Dehnavi, M. et al. Association between oxidative balance score and risk of postpartum depression in Iranian women: a prospective cohort study. Sci Rep 15, 8590 (2025). https://doi.org/10.1038/s41598-025-93206-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-93206-y