Abstract
Acute thyrotoxic myopathy (ATM) is a rare yet severe complication of thyrotoxicosis that affects the central nervous system and is associated with a high mortality rate if not diagnosed and treated promptly. Currently, the diagnosis of ATM primarily relies on clinical manifestations, and there is a lack of specific serological markers to support the diagnostic process. This study aimed to investigate the differences in serum CD40 levels between patients with acute thyrotoxic myopathy (ATM), those with Graves’ disease, and healthy controls, and to evaluate its potential as a diagnostic biomarker for differentiating ATM. Additionally, the study examined the correlation between serum CD40 levels and various parameters, including the ATM symptom score (ATMSS), FT3, FT4, TSH, TGAb, TRAb, and TPOAb. This retrospective cross-sectional study included 17 patients with ATM, 17 patients with Graves’ disease, and 17 healthy controls, all recruited from the First Affiliated Hospital of Guangxi Medical University between January 2022 and August 2024. Clinical evaluations, serum thyroid hormone and related antibody testing, and CD40 level measurements were conducted. The predictive value of CD40, FT3, FT4, TSH, TGAb, TRAb, and ATMSS for ATM was assessed using receiver operating characteristic (ROC) curve analysis. Spearman correlation analysis was performed to explore the relationship between CD40 levels and ATMSS, FT3, FT4, TSH, TGAb, TRAb, and TPOAb. Serum levels of CD40[259.17(227.50,378.03)vs.190.71(174.08,198.96)vs. 166.98(142.94,175.90)], FT3[28.34(17.13,37.65) vs. 8.82(6.36,21.00) vs. 5.02(3.93,5.45)], and FT4 [67.58(37.53,77.23)vs.27.03(15.96,47.05)vs.16.82(13.59,17.90)]in ATM patients were significantly higher than those in Graves’ disease patients and the control group, with statistically significant differences (P < 0.01). ROC analysis demonstrated that CD40 has high predictive value for distinguishing between GD and ATM, with an area under the curve (AUC) of 0.99, and both sensitivity and specificity of 94.1%. Correlation analysis revealed a positive correlation between CD40 and the ATM symptom score (ATMSS) (r = 0.72, P < 0.01). Positive correlations were also observed between CD40 and FT3 (r = 0.53, P < 0.01) and FT4 (r = 0.56, P < 0.01). TSH showed a negative correlation trend with CD40 (r = -0.21, P = 0.23), but this difference was not statistically significant. No significant correlations were found between CD40 and TGAb (r = 0.10, P = 0.59), TRAb (r = 0.26, P = 0.13), or TPOAb (r = 0.06, P = 0.72). Elevated serum CD40 levels are associated with the severity of ATM symptoms and may serve as a potential biomarker for ATM. The role of CD40 as an adjunct in the early diagnosis of ATM holds clinical significance, offering new insights into the differential diagnosis and treatment of ATM.
Similar content being viewed by others
Introduction
Acute Thyrotoxic Myopathy (ATM), also known as Acute Thyrotoxic Bulbar Palsy, is a rare and severe manifestation of thyrotoxicosis involving the central nervous system. If not promptly recognized and treated, the mortality rate associated with this condition is extremely high, highlighting the urgent need for improved diagnosis and management. Currently, the medical community faces a significant challenge due to the lack of standardized guidelines and consensus for the diagnosis and treatment of ATM worldwide. The diagnosis of ATM primarily relies on the patient’s clinical manifestations and the exclusion of other diseases that may cause bulbar palsy symptoms, after which a diagnosis of ATM can be confirmed1. ATM may occur concurrently with thyroid storm, with clinical features including dysphagia, dysphonia, hoarseness, aspiration during drinking, respiratory distress, and potentially fatal respiratory muscle paralysis leading to aspiration pneumonia2.
The insidious onset, rapid progression, and high mortality rate of ATM pose a serious threat to patients’ lives and health. Currently, the early differentiation and treatment of ATM face significant challenges due to lack of reliable serological markers to aid in diagnosis. Therefore, the exploration of serological markers for ATM is crucial for improving diagnostic accuracy and providing important clinical evidence for the formulation of effective treatment strategies. The international demand for guidelines and consensus on diagnosis and treatment of ATM highlights the urgent need for global management strategies for this rare disease.
