Abstract
This study aims to construct a nomogram to predict the prognosis of patients with cervical spondylotic myelopathy (CSM). This study included 358 patients diagnosed with cervical spondylotic myelopathy. We collected serological indicators of patients at admission, including routine blood tests, biochemical tests, liver and kidney function, coagulation function and other laboratory tests. We used t-test, rank-sum test, chi-square test or Fisher test for inter-group comparison. We used univariate and multivariate logistic regression analysis to obtain independent predictors of poor prognosis of patients, and constructed the independent predictors into a nomogram using R language, and verified the predictive performance of the nomogram. The results showed that Platelets (PLT) (1.005 [1.001, 1.009], p = 0.021), Albumin (ALB) (0.891 [0.818, 0.97], p = 0.008), Aspartate aminotransferase (AST) (1.031 [1.002, 1.061], p = 0.035), Alanine aminotransferase (ALT) (0.958 [0.92, 0.998], p = 0.037), and Fibrinogen (FIB) (0.654 [0.464, 0.921], p = 0.015) were independent predictors. Good prediction performance with modest errors was shown by the nomogram in both the training and validation groups. ALB, AST, ALT, FIB, and PLT at admission may be independent predictors of the efficacy of ACDF in CSM patients. The nomogram constructed with these factors has good predictive performance. Serological indicators can be used as a supplement to cervical spine-related imaging indicators, allowing clinicians to better complete the admission diagnosis and treatment process and preoperative evaluation process, so that more postoperative patients can benefit.
Similar content being viewed by others
Introduction
Cervical spondylotic myelopathy (CSM) denotes the degenerative alterations in the cervical intervertebral discs and the consequent pathological changes that affect the spinal cord, nerve roots, vertebral arteries, sympathetic nerves and other surrounding tissues1,2. Year by year, cervical spondylosis is becoming more prevalent, and the despair and sleeplessness accompanying it have a negative impact on patients’ quality of life3,4. Nowadays, cervical spondylosis has emerged as a major public health concern5. Most patients initially experience numbness and a sense of heaviness in their limbs, which is then followed by unstable gait, difficulties in walking and the feeling of walking on cotton wool6,7. A cervical magnetic resonance imaging examination can clearly display the abnormalities in the spinal cord, the compressed areas within the spinal canal and the changes in the spinal cord’s morphology, playing a crucial role in the identification of cervical spondylosis and cervical spine injuries8,9,10. Conservative and surgical treatments are the main approaches for cervical spondylosis. If conservative treatment fails to improve symptoms, surgical intervention should be considered. Currently, cervical laminoplasty, laminectomy, posterior cervical fusion and anterior cervical discectomy and fusion (ACDF) are the three primary surgical options11,12,13. Difficulty in swallowing, pain at the surgical site and superficial wound infection are the most common postoperative problems14,15.
Nomogram is capable of expressing the correlations among variables in a predictive model by integrating diverse predictive indicators and plotting them on the same plane in a predetermined proportion16. Researchers highly value nomogram and often employ them to predict the outcomes of clinical treatment and prognosis17,18. Compared with other traditional prediction tools, nomograms have the advantages of incorporating continuous variables, high accuracy, simple use, and intuitive results19,20.
Xu et al.21proposed that radiographic predictors include C0–2 angle, C2–C7 cervical lordosis angle, C2 slope, T1 slope, and T1 slope minus cervical lordosis angle. Some researchers believe that indices such as the sagittal diameter of the C5 vertebral body can effectively predict the prognosis of CSM22. The anisotropic tensors and isotropic tensors shown by diffusion MRI can provide better prognostic utility23. However, many studies have not mentioned serological indicators. Wang et al.24retrospectively analyzed the clinical data of patients with early fever after posterior cervical laminoplasty. The results showed that serum WBC, CRP, PCT and ESR can be used as diagnostic indicators for early infection24. Lu et al.25 retrospectively studied 75 patients with multi-segment CSM and the results showed that triglycerides can be used as indicators to evaluate cervical sagittal balance. However, the limitation of their research is that it did not integrate a large number of serological indicators and was unable to comprehensively assess the patient’s overall condition, including nutritional status, coagulation function, electrolyte status, liver and kidney function, and other issues.
