Abstract
Oxidative stress and disruption of blood‒testis barrier permeability are considered key factors in the pathogenesis of testicular inflammation, degeneration, and functional impairment, which play crucial roles in male infertility. Antimicrobial peptides (AMPs) are internationally recognized as some of the most promising alternatives to antibiotics. However, the molecular mechanisms by which AMPs regulate oxidative stress and the blood‒testis barrier in the testis are still poorly understood. In this study, we orally administered 0.5 mg/kg antimicrobial peptide MPX (MPX) to mice for 20 and 40 days and evaluated its effects on Lipopolysaccharide LPS-induced testicular oxidative stress and blood‒testis barrier repair, and elucidateed the pharmacokinetics of MPX in mice. The experiment was divided into six groups, control, LPS, MPX, MPX + LPS, Polymyxin and Polymyxin + LPS, respectively. The results showed that oral administration of MPX effectively increased testicular Glutathione (GSH), Total superoxide dismutase (T-SOD), and Catalase (CAT) levels and reduced Nitric oxide (NO) and Malondialdehyde (MDA) levels in the testes and Lactate dehydrogenase (LDH) levels in serum; these findings were consistent with the oxidative stress parameters in the liver. MPX significantly upregulated the expression of Kelch-like ECH-associated protein 1 (Keap1), Nuclear factor erythroid 2-related factor 2 (Nrf2), and Glutamate cysteine ligase, modifier (GLCM) in the testes while downregulating the expression of Glutamate cysteine ligase, catalytic (GCLC) and Inducible nitric oxide synthase (iNOS), thus exerting a regulatory effect on oxidative stress. MPX also effectively increased sperm count and motility and counteracted the LPS-induced blood‒testis barrier damage, and its molecular mechanism involved upregulating the expression of Slug, which subsequently promoted high expression of Claudin, Occludin, Zonula occludens-1 (ZO-1), N-cadherin, and E-cadherin in the testes. After intragastric administration of FITC-MPX for 30 min, FITC-MPX was mainly distributed in the stomach and thoracic cavity, then showed multi-tissue distribution after 30 min. The fluorescence signal could be detected in the testis 1 h later, which confirmed that MPX had testicular targeting. Moreover, both intraperitoneal and intravenous injection of FITC-MPX also confirmed its testicular targeting ability. In conclusion, this study systematically evaluated the long-term effects of the orally administered antimicrobial peptide MPX on oxidative stress and the blood‒testis barrier in the male reproductive system. This study laid the foundation for the antimicrobial peptide MPX to be used in the treatment of male testicular inflammatory diseases.
Similar content being viewed by others
Introduction
Testicular infection and inflammation are major causes of male infertility, accounting for approximately 15% of male infertility cases1. Orchitis is a frequent inflammatory reproductive disease that causes male infertility and a decline in sperm quality2. A variety of substances can cause testicular damage and act as inducers of orchitis models, such as Cadmium, Fluoride and Lipopolysaccharide (LPS). LPS is a major structural and functional component of the gram-negative bacterial outer membrane. During bacterial infection, it activates signal transduction and initiates the inflammatory process. Consequently, it has been used as a common experimental method for evaluating inflammatory responses in both in vivo and in vitro models3. Jalilvand et al. found that orchitis model induced by LPS, resulting in decreased superoxide dismutase, catalase, and total thiol, and increase malondialdehyde4. LPS has also been reported to cause disorders of the Blood-testis barrier (BTB) and acute systemic inflammation5. In addition, bacterial infection or LPS exposure can also lead to reproductive dysfunction in female animals6. Oxidative stress and disruption of BTB permeability are considered key factors leading to testicular degeneration and functional impairments. BTB are crucial for the cellular mechanisms of spermatogenesis as they protect against detrimental cytotoxic agents, chemicals, and pathogens, thereby maintaining a sterile environment necessary for sperm development7. It is evident that oxidative stress and the BTB play a crucial role in the development of testicular inflammation, making it important to study their contributions to testicular inflammation.
Antimicrobial peptides (AMPs) are a class of structurally specific small-molecule peptides that serve as the first line of defence against invading pathogens and are integral components of the host’s innate immune system. They exhibit diverse biological functions, including antibacterial, antifungal, antiviral, anticancer, and antitumour activities8. AMPs play an important role in regulating the body’s oxidative stress. On one hand, AMPs can promote oxidative stress. Hou et al. found that the cationic antimicrobial peptide NRC-03 induced high oxygen consumption, reactive oxygen species (ROS) release, mitochondrial dysfunction, and apoptosis in oral cancer cells. NRC-03 induced apoptosis in oral squamous cells via CypD-mPTP axis-mediated mitochondrial oxidative stress9. On the other hand, AMPs can inhibit oxidative stress and enhance the body’s antioxidant capacity. Feeding piglets the antimicrobial peptide IAMP significantly upregulates serum immunoglobulin m (IgM) and superoxide dismutase (SOD), increases the expression levels of intestinal Zonula occludens-1 (ZO-1), and increases villus height10. However, whether orally administered antimicrobial peptides also have a regulatory effect on testicular oxidative stress and the blood‒testis barrier in the reproductive system of animals, as well as their underlying mechanisms, remains unknown.
The antimicrobial peptide MPX belongs to the mastoparan antimicrobial peptide family and consists of 14 amino acids with a net positive charge of + 4. It is highly concentrated in wasp venom and exhibits strong antibacterial activity against both gram-positive and gram-negative bacteria11,12. In our laboratory’s previous studies, we found that intraperitoneal injection of the antimicrobial peptide MPX effectively reduced LPS-mediated inflammation in testicular supporting cells, decreased the oxidative stress response, and enhanced blood‒testis barrier formation13. Moreover, we detected oral administration of antimicrobial peptide MPX ameliorated orchitis and inflammatory damage in other organs, regulated LPS- induced pneumonia and enteritis14. However, whether oral antimicrobial peptide MPX can maintain the structural integrity of the blood-testis barrier and reduce oxidative stress is still unknown.