The diagnosis of ATM primarily relies on clinical presentation and thyroid function assays. With advancements in immunological research, the diagnostic potential of immune-related biomarkers is increasingly recognized. CD40, a type I transmembrane glycoprotein expressed on B cells, plays a pivotal role in B cell biology and T cell activation. It is a key mediator in immune responses and is implicated in the pathogenesis of autoimmune disease. Previous studies have highlighted the critical role of CD40 in the pathogenesis, progression, and treatment of thyrotoxicosis3. CD40 is recognized as a susceptibility gene for autoimmune thyroid diseases (AITD), with its genetic polymorphisms linked to various subtypes of Graves’ disease4. In the management of ATM, treatment protocols similar to those for thyroid storm are employed. Current research suggests that the administration of glucocorticoids, intra-thyroidal steroid injections, and beta-blockers have all demonstrated efficacy5,6.Clinical evidence suggests that anti-CD40 therapy can normalize thyroid function, regardless of antithyroid drug (ATD) treatment, highlighting its role in the pathophysiology of hyperthyroidism7. Despite this, the relationship between serum CD40 levels and the progression of ATM remains poorly characterized. This study aims to elucidate serum CD40 expression in ATM and evaluate its potential in distinguishing ATM from Graves’ disease, with the goal of providing novel diagnostic insights for ATM.
Results
Clinical data comparison
The comparative analysis of clinical data among the three groups is shown in Table 1; Fig. 1. There were no statistically significant differences in age and BMI among the ATM, GD, and control groups (P > 0.05). The number of females did not differ significantly between the ATM group (14 cases), GD group (13 cases), and control group (13 cases). Serum levels of FT3, FT4, and CD40 were significantly higher in the ATM group compared to the GD and control groups, with statistically significant differences (P < 0.05). No significant differences were observed in TSH, TRAb, and TGAb levels between the ATM and GD groups (P > 0.05). The ATM symptom score (ATMSS) was significantly higher in the ATM group compared to the GD group (P < 0.05), with an average of 8.76 ± 5.11 in the ATM group and 1 ± 1.12 in the GD group.
Receiver operating characteristic curve analysis
In this study, we utilized Receiver Operating Characteristic (ROC) curve analysis to assess the predictive efficacy of various markers for ATM. The pertinent findings are presented in Table 2, and Fig. 2. The ROC analysis was conducted with the objective of accurately distinguishing ATM patients from those with Graves’ disease and from the Control group. Our results indicated that CD40, FT3, and FT4 all exhibited significant predictive value for ATM. Specifically, in the ROC curve analysis comparing the GD group with the ATM group, CD40 demonstrated an Area Under the Curve (AUC) of 0.99, with a sensitivity and specificity both at 94%; FT3 had an AUC of 0.92, with sensitivity and specificity of 94% and 82%, respectively; FT4 showed an AUC of 0.89, with both sensitivity and specificity at 88%. In contrast, TSH had an AUC of 0.45 with sensitivity and specificity of 18% and 77%, respectively; TGAb presented an AUC of 0.56, with sensitivity and specificity of 19% and 65%, respectively; TRAb had an AUC of 0.65, with sensitivity and specificity of 20% and 47%, respectively. In the ROC analysis comparing the Control group with the ATM group, CD40, FT3, FT4, and TRAb also demonstrated substantial diagnostic efficacy.
Spearman correlation analysis
The results of the correlation analysis are detailed in Table 3, and are visually presented in Figs. 3 and 4. ATMSS were assigned to both ATM and Graves’ disease group based on their clinical presentations. Positive correlations were observed between ATMSS scores and serum levels of FT3 (r = 0.62, P < 0.01), FT4 (r = 0.56, P < 0.01), TRAb (r = 0.30, P = 0.08), and CD40 (r = 0.72, P < 0.01), with statistically significant differences (P < 0.01). A significant inverse correlation was noted between CD40 and TSH (r=-0.21, P = 0.23). CD40 also correlated positively with FT3 (r = 0.53, P < 0.01), FT4 (r = 0.56, P <0.01), TRAb (r = 0.26, P = 0.13), and TGAb (r = 0.10, P = 0.59), although these associations did not reach statistical significance (P > 0.05).