Reviewing previous literature, imaging indicators are important indicators for determining prognosisand have been fully used in clinical practice21,22,23. However, there is currently no research that integrates patients’ preoperative serological indicators for prediction.The purpose of this study is to construct a nomogram model composed of serological indexes. Clinicians can comprehensively evaluate the patient’s overall condition based on the imaging factors and supplemented by the preoperative serological indexes, so as to predict the patient’s prognosis more accurately and comprehensively.
Materials and procedures
Section on patients
This is a retrospective study. Included were all patients treated at the Guilin Medical College affiliated hospital from January 2017 to January 2023 for anterior cervical discectomy and fusion (ACDF) and diagnosed with cervical spondylotic myelopathy (CSM). The following were the inclusion criteria: (1) Classical clinical signs of CSM, such as bilateral lower limb weakness, cotton wool feeling, and reduced muscle strength in the lower limbs or all four limbs; (2) Magnetic resonance imaging (MRI) confirms the presence of spinal cord compression and changes in the spinal cord signal; (3) ACDF surgery is being performed; and (4) Age greater than eighteen. The following were the exclusion criteria: (1) Concurrent large systemic diseases, such as disorders of the blood or immune systems; (2) Concurrent other spinal disorders, such as spinal TB, infection, tumour, etc.; (3) Limb dysfunction resulting from different aetiologies.
To minimize the potential confounding effects of imaging factors (such as inadequate spinal cord decompression or incomplete spinal realignment), all included patients underwent cervical MRI examinations both preoperatively and postoperatively. These assessments were independently evaluated by two senior physicians (LWC and LH) (Fig. 1).
Gathering and defining data
Clinical information and laboratory results, such as complete blood counts, blood biochemistry, and coagulation function tests, were gathered for this investigation. Gender, age, history of diabetes, history of heart disease, history of hypertension, history of alcohol use, and history of smoking were among the baseline clinical data.
At the time of the patient’s hospitalisation, the research also obtained the following laboratory data: The following information is relevant: the number of white blood cells (WBC), red blood cells (RBC), haemoglobin (HGB), mean corpuscular volume (MCV), neutrophils (NC), lymphocytes (LYM), monocytes (MONO), eosinophils (Eos), basophils (Baso), albumin (ALB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), creatinine (Cr), blood urea nitrogen (BUN), prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen (FIB), and further pertinent information.
Outcome
Functional deficits in the human body are typically assessed using the Japanese Orthopaedic Association Score (JOA)26. The JOA total score is a number between 0 and 17, where lower numbers correspond to more severe functional impairments. [(post-treatment score - pre-treatment score) / (17 - pre-treatment score)] × 100% is the formula for calculating the JOA recovery rate. A recovery rate of 100% indicates a “cure”, a rate greater than 60% indicates “significant improvement”, a rate of 25–60% indicates “effectiveness”, and a rate less than 25% indicates “ineffectiveness”. The “Poor prognosis group” in this study was defined as having a JOA recovery rate of less than 25% at three days postoperatively, whereas the “Good prognosis group” was classified as having the opposite result.
Analysis of statistics
Using SPSS 21.0 (SPSS Inc., Chicago, IL), the data in this study was initially randomly split into a training test and a validation test in a 7:3 ratio. The comparability of the baseline data was then confirmed by comparing it between the validation test and the training test.
The training test data was then split into two groups: one for the poor prognosis and the other for the good prognosis. The two groups’ clinical information and test results from the lab were compared. The Shapiro normalcy test was utilised to determine normality for continuous data. If the data followed a normal distribution, it was presented as mean ± standard deviation, and one-way analysis of variance (ANOVA) was used for group comparisons. If the data did not follow a normal distribution, it was presented as median (25th percentile, 75th percentile), and the Kruskal-Wallis test was used for group comparisons. We fill the missing values by interpolating the mean and median.