In this study, the model animals were exposed to LPS to simulate the changes in testicular function and expression of related inflammatory factors during bacterial infection, then explored the effective regulation of oral MPX on testicular oxidative stress and blood-testis barrier, and discussed its potential mechanism, proving that MPX can effectively accumulate in testis, in order to provide a certain theoretical basis for the prevention and treatment of bacterial orchitis. we evaluated orally administered MPX can effectively regulate testicular oxidative stress and the blood‒testis barrier and explored its potential mechanisms, and demonstrated that MPX could efficiently accumulate in the testis. This research aiming to provide new strategies for the prevention and treatment of acute orchitis through oral administration of antimicrobial peptides.
Materials and methods
Mice
Adult male Kunming mice (8–10 weeks old) were obtained from Henan Skebees Biotechnology Co., Ltd. (No. 041002000211). The mice were housed under standard laboratory conditions at 22 °C with a 12 h light or dark cycle and provided with standard pellet food and water ad libitum.
Ethical statement
The animal procedures in this study followed the “Principles of Laboratory Animal Care” published by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” developed by the Institute of Laboratory Animal Resources and published by the National Institutes of Health in 2011 (8th edition). All experimental procedures were conducted in strict accordance with the guidelines approved by the Animal Ethics Committee of Henan University of Science and Technology and Jilin University (2022HIST023).
Peptide synthesis and lipopolysaccharides (LPS)
MPX (H-INWKGIAAMAKKLL-NH2). The antibacterial peptide MPX was produced by Shanghai Jier Biochemical Company (China) using a solid-phase N-9-fluoromethoxycarbonyl (Fmoc) strategy and high-performance liquid chromatography (HPLC) purification, and its purity was as high as 98%. LPS (Escherichia coli 055: B5, Cat L8880) was purchased from Solarbio Biotechnology Co., Ltd. (China).
Feeding of mice and establishment of the mouse testicular inflammation model
Polycolistin antibiotics are the last line of defense in the treatment of multidrug-resistant gram-negative infections. Polymyxin B is a cationic peptide antibiotic that binds E. coli LPS and prevents inflammatory activation15. The doses of LPS, MPX, and the antibiotic control polymyxin B were determined based on preliminary work.
The mice were randomly divided into six groups (n = 10): (1) the negative control group, in which mice received oral gavage of 200 µL of saline for 20 (10 times) and 40 days (20 times, same for subsequent oral gavage); (2) the positive control group, in which mice received oral gavage of 200 µL of saline for 20 and 40 days followed by intraperitoneal injection of LPS (5 mg/kg, 1 injection, same for subsequent injections); (3) the MPX group, in which mice received oral gavage of 0.5 mg/kg MPX in 200 µL saline for 20 (10 times) and 40 days (20 times); (4) the MPX + LPS group, in which mice received oral gavage of MPX for 20 and 40 days followed by intraperitoneal injection of LPS (200 µL); (5) the polymyxin B group, in which mice received oral gavage of 25 mg/kg polymyxin B in 200 µL saline for 20 (10 times) and 40 days (20 times); and (6) the polymyxin B + LPS group, in which mice received oral gavage of polymyxin B for 20 and 40 days followed by intraperitoneal injection of LPS (200 µL) [2; 16].
After LPS treatment for 24 h, mice were sacrificed after isoflurane anesthesia. Testicular and epididymal tissues were fixed in 4% paraformaldehyde for histological analysis or postfixed in 2.5% glutaraldehyde for scanning electron microscopy observation of the testes. The remaining testicular and epididymal samples were stored at -80 °C for further analysis of expression, including that of oxidative stress-related proteins (Keep, CLCM, Nrf2, GCLC, and iNOS) and tight junction proteins (Claudin, Occludin, ZO-1, N-cadherin, E-cadherin, and Slug). Serum samples were used to measure lactate dehydrogenase (LDH) levels.
qRT-PCR
Total RNA was isolated from the testes using TRIzol reagent (Invitrogen, USA). Reverse transcription was performed using a PrimeScript™ RT Reagent Kit (Thermo Fisher, #00988159). PCR was conducted in a 10 µL reaction mixture containing 2 µL of cDNA, 0.1 µL of forward primer, 0.1 µL of reverse primer (Table 1), and 5 µL of SYBR Green PCR MasterMix (QIAGEN, #208054). The amplification conditions were as follows: initial denaturation at 95 °C for 30 s, denaturation at 95 °C for 5 s, annealing and extension at 60 °C for 20 s, repeated for 40 cycles. The relative expression levels of the target genes (ZO-1, Claudin-1, Occludin, Sox9, IBP, iNOS, Nrf2, Keap1, GCLC, GLCM) were normalized to the expression of the reference gene using the 2-ΔΔCt method.
Antioxidant test
Tissue (testis) was prepared and tested according to the GSH kit instructions (A006-1-1, Nanjing Jiangcheng Co., LTD.). During the testing process, a blank (distilled water control), a standard tube (20 µmol/LGSH standard) and a determination tube (sample to be tested) were prepared in a 4 mL centrifuge tube according to the instructions, mixed with a vortex mixer, and placed at room temperature for 5 min. A 1 cm optical diameter colorimetric cup was set at 420 nm, and the distilled water was zeroed. The absorbance of each tube was measured by spectrophotometer.
Tissue (testis) homogenate was prepared and tested according to the CAT kit instructions (A007-2-1, Nanjing Chiencheng Co., LTD.). 1 cm optical diameter quartz colorimetric dish was taken, UV 240 nm, and double steamed water was adjusted to zero for use. Add 0.02 mL of the treated sample to the bottom of the cupola, quickly pour 3 mL of the substrate solution preheated to 25 ℃ with OD between 0.5 and 0.55 into the cupola, immediately measure the absorbance at 240 nm, and record the A1 value. Do not take out the cupola. Immediately measure the absorbance again after 1 min and record the A2 value.
Total Superoxide Dismutase (T-SOD) determination: Preparation of tissue (testicles)) according to the T-SOD kit instructions (A001-1, Nanjing Jiangcheng Co., LTD.) detection. The relevant reagents were prepared in 4 mL centrifuge tubes, mixed in a vortex mixer, and bathed in water at 37 ℃ for 40 min. 2 mL color developer was added to each centrifuge tube, and 2 mL supernetting and 1 cm optical diameter colorimetric cups were taken at 550 nm. The distilled water was adjusted to zero, and the absorbance of each tube was measured by spectrophotometer.