CD40 correlation analysis graph. (a) Correlation analysis between FT3 and CD40; (b) Correlation analysis between FT4 and CD40; (c) Correlation analysis between TSH and CD40; (d) Correlation analysis between TGAb and CD40; (e) Correlation analysis between TRAb and CD40; (f) Correlation analysis between TPOAb and CD40.
ATMSS correlation analysis graph. (a) Correlation analysis between CD40 and ATMSS; (b) Correlation analysis between FT3 and ATMSS; (c) Correlation analysis between FT4 and ATMSS; (d) Correlation analysis between TSH and ATMSS; (e) Correlation analysis between TGAb and ATMSS; (f) Correlation analysis between TRAb and ATMSS; (g) Correlation analysis between TPOAb and ATMSS.
Discussion
The results of this study clearly show that compared with the GD and Control groups, serum levels of CD40, FT3, FT4, and TRAb were significantly elevated in the ATM group. These findings are consistent with previous literature, which reports that ATM is more common in patients with uncontrolled or poorly controlled hyperthyroidism, further validating the results of our study. Existing literature has reported a positive correlation between FT3 and FT4 levels and thyroid skeletal muscle function8, while excessive thyroid hormones in the serum of ATM patients are considered one of the key contributing factors to the development of the disease. Notably, this study is the first to conduct an in-depth analysis of the differences in serum CD40 levels between ATM patients and Graves’ disease patients, addressing a gap in the research in this field. ATM has an insidious onset, a critical course, and a high mortality rate, severely affecting the life and health of patients. Currently, ATM lacks specific serum markers for auxiliary diagnosis. Therefore, studying the serum markers of ATM has significant clinical value for its differential diagnosis and treatment. This not only provides a strong basis for the differential diagnosis of ATM but also has the potential to open new avenues for optimizing treatment plans and improving patient outcomes. ATM must be distinguished from myasthenia gravis, hypokalemic periodic paralysis, and other neuromuscular disorders due to its potential for misdiagnosis. Often, ATM’s bulbar palsy symptoms are overshadowed by other manifestations. A case report described a 38-year-old man admitted to the ICU with a hyperthyroid crisis complicated by cardiopulmonary failure and coagulopathy. Upon recovery, he was transferred to the neurology department for suspected neuromuscular disease, presenting with proximal muscle weakness and bulbar symptoms, including dysphagia. Electromyography confirmed the diagnosis of thyrotoxic myopathy9. Muscle weakness is a common manifestation of hyperthyroidism; however, when ATM presents solely with myopathic symptoms, it is often overlooked, leading to misdiagnosis. Some patients initially exhibit dysphagia, which may progress to limb weakness and aspiration pneumonia10,11,12. In addition, ATM is not only seen in adults, but there are also reports in the literature that it can occur in children13. Early identification of patients at risk for ATM is essential for timely intervention, which significantly improve prognosis and quality of life, leading to better treatment outcomes and a more favorable life experience for affected individuals. Resting-state functional magnetic resonance imaging (rs-fMRI) studies have found that there are abnormalities in brain regions of patients with ATM, and changes in functional connectivity14,15.When ATM patients develop bulbar palsy, the permeability of the blood-brain barrier is compromised, and the central nervous system disorders often originate from outside the brain16. Cytokines from peripheral blood penetrate the blood-brain barrier, entering the central nervous system and further activating T and B lymphocytes, which increases the occurrence and progression of neuroinflammation. CD40, a key factor in this pathophysiological process, plays a crucial role in the co-stimulation and activation of T and B lymphocytes17. Receiver Operating Characteristic (ROC) curve analysis demonstrated that CD40 exhibited superior discriminative power in differentiating the ATM group from the GD group, with both sensitivity and specificity reaching 94.1%. Additionally, CD40 demonstrated high diagnostic efficacy in distinguishing the ATM group from the Control group. The detection of serum CD40 expression is of great significance for the diagnosis and treatment of ATM, and CD40 may emerge as a potential biomarker for ATM. However, our study also has limitations. The high AUC value may indeed suggest that the model is overly idealized or at risk of overfitting. This study provides preliminary data support for future larger-scale research, and future studies can further validate the practical utility of CD40 in differentiating ATM from Graves’ disease by increasing the sample size.