Categorical data were described using frequencies (percentages), and group comparisons were performed using the chi-square test or Fisher’s exact test. A p-value less than 0.05 was considered statistically significant for all analyses.
To calculate the optimal cutoff values for clinical factors in the training group, SPSS 21.0 software can be used. When building a statistical model, optimal cutoff values are selected and tried to find the cutoff value that gives the best model fit. The clinical factors can be classified based on these cutoff values, and then univariate logistic regression analysis can be performed. Clinical factors with a p-value less than 0.1 in the univariate analysis can be included in the multivariate logistic regression analysis to evaluate independent predictors of good prognosis.
Based on the results of the multivariate logistic regression analysis, the training group data can be used to create a nomogram for the independent predictors using R Studio (version 4.2.2).
The predictive performance of the model can be evaluated using the ROC curve and the area under the curve (AUC). The model’s average error can be calculated using a calibration plot, and the clinical utility of the model can be analyzed using a decision curve analysis (DCA). Additionally, the validation group data can be analyzed and graphically presented to validate the effectiveness of the model.
Result
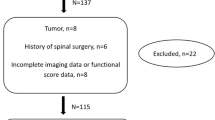
In this study, we collect a total of 379 patients with CSM. Twenty-one patients are excluded due to the aforementioned factors, resulting in a final inclusion of 358 patients for the retrospective study, as shown in Fig. 2. Among them, 250 patients are in the training test and 108 patients are in the validation test.
Comparison of baseline data between the training test and validation test shows that in the training test, there are 137 males (54.8%), with an age of 66 (55, 80) years. The numbers of patients with a history of hypertension, diabetes, heart disease, smoking, and alcohol consumption are 92 (36.8%), 75 (30%), 8 (3.2%), 74 (29.6%), and 97 (38.8%), respectively. In the validation test, there are 57 males (52.78%), with an age of 67.5(56, 76) years. The numbers of patients with a history of hypertension, diabetes, heart disease, smoking, and alcohol consumption are 36 (33.33%), 31 (28.7%), 4 (3.7%), 38 (35.19%), and 35 (32.41%), respectively. There are no statistically significant differences in baseline data between the two groups (p > 0.05), indicating the comparability of the data (Table 1).
The training test data is divided into two groups based on the recovery rate: the poor prognosis group (recovery rate < 25%) and the good prognosis group (recovery rate > 25%). Comparison between the two groups shows that Age, RBC, WBC, PLT, PT, APTT, and FIB have statistically significant differences (p < 0.05) (Table 2).
Univariate logistic regression analysis is performed for each clinical factor. The results show that Age (0.54 [0.301, 0.969], p = 0.039), RBC (0.388 [0.197, 0.764], p = 0.006), HGB (2.766 [1.035, 7.392], p = 0.043), WBC (2.255 [1.205, 4.218], p = 0.011), NC (0.478 [0.244, 0.935], p = 0.031), PLT (2.924 [1.625, 5.261], p < 0.001), ALB (0.253 [0.127, 0.506], p < 0.001), PT (0.393 [0.215, 0.717], p = 0.002), APTT (0.335 [0.167, 0.671], p = 0.002), and FIB (0.224 [0.085, 0.59], p = 0.002) are all independent predictors of prognosis (Table 3).
Multivariate logistic regression analysis is performed for factors with p < 0.1 in the univariate analysis. The results show that PLT (1.005 [1.001, 1.009], p = 0.021), ALB (0.891 [0.818, 0.97], p = 0.008), AST (1.031 [1.002, 1.061], p = 0.035), ALT (0.958 [0.92, 0.998], p = 0.037), and FIB (0.654 [0.464, 0.921], p = 0.015) are independent predictors (Table 3).