H&E staining
Histopathological examination was performed using haematoxylin and eosin (H&E) staining. Mice were anesthetized with isoflurane and euthanized by bloodletting, and the viscera were carefully removed. Tissues were fixed in 4% paraformaldehyde for at least 24 h, dehydrated in a graded alcohol series, cleared in xylene, and embedded in paraffin. Subsequently, tissue blocks were sectioned at a thickness of 5 μm, stained with H&E, and observed under an optical microscope at 200× magnification (Leica Microsystems GmbH, n = 6–9).
Immunohistochemistry and Immunofluorescence analysis
The testes were fixed in 4% paraformaldehyde for 24 h. After cryoprotection in 30% sucrose, frozen sections were cut to a thickness of 7 μm using Leica CM1950 (Leica Biosystems, Nussloch, Germany). The sections were incubated in PBS containing 3% H2O2 for 10 min to inhibit endogenous peroxidase activity. After blocking with 5% rabbit serum in PBS for 1 h at room temperature, the sections were incubated overnight at 4 °C with primary antibodies (Table 2). The sections were rinsed three times with PBS and incubated with an HRP-conjugated secondary antibody at room temperature for 30 min. HRP activity was visualized using the diaminobenzidine method, after which counterstaining was performed with haematoxylin, and the sections were observed under a microscope. Fluorescence images were acquired using a Nikon ECLIPSE Ti fluorescence microscope (Nikon Corporation, Japan) with NIS-Elements BR 3.0 software. Immunohistochemical results were collected and analysed using Ippwin 32 software12. For immunofluorescence analysis, the sections were incubated with the corresponding secondary antibodies in the dark at room temperature for 1 h, and stained with DAPI for 15 min. Images were examined and acquired using a confocal laser microscope (ZEISS LSM 780, Germany). Antibody information and working dilutions are shown in Table 2.
Transmission electron microscopy examination
Testicular tissues were fixed in 2.5% glutaraldehyde fixative for approximately 30 min until the testicular tissue hardened. Secondary fixation and staining were performed using osmium tetroxide after washing three times with 0.1 M PBS. After rinsing with deionized water, the tissues were stained overnight at 4 °C with 1% uranyl acetate in 1% ethanol. The tissues were then dehydrated in increasing concentrations of ethanol and graded acetone solutions and embedded in epoxy resin. Ultrathin Sect. (60 nm) were prepared using an Ultratome (Leica, Reichert Ultracuts). Finally, the ultrathin sections were examined using a Hitachi-7500 transmission electron microscope (Hitachi) in the Electron Microscopy Laboratory of Henan University of Science and Technology17.
Western blot
Testicular tissues (approximately 100 mg) were collected and homogenized in RIPA lysis buffer containing protease inhibitors using a tissue homogenizer in a cold environment. The protein concentrations were determined using a Pierce™ Rapid Gold BCA Protein Quantification Kit (Thermo, #A53226). Protein lysates (approximately 30 µg) were separated by sodium dodecyl sulfate‒polyacrylamide gel electrophoresis (SDS‒PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes at a constant current of 200 mA for 90 min. Subsequently, the PVDF membranes were incubated in skim milk for 2 h and then incubated overnight with primary antibodies (Table 2) at 4 °C. After washing three times, the membranes were incubated with secondary antibodies for 2 h. Finally, the target proteins were detected using an enhanced chemiluminescence substrate (SuperSignal™ West Pico PLUS, Thermo, #34577).
Sperm counting
Sperm isolation from the epididymal tail was performed by collecting the epididymal tail and placing it in 1 mL of human tubal fluid (HTF) medium. Multiple small incisions were made in the tail using fine scissors, and the tissue was incubated at 37 °C in a 5% CO2 incubator for 15 min to disperse the sperm. The suspension was gently pipetted up and down, and the resulting sperm suspension was passed through a 70 μm filter. The sperm suspension was then diluted 1:50, and 20 µL of the diluted suspension was placed onto a counting chamber. A coverslip was placed on top, and sperm counting was performed under a 20x microscope. ImageJ (NIH) with the Cell Counter plugin was used to count the sperm at least three times per mouse, with a minimum of five squares counted each time. The average value was used for statistical analysis18.
Sperm scoring
Tissue sections fixed in Bouin’s solution (4 h) were stained with haematoxylin and eosin (testes) or Sirius Red (epididymides). The adjusted method of the classical Johnsen scoring system for sperm development (Johnsen, 1970) was used. By evaluating 200 cross-sections of seminiferous tubules per mouse and recording the most advanced recognizable germ cell type in each tubular cross-section, the percentage of tubular cross-sections displaying respective stages of germ cells was determined to assess spermatogenic impairment. Accordingly, elongated spermatids (ES) correlated with Johnsen scores of 10−8; round spermatids (RS) correlated with Johnsen scores of 7–6; pachytene spermatocytes (PScs) correlated with Johnsen scores of 5−4; spermatogonia (SG) correlated with a Johnsen score of 3; Sertoli cells only (SCO) correlated with a Johnsen score of 2; and complete absence of germinal epithelium correlated with a Johnsen score of 116.
MPX distribution in vivo
The mice were randomly divided into 4 groups with 10 mice in each group. The experimental groups were administered FITC-MPX (2.5 mg/kg) by intraperitoneal injection, intravenous injection, in situ injection and intragastric administration, respectively. The control group was given normal saline in the same way. Fluorescence signals in the mice were detected by In-vivo imaging Systems (AniView 100, Boluteng, China) at 2 min, 5 min, 10 min, 15 min, 25 min, 30 min, 45 min, 60 min, 90 min and 2 h after administration, and urine was collected at the same time. After 2 h, mice were anesthetized with isoflurane and euthanized by bloodletting and the organs were collected, then the fluorescence signals of the organs and urine were detected. Imaging mode is FITC (excitation wavelength is 488 nm, emission wavelength is 535 nm).
Statistical analysis
Data were analysed for significant differences using repeated-measures models followed by one-way analysis of variance (ANOVA) and subsequent multiple comparison tests. The results are presented as the mean ± SD. Statistical analysis was performed using GraphPad Prism 8 software. Differences were considered significant and highly significant when indicated as *p < 0.05, **p < 0.01, #p < 0.05, and ##p < 0.01.