CD40, a type I transmembrane glycoprotein belonging to the TNF receptor superfamily, triggers multiple downstream pathways upon ligand binding, eliciting pathological changes. The expression of CD40 lacks high specificity, as it may change in various immune responses and disease states. Studies have shown that CD40 expression is elevated in autoimmune diseases such as Graves’ disease, Sjögren’s syndrome, and systemic lupus erythematosus18,19. CD40 also plays a role in the pathophysiology of thyroid-associated ophthalmopathy (TAO), where TRAb stimulates CD40 overexpression in orbital fibroblasts, thereby exacerbating the condition20. Anti-CD40 monoclonal antibodies, used in hyperthyroidism treatment, disrupt CD40-ligand co-stimulation, thereby mitigating harmful reactions. Furthermore, CD40 SNPs correlate with serum TRAb levels4. Our study reveals significantly upregulated serum CD40 levels in ATM patients, suggesting its involvement in disease progression. This reflects immune-metabolic disturbances, enhanced T and B cell costimulatory responses, increased pathogenic autoantibody release by plasma cells, and disruption of thyroid metabolism. Additionally, serum CD40 levels positively correlate with ATMSS, underscoring its pivotal role in disease development. The clinical symptoms of ATM are evident, but due to its insidious onset and susceptibility to misdiagnosis, we believe that the adjunctive role of CD40 in the early diagnosis of ATM still holds significant clinical value. Clinically, ATM is considered a complication of hyperthyroidism, and we observed that serum CD40 expression in ATM patients was significantly higher than in Graves’ disease patients. This difference provides a potential reference for further clinical diagnosis. Though no literature directly links CD40 to ATM pathophysiology, it is established that AITD, including Graves’ disease, arises from immune dysregulation that leads to organ-specific T-cell autoimmunity and thyroid autoantibody production. Graves’ disease exemplifies the most prevalent AITD, with T-cell activation governed by CD40, a co-stimulatory molecule expressed on T cells and requiring CD40L binding for full activation of B cells. ATM, as a complication of Graves’ disease, likely shares similar immunopathogenic mechanisms. Our findings reveal elevated serum CD40 levels in ATM patients compared to those with Graves’ disease and healthy controls, suggesting a potential role for CD40 in ATM pathogenesis. Overexpression of CD40 may drive excessive T-cell activation, as reflected by lymphocytic infiltration in the thyroid gland. CD40, a pivotal molecule in adaptive immunity, is implicated in various inflammatory responses and is associated with diseases such as atherosclerosis, epilepsy, and Alzheimer’s21,22. Both in vitro and in vivo studies have demonstrated CD40’s role in upregulating inflammatory cytokines23. Elevated serum CD40 in ATM patients may thus indicate a systemic pro-inflammatory state.
TRAb are pivotal in the pathogenesis of hyperthyroidism, with patients exhibiting increased serum TRAb levels and reciprocally decreased TSH, alongside elevated FT3 and FT4 levels. Retrospective analyses have linked sustained TSH suppression and elevated FT4 with increased all-cause mortality in Graves’ disease24. While CD40 is not directly implicated in thyroid hormone synthesis, it modulates hormone levels through its influence on immune-mediated thyroidal damage. Heightened CD40-driven autoimmunity stimulates plasma cells to secrete increased TRAb, which, by mimicking TSH activity, activate the TSHR and precipitate thyroid hyperplasia and hyperthyroidism. ATM predominantly affects individuals with uncontrolled or inadequately managed hyperthyroidism, presenting with significantly higher serum FT3 and FT4 levels than those with Graves’ disease. This phenomenon may suggest a more intense immune response and abnormal regulation of thyroid hormone synthesis and secretion in ATM. ATM may involve more active autoimmune attacks, leading to excessive secretion and release of thyroid hormones, thereby causing more severe symptoms of hyperthyroidism. Furthermore, this also indicates that clinical treatment and management of ATM patients require closer attention and more aggressive interventions to effectively control thyroid hormone levels, alleviate symptoms, and prevent the occurrence of complications. In this study, we observed that both patients with ATM and Graves’ disease exhibited reduced serum levels of TSH; however, this difference was not statistically significant. Similarly, the levels of TRAb between the two groups did not show a statistically significant difference. The small sample size may be one of the reasons for the lack of statistical significance in these differences. Previous research has indicated that there is a positive correlation between serum CD40 ligand and serum TRAb levels in children, but no correlation with serum thyroglobulin levels25. Studies have also indicated a positive correlation between FT3:FT4 ratios, TRAb, and thyroid volume26, and a similar trend between CD40 and thyroid hormone levels, though this was not statistically significant in our study. Our study revealed a positive correlation between CD40 levels and ATM symptomatology, along with FT3, FT4, and TRAb levels, suggesting that worsening bulbar palsy symptoms in ATM patients may be accompanied by notable fluctuations in thyroid hormone-related indices.