To further validate the predictive ability of these factors for CSM patients, we construct a nomogram model using R Studio software based on the results of the multivariate analysis (ALB, AST, ALT, FIB, PLT). The nomogram is constructed using the training test data, as shown in Fig. 3. The ROC curve is plotted to validate the predictive performance of the line plots in the training group (Fig. 4A), with a C-index of 0.711, indicating good prediction. The calibration plot of the training group (Fig. 5A) shows an error of 0.015, indicating good consistency between the model and the actual situation. The decision curve analysis (DCA) of the training group (Fig. 6A) shows good clinical benefits between the threshold of 0.01–0.70. The validation group data is used for validation, and the ROC curve (Fig. 4B) shows a C-index of 0.671. The calibration plot (Fig. 5B) shows an average error of 0.052, and the DCA (Fig. 6B) shows good clinical benefits between the threshold of 0.01–0.99. It can be seen that the line plots perform well in both the training and validation groups, with small errors and benefiting most clinical patients.
Discussion
Cervical Spondylotic Myelopathy represents the most severe form of cervical spondylosis. Owing to its insidious onset and substantial individual variations, the manifestations of spinal cord damage are multifarious, and the development speed, trend, and outcome also differ27,28,29. Consequently, the enhancement of limb function following surgery is our primary concern.
The study reported that male gender, age over 50 years, and the number of affected intervertebral discs are crucial risk factors for increased drainage after ACDF30. Inoue Takaki et al.31 discovered that the reduction of the sagittal plane vertical axis of C2-7 vertebral bodies is associated with decreased cervical lordosis after CSM surgery. Huang Shengsheng et al.32 suggested that high signal intensity on T2WI magnetic resonance imaging is a risk factor for postoperative ischemia-reperfusion injury in CSM patients. Yang Yiyuan et al.33found that a lower preoperative Cobb angle of C2-7 vertebral bodies might be related to postoperative kyphosis in CSM patients undergoing laminoplasty. A cohort study identified age, male gender, and relative socioeconomic deprivation as independent risk factors for CSM occurrence34. A literature review indicated that diabetes is a risk factor for poor neurological recovery after cervical spine surgery35. Some scholars have utilized machine learning methods to verify that postoperative infection, increased age, increased BMI, longer operation time, and decreased preoperative hematocrit and white blood cell count can be used to predict the occurrence of reoperation and readmission events36. Wang Jie et al.37 proposed that the maximum spinal canal injury and maximum spinal cord compression can serve as predictors of postoperative prognosis improvement in CSM.
In this study, ALB, AST, ALT, FIB, and PLT were identified as independent predictors of limb function improvement following ACDF. ALB is a crucial indicator reflecting the nutritional status and inflammatory response of patients38. Frequently, low albumin levels are associated with chronic diseases, inflammation, or malnutrition, which may have an impact on postoperative tissue repair, bone healing, and immune response39,40. Patients with low ALB levels are more likely to be susceptible to infection, experience delayed tissue repair, or develop postoperative complications41. AST and ALT serve as indicators of liver function and mirror the damage to liver cells42. Preoperative abnormal liver function can result in systemic metabolic disorders and an increased release of inflammatory mediators, thereby affecting postoperative recovery43. Impaired liver function may delay recovery and raise the risk of complications. Moreover, liver metabolic disorders can disrupt bone metabolism and influence the bone fusion process44,45. FIB is a key component of the coagulation system, and elevated fibrinogen levels may be harmful to bone health46. Fibrinogen inhibits the formation of osteoclasts from precursor cells47. Changes in PLT may reflect systemic inflammation or coagulation abnormalities48. Elevated PLT levels may suggest chronic inflammation or reactive hyperplasia and are associated with a poor prognosis49.
Consequently, the above factors directly or indirectly influence the following aspects: postoperative tissue repair and bone fusion; the nutritional and inflammatory status significantly affects soft tissue healing and intervertebral cage bone fusion; and the risk of postoperative complications such as infection, thrombosis, or bleeding. It is evident from the research results that in clinical practice, more attention should be paid to patients’ nutritional supplementation to increase preoperative ALB levels and enhance postoperative recovery ability. Simultaneously, the management of inflammation and hypercoagulation should be emphasized, and it is necessary to explore the possibility of intervening in abnormal preoperative FIB and PLT levels through drugs (such as anticoagulants or anti-inflammatory drugs) in the future. Furthermore, preoperative treatment should be adjusted to stabilize AST and ALT levels. We compared the previous literature with our research results and drew a table (Table 4). Compared with previous prediction models, scholars have focused more on imaging results. Our model attaches greater importance to the patient’s general condition and systemic nutritional status, which were rarely considered in previous models. By concentrating on laboratory indicators and the patient’s physical condition, better preoperative management can be initiated immediately upon admission, benefiting more patients.