Results
LPS promoted testicular oxidative stress and disrupted the blood‒testis barrier in mice
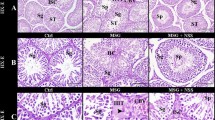
Histological analysis of testicular and epididymal tissue stained with H&E revealed that LPS treatment resulted in sloughing and thinning of the seminiferous epithelium, premature release of germ cells from the epithelium, and the presence of numerous mature spermatozoa within the lumen, indicative of a typical inflammatory response (Fig. 1A). Sperm scores (Johnsen scores) were significantly lower in the LPS-treated group compared to the negative control group (Fig. 1B, **p < 0.01). LPS-induced damage in the epididymides was characterized by a reduced sperm count, decreased numbers of cilia, and the presence of inflammatory cells and red blood cells within the epididymal duct (Fig. 1C). Sperm counting in the epididymides demonstrated a significant reduction in sperm count after LPS treatment (Fig. 1D, **p < 0.01). The levels of malondialdehyde (MDA), the end product of lipid peroxidation, and lactate dehydrogenase (LDH) reflect the extent of cellular oxidative stress damage, while superoxide dismutase (SOD) functions as an antioxidant and antiaging agent. The results showed that LPS treatment significantly increased MDA levels in the testes (Fig. 1E, *p < 0.05), decreased T-SOD levels in the testes (Fig. 1F, *p < 0.05), and increased LDH levels in the blood (Fig. 1G, **p < 0.01), indicating enhanced oxidative stress. Transmission electron microscopy analysis of the blood‒testis barrier revealed that LPS treatment significantly increased the permeability of the barrier (Fig. 1H) and widened the gap within the blood‒testis barrier (Fig. 1I, **p < 0.01). These results indicate that intraperitoneal injection of 5 mg/kg LPS effectively promotes testicular oxidative stress and disrupts the blood‒testis barrier in mice.
Effects of LPS on testicular oxidative stress and the blood–testis barrier in mice. (A) H&E staining was used to observe the pathological injury of mouse testes induced by LPS. (B) Sperm score (Johnsen’s score). (C) H&E staining was used to observe the pathological injury of mouse epididymides induced by LPS. (D) Sperm counting of the epididymides after LPS treatment. (E) Levels of MDA in the testes after LPS treatment. (F) Levels of T-SOD in the testes after LPS treatment. (G) Levels of LDH in the blood after LPS treatment. (H) Transmission electron microscopy analysis of the effect of LPS on the blood‒testis barrier. (I) BTB width measurement after LPS treatment. MDA malondialdehyde, LDH lactate dehydrogenase, T-SOD total superoxide dismutase, n = 10 mice/group).
Oral administration of MPX effectively reduced oxidative stress in mice
To investigate the effects of MPX on testicular oxidative stress, mice were orally administered MPX for 20 and 40 days and then intraperitoneally injected with 5 mg/kg LPS. The levels of GSH, T-SOD, CAT, NO, MDA, and LDH in the testes were measured. There was no significant difference compare MPX and polymyxin B to control, but the LPS could significantly increased the levels of GSH, T-SOD, NO, MDA and CAT. Compare MPX and polymyxin B to LPS, oral administration of MPX significantly increased the levels of GSH, T-SOD, and CAT in the testes (*p < 0.05, **p < 0.01, Fig. 2A, B, C). Similarly, oral administration of polymyxin B also effectively increased the level of T-SOD in the testes (#p < 0.05, ##p < 0.01, Fig. 2B). Conversely, oral administration of MPX significantly reduced the levels of NO and MDA in the testes (*p < 0.05, **p < 0.01, Fig. 2D, E) and decreased LDH activity in the blood (**p < 0.01, Fig. 2F). Oral administration of polymyxin B also effectively reduced the levels of MDA in the testes and LDH activity in the blood (#p < 0.05, ##p < 0.01, Fig. 2E, F). Additionally, MPX increased the levels of GSH and the activity of T-SOD in the liver (*p < 0.05, Fig. 3A, B) while decreasing the levels of NO and MDA in the liver (*p < 0.05, **p < 0.01, Fig. 3C, D). In conclusion, long-term administration of MPX effectively reduces LPS-induced oxidative stress in the testes and livers of mice.
Effect of oral administration of MPX on LPS-induced oxidative stress at Days 20 and 40. (A–F) The levels of GSH, T-SOD, CAT, NO, MDA, and LDH were measured in the testes at Days 20 and 40 with an oxidative stress kit (*p < 0.05, **p < 0.01, * represented MPX treatment group vs. LPS group; #p < 0.05, ##p < 0.01, # represented Polymyxin B treatment group vs. LPS group). GSH glutathione, T-SOD total superoxide dismutase, CAT catalase, NO nitric oxide, MDA malondialdehyde, LDH lactate dehydrogenas, n = 10 mice/group).
Effect of the antimicrobial peptide MPX on LPS-induced oxidative stress in the livers of mice. (A–D) The levels of GSH, T-SOD, NO and MDA were measured in the liver at Days 20 and 40 after LPS treatment (*p < 0.05, **p < 0.01, * represented MPX treatment group vs. LPS group; #p < 0.05, ##p < 0.01, # represented Polymyxin B treatment group vs. LPS group). GSH glutathione, T-SOD total superoxide dismutase, NO nitric oxide, MDA malondialdehyde, n = 10 mice/group).
MPX reduced testicular oxidative stress by modulating the Nrf2 signalling pathway
Keap1, GLCM and GCLC play important roles in oxidative stress. Compared with the LPS-only group, the MPX + LPS group exhibited increased mRNA expression of Keap1 and GLCM in the testes (*p < 0.05, Fig. 4A, C) but reduced mRNA expression of GCLC and iNOS (*p < 0.05, Fig. 4B, D). Nrf2 is a key transcription factor involved in antioxidant responses. Under oxidative stress, Nrf2 is activated and translocates into the nucleus to bind to the antioxidant response element (ARE) and drive the expression of antioxidant genes19. Immunohistochemical analysis in this study revealed that both MPX and polymyxin B significantly increased the positive area of Nrf2 in the testes (**p < 0.01, ##p < 0.01, Fig. 4E, F). Furthermore, MPX reduced the number of iNOS-positive cells and the positive area ratio in iNOS-positive cells (*p < 0.05, Fig. 4G, H). In conclusion, compared to polymyxin B, MPX exhibited superior antioxidant effects and was able to modulate testicular oxidative stress through the Keap1/Nrf2 signalling pathway.