Thyroglobulin (TG) is a thyroid-specific glycoprotein essential for thyroid hormone synthesis. TGAb targets TG and is commonly detected in autoimmune thyroid diseases, particularly in Hashimoto’s thyroiditis and Graves’ disease. Clinical research on TGAb primarily focuses on its predictive value for central lymph node metastasis in thyroid cancer patients27,28,29. Additionally, TGAb has been implicated as a potential protective factor in metabolic disorder-related fatty liver30. Currently, there are no reports on TGAb’s role in predicting ATM. Notably, our study observed higher serum TGAb levels in ATM patients (31.20%) compared to Graves’ disease patients (17.67%), although this difference was not statistically significant. No correlation was identified between serum CD40 and serum TGAb levels.
This study acknowledges certain limitations. We acknowledge that ATM is a rare complication of hyperthyroidism, and as such, the collection of a sufficient sample size presents significant challenges. Due to the low incidence of ATM, the sample size in our study was relatively small, and further validation with a larger cohort of patients is needed. This may also explain why the serum TRAb levels in ATM patients were higher than those in the Graves’ disease group, yet the difference was not statistically significant. The body mass index, age, and gender distribution among the three groups showed no significant differences, which helped minimize potential confounding factors. Additionally, there were no differences in serum potassium levels between the groups, and neither the GD nor ATM patients exhibited symptoms of hypokalemic periodic paralysis. Although the current sample size is limited, we hope this study can offer new diagnostic perspectives for the early diagnosis of ATM. The etiology of ATM remains elusive, and the multifaceted pathophysiology of the condition suggests that the predictive accuracy of individual biomarkers, including CD40, is suboptimal and requires refinement. Future research should explore the combinatorial use of CD40 with other biomarkers to enhance the predictive and diagnostic accuracy of ATM, thereby increasing its clinical utility. Additionally, as CD40 is a cytokine indicative of autoimmune disease activity, its serum levels are influenced by diverse inflammatory mediators. Elevated serum CD40 levels in ATM patients may indirectly indicate a state of systemic inflammation, highlighting the complex interplay between inflammation and autoimmune thyroid disorders. We plan to expand the sample size in future studies and use additional clinical data to validate our preliminary findings.
In conclusion, this investigation marks the first application of serum CD40 levels for the differential diagnosis and prognostic stratification of ATM. Our findings indicate that CD40 expression is upregulated in ATM patients and positively correlates with ATMSS. This correlation suggests that increased CD40 levels may reflect greater symptom severity in ATM, implying that CD40 inhibition could emerge as a novel therapeutic strategy. Collectively, our results provide a new perspective for the diagnostic and therapeutic approaches to ATM.