This study has the following advantages: (1) It is the first time that serology is used as a predictive indicator to construct a nomogram, emphasizing its convenience, speed, ease of use, and certain practicality and predictive potential. (2) Serological predictive factors can be used as a supplement to imaging predictive factors to comprehensively predict patient prognosis. (3) The models constructed in the training group and validation group of this study showed good predictive performance, small average error, and significant clinical benefit. Nevertheless, this study also has the following limitations: (1) It is a single-center study and further external validation using multicenter data is required. (2) We must admit that this study has inherent limitations. Our study did not include imaging factors related to the cervical spine. Serological factors cannot replace the predictive effect of imaging factors on the prognosis of CSM, so the scope of application is limited. Our further research will consider integrating imaging indicators and serological indicators, and verify them through multicenter data to obtain a more powerful nomogram model.
Conclusion
ALB, AST, ALT, FIB, and PLT at admission may be independent predictors of the efficacy of ACDF in CSM patients. The nomogram constructed with these factors has good predictive performance. Serological indicators can be used as a supplement to cervical spine-related imaging indicators, allowing clinicians to better complete the admission diagnosis and treatment process and preoperative evaluation process, so that more postoperative patients can benefit.
JX is responsible for writing articles, conducting statistical analysis, reviewing articles, and creating images.
DGY and HL are responsible for collecting data and conducting statistical analysis.
All authors reviewed the manuscript.
All authors read and approved the final manuscript.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- CSM:
-
Cervical spondylotic myelopathy
- JOA:
-
Japanese Orthopedic Association Score
- ACDF:
-
Anterior cervical discectomy and fusion
- WBC:
-
White blood cell count
- RBC:
-
Red blood cell count
- HGB:
-
Hemoglobin
- MCV:
-
Mean corpuscular volume
- NC:
-
Neutrophils
- LYM:
-
Lymphocyte
- MONO:
-
Monocyte
- PLT:
-
Platelet
- Eos:
-
Eosinophils
- Baso:
-
Basophil
- ALB:
-
Albumin
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- TBIL:
-
Total bilirubin
- PT:
-
Prothrombin time
- APTT:
-
Activated partial thrombin time
- FIB:
-
Fibrinogen
- Cr:
-
Creatinine
- BUN:
-
Blood urea nitrogen
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- AUC:
-
The area under the curve
- DCA:
-
Decision curve analysis
- ROC:
-
Receiver operating characteristic
References
Davies, B. M., Mowforth, O. D., Smith, E. K. & Kotter, M. R. Degenerative cervical myelopathy. BMJ (Clinical Res. ed). 360, k186 (2018).
Baogan, P. DePalma Michael J,Cervical disc degeneration and neck pain[. J] J. Pain Res. 11, 2853–2857 (2018).
Zhong Gui-Qin,Lin Bi-Hua,Chen Yu-Xuan et al. Analysis of Factors Correlated with Postoperative Kinesiophobia in Patients with Cervical Spondylotic Myelopathy: A Cross-Sectional Survey.[J].Neuropsychiatr Dis Treat, 19: 1755–1761. (2023).
Bisson Erica, F. et al. Sleep disturbances in cervical spondylotic myelopathy: prevalence and postoperative outcomes-an analysis from the quality outcomes Database.[J].Clin spine Surg, 36: 112–119. (2023).
Guadarrama-Ortiz et al. Parménides,Ruíz-Rivero César Osvaldo,Capi-Casillas Deyanira. Demographic and Clinical Characteristics of Patients With Cervical Spine Degeneration Reveal Frequent Cervicolumbar Tandem Spinal Stenosis in Mexico.[J].Int J Spine Surg, 17: 670–677. (2023).