Mechanism by which MPX reduces LPS-induced testicular oxidative stress. (A–D) mRNA expression of Keap1, GLCM, GCLC and iNOS in the testes at Days 20 and 40 after MPX treatment. (E) Immunohistochemical analysis of the protein expression of Nrf2 in the testes at Days 20 and 40 after MPX treatment. (F) Measurement area (%) of Nrf2 expression in the testes. (G) Immunohistochemical analysis of the protein expression of iNOS in the testes at Days 20 and 40 after MPX treatment. (H) Measurement area (%) of iNOS expression in the testes (*p < 0.05, **p < 0.01, * represented MPX treatment group vs. LPS group; #p < 0.05, ##p < 0.01, # represented Polymyxin B treatment group vs. LPS group) (Kelch-like ECH-associated protein 1,Keap1; Nuclear factor erythroid 2-related factor 2, Nrf2; GLCM glutamate cysteine ligase, modifier, GCLC glutamate cysteine ligase, catalytic, n = 10 mice/group).
MPX administration repaired blood testis barrier damage
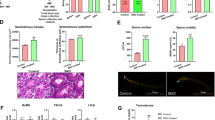
H&E staining of testicular tissue revealed that long-term administration of MPX alleviated LPS-induced testicular injury, as indicated by restoration of an intact seminiferous tubule structure, a thickened and densely packed germinal epithelium, and an increased sperm count. In contrast, long-term administration of polymyxin B did not effectively alleviate LPS-induced testicular injury, resulting in thinning of the germinal epithelium, loss of germ cells, and structural disarray (Fig. 5A). MPX significantly increased the height of the germinal epithelium (*p < 0.05, Fig. 5B) and significantly improved Johnsen scores, demonstrating better efficacy than polymyxin B (**p < 0.01, #p < 0.05, Fig.5C). TEM examination of ultrathin sections of the testes revealed that MPX effectively repaired LPS-induced tight junction (TJ) barrier damage, resulting in intact and compact TJ structures and reduced mitochondrial swelling in supporting cells (Fig. 5D). The width of the TJ gap was significantly decreased with MPX treatment (*p < 0.05, Fig. 5E). Overall, MPX effectively protected against LPS-induced TJ barrier damage and enhanced TJ tightness.
Effect of oral administration of the antimicrobial peptide MPX on LPS-induced blood‒testis barrier damage. (A) H&E staining was used to observe the therapeutic effect of MPX on LPS-induced testicular pathological injury on Days 20 and 40. (B) Sperm counting statistics of the epididymides after MPX treatment at Days 20 and 40. (C) Sperm scoring after MPX treatment (Johnsen’s score) at Days 20 and 40. (D) Transmission electron microscopy was used to observe the effect of MPX treatment on LPS-induced tight junction (TJ) barrier damage at Days 20 and 40. (E) Effect of MPX treatment on BTB width at Days 20 and 40 (n = 10 mice/group).
MPX repairs the blood testis barrier damage by increasing the expression of tight junction proteins
In the mammalian testis, the blood‒testis barrier (BTB) exhibits a unique ultrastructure. The BTB consists of coexisting tight junctions (TJs), basal ectoplasmic specializations (basal ESs), desmosomes, and gap junctions between adjacent Sertoli cells beneath the basal lamina of the seminiferous tubules20. TJs, composed of proteins such as claudin, occludin, N-cadherin, and ZO-1, play a crucial role in maintaining the integrity of the BTB. qRT‒PCR analysis revealed that oral administration of MPX significantly increased the expression of the claudin, occludin, and ZO-1 genes, consistent with the findings in the polymyxin B group (*p < 0.05, **p < 0.01, #p < 0.05, ##p < 0.01, Fig. 6A–C). Western blot results showed that MPX significantly increased the expression of N-cadherin, E-cadherin, and Slug (*p < 0.05, **p < 0.01, ##p < 0.01, Fig. 6D, F, G, I). MPX also increased the expression of Occludin and Vimentin, but the differences in greyscale analysis were not significant (*p > 0.05, Fig. 6E, H). Polymyxin B significantly inhibited the expression of TWIST1 (#p < 0.05, Fig. 6J). Immunofluorescence staining of testicular tissue further confirmed that compared to the LPS group, MPX significantly increased the expression of Claudin-1, N-cadherin, and ZO-1, while the polymyxin B group showed significant increases in Claudin-1 and N-cadherin expression (*p < 0.05, **p < 0.01, #p < 0.05, ##p < 0.01, Fig. 7A-D). These results indicate that MPX administration in mice repairs LPS-induced BTB damage by increasing the expression of barrier proteins.
Effect of MPX on the expression of tight junction proteins after blood‒testis barrier injury induced by LPS at Days 20 and 40. (A-C) mRNA expression of Claudin, Occludin, and ZO-1 in testicular tissue at Days 20 and 40 after MPX treatment. (D) Protein expression of N-cadherin, E-cadherin, Slug, Occludin, Vimentin, and TWIST1 in testicular tissue at Days 20 and 40 after MPX treatment. (E–J) Quantitative analysis of protein expression at Days 20 and 40 after MPX treatment (*p < 0.05, **p < 0.01, * represented MPX treatment group vs. LPS group; #p < 0.05, ##p < 0.01, # represented Polymyxin B treatment group vs. LPS group; n = 10 mice/group).
Immunofluorescence staining revealed the effect of MPX on the expression of tight junction proteins. (A) Protein expression of Claudin-1, N-cadherin, and ZO-1 in testicular tissue after MPX treatment at Days 20 and 40. (B–D) Analysis of the relative fluorescence intensity of Claudin-1, N-cadherin, and ZO-1 after MPX treatment at Days 20 and 40. (*p < 0.05, **p < 0.01, * represented MPX treatment group vs. LPS group; #p < 0.05, ##p < 0.01, # represented Polymyxin B treatment group vs. LPS group; n = 10 mice/group).