Materials and methods
General information
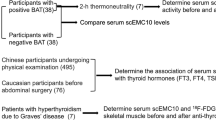
This retrospective study included 17 patients with ATM(ATM group), 17 patients with Graves’ disease(GD group), and 17 healthy individuals(Control group) undergoing routine physical exams at the First Affiliated Hospital of Guangxi Medical University from January 2022 to August 2024. The diagnostic criteria for hyperthyroidism followed the “Diagnosis and Management Guidelines for Hyperthyroidism and Other Causes of Thyrotoxicosis” published by the American Thyroid Association (ATA) in 201631.The main diagnostic criteria for hyperthyroidism include: (1) Clinical symptoms such as weight loss, palpitations, fatigue, and increased appetite; (2) Laboratory findings, including elevated serum FT3 and FT4 levels and suppressed TSH levels; (3) Radiological findings, such as increased thyroid ultrasound or radioactive iodine uptake; (4) Thyroid antibody testing, with positive TRAb (thyroid-stimulating hormone receptor antibody) results. All ATM and Graves’ disease patients included in this study strictly met the diagnostic criteria for hyperthyroidism. Inclusion criteria: (1) The ATM group met the diagnostic criteria for hyperthyroidism and exhibited at least one medullary paralysis symptom, including dysphagia, hoarseness, dysarthria, and respiratory muscle paralysis; (2) Graves’ disease patients presented with thyroid dysfunction without accompanying thyroid-related myopathy; (3) The control group had normal thyroid function and no other underlying diseases. Exclusion criteria: (1) Secondary hyperthyroidism; (2) Coexisting autoimmune diseases other than hyperthyroidism, diabetes, hematologic disorders, or acute infectious diseases; (3) Patients with myasthenia gravis or chronic thyrotoxic myopathy; (4) Patients with liver or kidney dysfunction; (5) Those with a history of surgery or trauma in the past three months; (6) Pregnancy. This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Guangxi Medical University, with the ethical approval number 2024-E779-01. This study was conducted in accordance with the “Guidelines for Ethical Review of Clinical Research Involving Humans (2020 Edition)” issued by the National Health Commission of China. The data used in the study underwent de-identification to ensure that no personal identifiable information was included. Data processing and usage strictly adhered to relevant confidentiality and privacy protection regulations to ensure the effective protection of participants’ privacy rights. As this study is a retrospective analysis, the serum samples collected were from routine clinical samples obtained from healthy individuals undergoing physical exams and patients during their regular treatment, and therefore no additional sampling or direct intervention was involved. In accordance with the ethical standards set forth in the Declaration of Helsinki and its subsequent revisions, the Medical Ethics Committee of the First Affiliated Hospital of Guangxi Medical University approved and waived the requirement for patient informed consent for this study.
Clinical assessment
A comprehensive evaluation of bulbar paralysis symptoms was conducted in patients diagnosed with ATM. Leveraging extensive clinical experience in ATM diagnosis and management, our team devised a systematic scoring method for bulbar paralysis in ATM, designated as the ATM Symptom Score (ATMSS), in 201232.An ATMSS threshold greater than 3 indicates the presence of bulbar paralysis symptoms. We collected demographic data and laboratory findings from these patients.
Serum marker detection
In this study, blood samples were collected from fasting patients using 3 mL coagulation activator tubes. Subsequently, the samples were centrifuged at 3000 revolutions per minute (rpm) for 5 min in a refrigerated centrifuge to separate the serum. The nursing staff responsible for blood collection were all employed nurses at our hospital, who had undergone rigorous professional training in specimen collection and were proficient in standardized phlebotomy techniques and procedural protocols. The levels of free triiodothyronine (FT3), free thyroxine (FT4), thyroid-stimulating hormone (TSH), thyroglobulin antibody (TGAb), and thyroid receptor antibody (TRAb) in serum, as well as anti-thyroid peroxidase antibody (TPOAb), were measured using the chemiluminescent immunoassay method (CLIA) from Roche Diagnostics, Germany. Serum potassium (K) was measured using the ion-selective electrode (ISE) method, also provided by Roche Diagnostics, Germany.The normal reference ranges for these indicators were as follows: FT3, 3.08–6.50 pmol/L; FT4, 9.65–21.88 pmol/L; TSH, 0.4–5.86 mIU/L; TGAb, < 30%; TRAb, 0-1.5 IU/L, TPOAb,0-10IU/ml; K,3.5-5.3mmol/L. Additionally, the expression levels of CD40 were quantified using an enzyme-linked immunosorbent assay (ELISA) kit from SAB Company.
Statistical method
Statistical analyses were conducted using SPSS version 26. Quantitative data conforming to a normal distribution are expressed as the mean ± standard deviation, with intergroup comparisons performed using independent samples T-tests. Gender comparison was performed using the chi-square test. For quantitative data that did not follow a normal distribution, the median (M) with interquartile range (P25, P75) was used. Group comparisons were performed using the Mann-Whitney U test. Correlation analysis for non-normally distributed data was conducted using Spearman’s correlation. A p-value of < 0.05 was considered statistically significant.
Data availability
The datasets used and/or analysed during the current study availble from the corresponding author on reasonable request.