The distribution of MPX in testicular tissue
In intraperitoneal injection mode, FITC-MPX was mainly concentrated in the abdominal cavity, and it can be effectively delivered into the blood circulation after 1 h, then distributed into multiple organs 2 h later. FITC-MPX showed systemic distribution after intravenous injection, and the duration of systemic fluorescence signal was more than 2 h. FITC-MPX could be retained in the testes for 2 h after testicular injection in situ, then entered into the bloodstream showing systemic distribution. FITC-MPX was mainly distributed in the stomach and thorax within 30 min of gavage, and showed multi-tissue distribution after 30 min. It was delivered into testes after 1 h, suggesting that MPX could be delivered into the testis by digestive administration (Fig. 8A). In addition, we further examined the fluorescence signals in the organs of different experimental groups of mice, and found that FITC-MPX was mainly distributed in the intestine in different groups, followed by testes and kidneys, with no or very little distribution in liver, spleen, lung, and heart (Fig. 8B). Furthermore, the fluorescence signal in the intestine was further detected, and revealed that FITC-MPX mainly concentrated in the jejunum in the abdominal and intravenous injection groups; In situ injection of FITC-MPX into testes mainly distributed in jejunum and colon; All positive distribution were found in the gavage group (Fig. 8C). Finally, we also tested the fluorescence signals in urine, showing that there were a large number of fluorescence signals in the urine of the intraperitoneal injection and intravenous injection groups, the fluorescence intensity in the urine of the in situ injection group was weak, and the fluorescence intensity in the urine of the intragastric group was the lowest (Fig. 8D).
Discussion
Intraperitoneal injection of LPS can induce acute systemic inflammation, impair male reproductive function, lower testosterone levels, compromise spermatogenesis, enhance testicular oxidative stress, damage the blood‒testis barrier, and significantly downregulate the expression of intercellular adhesion molecule 1, tight junction protein 1, and gap junction α-1 protein5. In this study, we found that oral administration of MPX upregulates the expression of Keap1, GLCM, and Nrf2 in the testes; downregulates the expression of GCLC and iNOS; increases the levels and activity of antioxidant stress markers, such as GSH, T-SOD, and CAT; and reduces the levels of NO and MDA, thus exerting a regulatory effect on oxidative stress. MPX also promotes the high expression of Claudin-1, Occludin, ZO-1, N-cadherin, and E-cadherin in the testes, thereby repairing LPS-induced blood‒testis barrier injury. Moreover, MPX can be effectively delivered into the blood circulation, and mainly distributed in the digestive tract, male urogenital tract. It had a good tendency to the testis, and it was metabolized by the kidney and excreted in the urine finally.
Reducing testicular tissue oxidative stress and improving testicular function and spermatogenesis are effective approaches for treating male reproductive disorders. Hesperidin has a protective effect on paclitaxel-induced testicular toxicity through regulating oxidative stress, apoptosis, inflammation and endoplasmic reticulum stress, PTX could decrease antioxidant enzyme (superoxide dismutase, catalase, and glutathione peroxidase) activities and increase malondialdehyde level were regulated, and the severity of oxidative stress was reduced21, this effect is very similar to MPX. Shafiey et al. found Rivaroxaban counteracted oxidative stress and histopathological aberrations22. Oral MPX can also counteract LPS-induced oxidative stress and histopathological abnormalities of the testis. Doxorubicin (DOX) is a powerful chemotherapeutic agent used in many types of malignancies. However, its use results in testicular damage. Treatment with liraglutide significantly abrogated DOX-induced testicular damage by restoring testicular architecture, increasing sperm count and sperm motility, and decreasing sperm abnormalities as compared to DOX group23. MPX administration repaired blood testis barrier damage, significantly improved Johnsen scores, similar to the effect of liraglutide. Melatonin receptor 1/melatonin receptor 2 (MT1/MT2) is primarily expressed in the interstitial cells of the human and mouse testes. Melatonin stimulates SIRT1/Nrf2 signalling by activating MT1/MT2 to prevent cisplatin-induced Leydig cell oxidative stress and apoptosis, thereby improving mouse testicular function24. Piceatannol can counteract the decrease in spermatogenesis, testicular pathological changes, and oxidative stress state in rats after Cd treatment. It reverses the significant downregulation of the mRNA and protein expression levels of Nrf2, haem oxygenase HO1, Gamma-glutamylcysteine synthetase (γGCS) and Recombinant NADH Dehydrogenase (NQO1) induced by Cd treatment. This confirms that piceatannol has a significant protective effect against Cd-induced testicular dysfunction by regulating the Nrf2-Keap1 signalling pathway25. In this study, we found that oral administration of MPX effectively upregulates the expression of Keap1, Nrf2, and GLCM in the testes; downregulates the expression of GCLC and iNOS; increases the levels of GSH, T-SOD, and CAT in the testes; and reduces the levels of nitric oxide (NO) and MDA, thereby exerting a regulatory effect on oxidative stress.
Enhancing blood‒testis barrier (BTB) integrity effectively promotes testicular injury repair. Exposure to Manganese ion (Mn2+) can cause testicular damage, resulting in male germ cell disorders. Nine-spice beetle extract (Coridius chinensis, CcE), a traditional Chinese medicine, has been found by Cen et al. to significantly inhibit the Mn2+-induced downregulation of blood‒testis barrier biomarkers, including occludin, claudin-1, ZO-1, and junctional adhesion molecule 1, in SD rats after oral administration, and these effects are closely associated with downregulation of adhesion kinase (FAK) and c-Src expression26. Lycium barbarum polysaccharide (LBP), a major bioactive component of goji berries, has a protective effect on the male reproductive system. Hu et al. found that in vitro treatment of testicular support cells with LBP effectively alleviates the decrease in proliferation activity and depolarization of SCs under heat stress. It increases the expression of occludin and ZO-1, promotes the expression of androgen receptor (AR), and enhances the phosphorylation of Akt27. Schisandra chinensis Baillon (SC), known for its antioxidant and anti-inflammatory activities, is widely used in various medical treatments. Karna et al. found that in the treatment of varicocele, SC significantly increases testicular weight; sperm count; sperm vitality; serum testosterone levels; the Johnsen score; germ cell density; and SOD, glutathione peroxidase (GPX), catalase, and steroidogenic acute regulatory protein (StAR) levels in rats. It also reduces testicular dysfunction. SC can also increase sperm vitality in humans, establishing the therapeutic potential of SC in the treatment of varicocele and prevention of infertility28.
In recent years, increasing evidence from scholars has shown that bioactive peptides can effectively regulate the permeability of the blood‒testis barrier (BTB). The bioactive laminin globular domain peptide 3/4/5 (LG3/4/5), derived from the C-terminus of the laminin α2 chain, is a major component of the basement membrane protein29. The LG3/4/5 peptide shares similarity with the NC1 peptide and acts through the mTORC1/p-rpS6/p-Akt1/2 signalling pathway. Unlike the F5 and NC1 peptides, the LG3/4/5 peptide has been shown in in vitro RNAi studies to promote BTB integrity, which is disrupted in Sertoli cell-specific laminin α2 chain knockout30. Overexpression of p-FAK-Y397F (a constitutively inactive form of the p-FAK-Tyr397Phe mutant) or p-FAK-Y407E (a constitutively active form of the p-FAK-Tyr407Glu mutant) on the Sertoli cell epithelium promotes the integrity of the Sertoli cell tight junction (TJ) barrier, making it tighter, suggesting that the permeability of the blood‒testis barrier can be altered by modulating the expression of peptides31. Conversely, some bioactive peptides can increase the permeability of the blood‒testis barrier, leading to sperm detachment. For example, the synergistic interaction of neuronal Wiskott-Aldrich syndrome protein (N-WASP) and actin-related protein 3 (Arp3) with the epidermal growth factor receptor pathway substrate 8 (Eps8) protein provides the plasticity required for F-actin remodelling in testicular Sertoli cells32. This change in the filaments causes them to be no longer densely confined to the basal ES/BTB, resulting in increased BTB permeability and sperm detachment33,34. Mao et al. found that compared to a control group of mice injected with empty pCI-neo, an experimental group of mice injected with p-rpS6-MT and F5 peptides showed significantly enhanced BTB permeability. Moreover, overexpression of p-rpS6 or the F5 peptide disrupted the cytoskeleton and effectively caused BTB leakage35. In this study, we found that oral administration of MPX effectively promotes high expression of Claudin-1, Occludin, ZO-1, N-cadherin, and E-cadherin in the testes; protects against LPS-induced BTB damage; and promotes testicular injury repair.
Conclusions
In this study, it was found that MPX could reducing the levels of NO and MDA, and thus exerted a regulatory effect on oxidative stress. In addition, MPX had a protective effect on LPS-induced BTB injury by promoting the high expression of Claudin-1, Occludin, ZO-1, N-cadherin, E-cadherin in testicular tissue. Interestingly, MPX was testicle-targeting and can be effectively recruited into the testicles in vivo to perform its role. In general, MPX had significant advantages in the prevention and treatment of orchitis, repair of BTB, regulation of testicular oxidative stress. MPX is an effective strategy for male testicular damage, and provides a useful reference for the clinical application of antimicrobial peptides.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
References
Huang, C. et al. Methane Ameliorates Lipopolysaccharide-Induced Acute Orchitis by Anti-Inflammatory, Antioxidative, and Antiapoptotic Effects Via Regulation of the Pk2/Pkr1 Pathway. Oxid. Med. Cell Longev. 7075836 (2020).
Guo, Q. et al. The gut microbiota contributes to the development of Lps-Induced orchitis by disrupting the Blood-Testosterone barrier in mice. Reprod. Sci. 31, 3379–3390 (2024).
Winnall, W. R., Muir, J. A. & Hedger, M. P. Differential responses of epithelial Sertoli cells of the rat testis to Toll-Like receptor 2 and 4 ligands: implications for studies of testicular inflammation using bacterial lipopolysaccharides. Innate Immun. 17, 123–136 (2011).
Jalilvand, N. et al. The Effects of Olibanum On Male Reproductive System Damage in a Lipopolysaccharide Induced Systemic Inflammation Model in Rat. Heliyon. 10, e36033 (2024).
Shen, P. et al. Lps-Induced systemic inflammation caused Mpoa-Fsh/Lh disturbance and impaired testicular function. Front. Endocrinol. (Lausanne). 13, 886085 (2022).
Shimizu, T. et al. Involvement of lipopolysaccharide in ovarian cystic follicles in dairy cow: expressions of Lps receptors and Steroidogenesis-Related genes in follicular cells of cystic follicles. Anim. Reprod. Sci. 195, 89–95 (2018).
Justin, M. J. & Jain, S. K. The protective role of L-Cysteine in the regulation of Blood-Testis barrier Functions-a brief review. Genes (Basel) 15. (2024).
Engelberg, Y. & Landau, M. The human Ll-37(17–29) antimicrobial peptide reveals a functional supramolecular structure. Nat. Commun. 11, 3894 (2020).
Hou, D. et al. Cationic antimicrobial peptide Nrc-03 induces oral squamous cell carcinoma cell apoptosis via Cypd-Mptp Axis-Mediated mitochondrial oxidative stress. Redox Biol. 54, 102355 (2022).
Liu, N., Ma, X. & Jiang, X. Effects of immobilized antimicrobial peptides on growth performance, serum biochemical index, inflammatory factors, intestinal morphology, and microbial community in weaning pigs. Front. Immunol. 13, 872990 (2022).
Wang, L. et al. The antimicrobial peptide Mpx kills Actinobacillus pleuropneumoniae and reduces its pathogenicity in mice. Vet. Microbiol. 243, 108634 (2020).
Henriksen, J. R., Etzerodt, T., Gjetting, T. & Andresen, T. L. Side chain hydrophobicity modulates therapeutic activity and membrane selectivity of antimicrobial peptide Mastoparan-X. PLoS One. 9, e91007 (2014).
Zhu, C. L. et al. Antimicrobial peptide Mpx attenuates Lps-Induced inflammatory response and Blood-Testis barrier dysfunction in Sertoli cells. Theriogenology 189, 301–312 (2022).
Zhu, C. L. et al. Oral Administration of Antimicrobial Peptide Mpx Ameliorates Orchitis and Inflammatory Damage in Other Organs. Anim. Zoonoses. (2024).
Saito, M. et al. Polymyxin B agonist capture therapy for intrauterine inflammation: Proof-of-Principle in a fetal ovine model. Reprod. Sci. 21, 623–631 (2014).