References
Boddu, N. J., Badireddi, S., Straub, K. D., Schwankhaus, J. & Jagana, R. Acute thyrotoxic bulbar myopathy with encephalopathic behaviour: An uncommon complication of hyperthyroidism. Case Rep. Endocrinol. 2013, 369807. https://doi.org/10.1155/2013/369807 (2013).
Zhou, H. et al. Clinicial analysis of 69 patients with acute hyperthyroid myopathy and its treatment. Chin. J. Endocr. Metab. 28, 896 (2012).
Wagner, A. H., Klersy, A., Sultan, C. S. & Hecker, M. Potential role of soluble CD40 receptor in chronic inflammatory diseases. Biochem. Pharmacol. 217. https://doi.org/10.1016/j.bcp.2023.115858 (2023).
Jiang, H. et al. Compelling evidence linking CD40 gene with graves’ disease in the Chinese Han population. Front. Endocrinol. 12, 759597. https://doi.org/10.3389/fendo.2021.759597 (2021).
Fu, S. E. et al. Acute thyrotoxic myopathy combined with neck pain: A case report. Neuroendocrinol. Lett. 44, 427–431 (2023).
Mathew, B., Devasia, A. J., Ayyar, V., Thyagaraj, V. & Francis, G. A. Thyrotoxicosis presenting as acute bulbar palsy. J. Assoc. Phys. India 59, 386–387 (2011).
Kahaly, G. J. et al. A novel Anti-CD40 monoclonal antibody, Iscalimab, for control of graves Hyperthyroidism-A Proof-of-Concept trial. J. Clin. Endocrinol. Metab. 105. https://doi.org/10.1210/clinem/dgz013 (2020).
Fu, S. E. et al. Chronic thyrotoxic myopathy development is associated with thyroid hormone sensitivity index, predicted by lower-limb fatigue and the squat-up test. Sci. Rep. 14, 24364. https://doi.org/10.1038/s41598-024-76273-5 (2024).
Ali, A., Mostafa, W., Fernandez, C., Ahmad, H. & Htwe, N. Apathetic thyroid storm with cardiorespiratory failure, pulmonary embolism, and coagulopathy in a young male with graves’ disease and myopathy. Case Rep. Endocrinol. 8896777. https://doi.org/10.1155/2020/8896777 (2020).
Noto, H., Mitsuhashi, T., Ishibashi, S. & Kimura, S. Hyperthyroidism presenting as dysphagia. Int. Med. 39, 472–473. https://doi.org/10.2169/internalmedicine.39.472 (2000).
Okada, H. & Yoshioka, K. Thyrotoxicosis complicated with dysphagia. Int. Med. 48, 1243–1245. https://doi.org/10.2169/internalmedicine.48.2202 (2009).
Alwithenani, R., Andrade, D. M., Zhang, L. & Gomez-Hernandez, K. E. Myopathic dysphagia caused by thyrotoxicosis: A case report and review of the literature. Endocrinol. Diabetes Metab. Case Rep. https://doi.org/10.1530/edm-21-0175 (2022).
Rajendiran, R. et al. Thyrotoxic dysphagia in a child. Indian J. Pediatr. 88, 1046. https://doi.org/10.1007/s12098-021-03870-x (2021).
Li, Y. et al. The Resting-State brain network functional connectivity changes in patients with acute thyrotoxic myopathy based on independent component analysis. Front. Endocrinol. 13, 829411. https://doi.org/10.3389/fendo.2022.829411 (2022).
Kuang, Y. et al. Abnormal brain regional activity in acute thyrotoxic myopathy assessed by resting-state functional MRI. Acta Radiol. 65, 1347–1358. https://doi.org/10.1177/02841851241280115 (2024).
Gilhus, N. E. & Deuschl, G. Neuroinflammation—a common thread in neurological disorders. Nat. Rev. Neurol. 15, 429–430. https://doi.org/10.1038/s41582-019-0227-8 (2019).
Zhou, Y., Richmond, A. & Yan, C. Harnessing the potential of CD40 agonism in cancer therapy. Cytokine Growth Factor Rev. 75, 40–56. https://doi.org/10.1016/j.cytogfr.2023.11.002 (2024).
Jobling, K. & Ng, W. F. CD40 as a therapeutic target in Sjögren’s syndrome. Expert Rev. Clin. Immunol. 14, 535–537. https://doi.org/10.1080/1744666x.2018.1485492 (2018).
Wangriatisak, K. et al. CD4(+) T-cell Cooperation promoted pathogenic function of activated Naïve B cells of patients with SLE. Lupus Sci. Med. 9. https://doi.org/10.1136/lupus-2022-000739 (2022).
Görtz, G. E. et al. Pathogenic phenotype of adipogenesis and hyaluronan in orbital fibroblasts from female graves’ orbitopathy mouse model. Endocrinology 157, 3771–3778. https://doi.org/10.1210/en.2016-1304 (2016).
Daub, S., Lutgens, E., Münzel, T. & Daiber, A. CD40/CD40L and related signaling pathways in cardiovascular health and Disease-The pros and cons for cardioprotection. Int. J. Mol. Sci. 21. https://doi.org/10.3390/ijms21228533 (2020).
Ots, H. D., Tracz, J. A., Vinokuroff, K. E. & Musto, A. E. CD40-CD40L in neurological disease. Int. J. Mol. Sci. 23. https://doi.org/10.3390/ijms23084115 (2022).
Reiche, M. E. et al. Adipocytes control hematopoiesis and inflammation through CD40 signaling. Haematologica 108, 1873–1885. https://doi.org/10.3324/haematol.2022.281482 (2023).
Okosieme, O. E. et al. Primary therapy of graves’ disease and cardiovascular morbidity and mortality: A linked-record cohort study. Lancet Diabets Endocrionol. 7, 278–287. https://doi.org/10.1016/s2213-8587(19)30059-2 (2019).
Metwalley, K. A., Farghaly, H. S., Raafat, D. M., Ismail, A. M. & Saied, G. M. Soluble CD40 ligand levels in children with newly diagnosed graves’ disease. J. Clin. Res. Pediatr. Endocrinol. 12, 197–201. https://doi.org/10.4274/jcrpe.galenos.2019.2019.0108 (2020).
Minasyan, M. et al. fT3:fT4 ratio in graves’ disease—correlation with trab level, goiter size and age of onset. Folia Med. Cracov. 60, 15–27. https://doi.org/10.24425/fmc.2020.135010 (2020).
Min, Y. et al. Preoperatively predicting the central lymph node metastasis for papillary thyroid Cancer patients with Hashimoto’s thyroiditis. Front. Endocrinol. 12, 713475. https://doi.org/10.3389/fendo.2021.713475 (2021).
Wu, X. et al. Predictive value of FNA-Tg and TgAb in cervical lymph node metastasis of papillary thyroid carcinoma. Technol. Cancer Res. Treat. 21, 15330338221127605. https://doi.org/10.1177/15330338221127605 (2022).
Zhao, Y. et al. Prognostic value of postoperative anti-thyroglobulin antibody in patients with differentiated thyroid cancer. Front. Endocrinol. 15, 1354426. https://doi.org/10.3389/fendo.2024.1354426 (2024).
Zhang, X. et al. The role of thyroid hormones and autoantibodies in metabolic dysfunction associated fatty liver disease: TgAb May be a potential protective factor. Front. Endocrinol. 11, 598836. https://doi.org/10.3389/fendo.2020.598836 (2020).
Ross, D. S. et al. American Thyroid Association Guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 26, 1343–1421. https://doi.org/10.1089/thy.2016.0229 (2016).
Zhou, H. Y., Liang, X. H., Qin, S. Z., Qin, Y. F., Zhou, J., Zhang J. & Luo, Z. J. In The 11th National Endocrinology Academic Conference of the Chinese Medical Association: Proceedings 109–109 (Chinese Medical Association).
Funding
This work was supported by National Natural Science Foundation of China (82260159;81860146).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design.Date acquisition and analysis were conducted by C.W., Y.Z., and T.W., M.G., J.Q. Manuscript revision was carried out by Z.L. The final manuscript was reviewwd and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
This study is retrospective in nature, with all data anonymized to exclude any personally identifiable information, thereby waiving the requirement for patient informed consent.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki. This study was conducted with the approval of the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University, under the ethical review number 2024-E779-01.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, C., Zhang, Y., Wang, T. et al. Elevated serum CD40 as a potential biomarker for the differential diagnosis of acute thyrotoxic myopathy. Sci Rep 15, 8467 (2025). https://doi.org/10.1038/s41598-025-93522-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-93522-3