Guo, Q. et al. The protective role of Phlorizin against Lipopolysaccharide-Induced acute orchitis in mice associated with changes in gut microbiota composition. Front. Vet. Sci. 11, 1340591 (2024).
Cai, Z. et al. Alkbh5 in mouse testicular Sertoli cells regulates Cdh2 Mrna translation to maintain Blood-Testis barrier integrity. Cell. Mol. Biol. Lett. 27, 101 (2022).
Kahrl, A. F. et al. Selection On Sperm Count, but Not On Sperm Morphology Or Velocity, in a Wild Population of Anolis Lizards. Cells-Basel. 10 (2021).
Zhang, Q. et al. Activation of Nrf2/Ho-1 signaling: an important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 34, 43–63 (2021).
Shen, Y. et al. Bibliometric and visual analysis of Blood-Testis barrier research. Front. Pharmacol. 13, 969257 (2022).
Ileriturk, M. et al. Hesperidin has a protective effect on Paclitaxel-Induced testicular toxicity through regulating oxidative stress, apoptosis, inflammation and Endoplasmic reticulum stress. Reprod. Toxicol. 118, 108369 (2023).
Shafiey, S. I., Abo-Saif, A. A., Abo-Youssef, A. M. & Mohamed, W. R. Protective effects of Rivaroxaban against Cisplatin-Induced testicular damage in rats: impact on oxidative stress, coagulation, and P-Nf-Kappab/Vcam-1 signaling. Food Chem. Toxicol. 169, 113419 (2022).
Alafifi, S. A., Wahdan, S. A., Elhemiely, A. A., Elsherbiny, D. A. & Azab, S. S. Modulatory effect of liraglutide on Doxorubicin-Induced testicular toxicity and behavioral abnormalities in rats: role of testicular-Brain axis. Naunyn Schmiedebergs Arch. Pharmacol. 396, 2987–3005 (2023).
Zhang, J. et al. Activation of Mt1/Mt2 to Protect Testes and Leydig Cells Against Cisplatin-Induced Oxidative Stress through the Sirt1/Nrf2 Signaling Pathway. Cells-Basel. 11 (2022).
Shi, X. & Fu, L. Piceatannol inhibits oxidative stress through modification of Nrf2-Signaling pathway in testes and attenuates spermatogenesis and steroidogenesis in rats exposed to cadmium during adulthood. Drug Des. Devel Ther. 13, 2811–2824 (2019).
Cen, C., Wang, F., Xiong, K., Jiang, L. & Hou, X. Protective effects of Coridius chinensis extracts on rat reproductive damage induced by manganese. Andrologia 54, e14326 (2022).
Hu, S., Liu, D., Liu, S., Li, C. & Guo, J. Lycium Barbarum Polysaccharide Ameliorates Heat-Stress-Induced Impairment of Primary Sertoli Cells and the Blood-Testis Barrier in Rat Via Androgen Receptor and Akt Phosphorylation. Evid. Based Complement. Alternat. Med. 5574202. (2021).
Karna, K. K., Choi, B. R., Kim, M. J., Kim, H. K. & Park, J. K. The effect of schisandra chinensis baillon on Cross-Talk between oxidative stress, Endoplasmic reticulum stress, and mitochondrial signaling pathway in testes of Varicocele-Induced Sd rat. Int. J. Mol. Sci. 20, (2019).
Siu, M. K. & Cheng, C. Y. Dynamic Cross-Talk between cells and the extracellular matrix in the testis. Bioessays 26, 978–992 (2004).
Gao, Y. et al. Regulation of the Blood-Testis barrier by a local axis in the testis: role of laminin Alpha2 in the basement membrane. FASEB. J. 31, 584–597 (2017).
Lie, P. P. et al. Focal adhesion Kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on Blood-Testis barrier dynamics in the rat. Proc. Natl. Acad. Sci. U. S. A. 109, 12562–12567 (2012).
Meng, Z., Liu, Y., Zhou, J., Zheng, B. & Lv, J. Drug transport across the Blood-Testis barrier. Am. J. Transl. Res. 14, 6412–6423 (2022).
Su, L., Mruk, D. D., Lie, P. P., Silvestrini, B. & Cheng, C. Y. A peptide derived from Laminin-Gamma3 reversibly impairs spermatogenesis in rats. Nat. Commun. 3, 1185 (2012).
Gao, Y., Mruk, D. D., Lui, W. Y., Lee, W. M. & Cheng, C. Y. F5-Peptide induces aspermatogenesis by disrupting organization of Actin- and Microtubule-Based cytoskeletons in the testis. Oncotarget 7, 64203–64220 (2016).
Mao, B. et al. F5-Peptide and Mtorc1/Rps6 effectively enhance Btb transport function in the Testis-Lesson from the adjudin model. Endocrinology. 160, 1832–1853 (2019).
Funding
This work was supported by Natural Science Foundation of Henan (222300420043), National Natural Science Foundation of China (32172862 and 32172803), the National Key R&D Program of China (2021YFD1301200), Science and Technology Innovative Research Team in Higher Educational Institutions of Henan Province (24IRTSTHN035), the joint fund of science and technology research and development plan in Henan province (225200810044).
Author information
Authors and Affiliations
Contributions
Conceptualization, C.Z. and L.W.; supervision, C.L.;methodology, Y.B. and J.H.; formal analysis, X.X., R.Y. and B.Z.; investigation, W.Z.; resources, H.Z.; data curation, X.Z.,H.S. and W.L.; writing-original draft preparation, Y.W. and K.D.; writing-review and editing, X.Z. and J.H.; visualization, Y.B. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
The study was conducted in strict accordance with the guidelines approved by the Animal Ethics Committee of Henan Institute of Science and Technology and Jilin University.This study was approved by the Ethics Committee of Henan University of Science and Technology (LLSC2022045). Animal experiments are conducted according to ARRIVE (Animals in Research: Reporting in Vivo Experiments) guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, C., Liao, C., Bai, Y. et al. Oral administration of antimicrobial peptide MPX can effectively regulate LPS-mediated testicular oxidative stress and blood‒testis barrier damage. Sci Rep 15, 9422 (2025). https://doi.org/10.1038/s41598-025-93507-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-93507-2