Fotakopoulos George,Georgakopoulou Vasiliki Epameinondas,Lempesis et al. Pathophysiology of cervical myelopathy (Review).[J].Biomed Rep, 19: 84. (2023).
Nguyen Alyssa, N. et al. Uncommon Presentation Cerv. Myelopathy [J] Cureus, 15: e45609. (2023).
Khan Ali Fahim,Mohammadi Esmaeil,Haynes Grace et al. Evaluating tissue injury in cervical spondylotic myelopathy with spinal cord MRI: a systematic review[. J] Eur. Spine J., (2023).
Atchut Kauthankar Akshada,Shetty Lathika,Ravichandran kayalvizhi,role of diffusion tensor imaging in stenotic and non-stenotic spinal canal[. J] J. Med. Imaging Radiat. Sci., 54: 699–706. (2023).
Hesni Susan, B., David, S. & Asif The imaging of cervical spondylotic myeloradiculopathy[. J] Skeletal Radiol. 52, 2341–2365 (2023).
Yang Eunice, M. P. V. et al. Cervical laminoplasty versus laminectomy and posterior cervical fusion for cervical myelopathy: propensity-matched analysis of 24-month outcomes from the. Qual. Outcomes Database [J] J. Neurosurg. Spine. 39, 671–681 (2023).
Porche Ken,Vaziri Sasha,Stein Alan. The effect of myelopathic symptoms on hospital costs, length of stay, and discharge location in anterior cervical discectomy and fusion[. J] Neurosurg. Focus. 55, E8 (2023).
Singleton Ian, M. et al. Laminoplasty in Motion: Evolving Techniques and Complications[ (J].J Neurol Surg A Cent Eur Neurosurg, 2023).
Saheb Ricardo Lucca Cabarite,Soeira Thabata Pasquini,Moratelli Lucas. EARLY COMPLICATIONS OF SURGICAL TREATMENT OF CERVICAL SPONDYLOTIC MYELOPATHY.[J]. Acta Ortop. Bras. 31, e260397 (2023).
Chan Ryan Wing-Yuk,Chiang Yung-Hsiao,Lin Hsiu-Chen. et al. Postoperative 30-Day comparative complications of multilevel anterior cervical discectomy and fusion and laminoplasty for cervical spondylotic myelopathy: an evidence (2023). in Reaching Consensus.[J].Diagnostics (Basel), 13: undefined .
Wu Linghong,Peng Xiaozhong,Zhuo Xianglong et al et al. Development and Validation of a Risk-Prediction Nomogram for Preoperative Blood Type and Antibody Testing in Spinal Fusion Surgery[ (J].Orthop Surg, 2023).
Zheng Jianhu,Gao Yan,Yu Wenlong. Development and validation of a nomogram for predicting new vertebral compression fractures after percutaneous kyphoplasty in postmenopausal patients.[J]. J. Orthop. Surg. Res. 18, 914 (2023).
Zhong Huohu,Liu Yingchao,Liu Piaopiao. Risk Estimation for postoperative nausea and vomiting: development and validation of a nomogram based on point-of-care gastric ultrasound[. J] BMC Anesthesiol. 23, 393 (2023).
Xie, W., Li, Y., Meng, X. & Zhao, M. Machine learning prediction models and nomogram to predict the risk of in-hospital death for severe DKA: A clinical study based on MIMIC-IV, eICU databases, and a college hospital ICU. Int. J. Med. Inf. 174, 105049. https://doi.org/10.1016/j.ijmedinf.2023.105049 (2023).
Hu, C. & Nomogram A better method for evaluating MVD risk. Int. J. Cardiol. 411, 132283. https://doi.org/10.1016/j.ijcard.2024.132283 (2024).
Xu, S. et al. Exploration on sagittal alignment and clinical outcomes after consecutive three-level hybrid surgery and anterior cervical discectomy and fusion: a minimum of a 5-year follow-up. J. Orthop. Surg. Res. 15 (1), 79. https://doi.org/10.1186/s13018-020-01589-7 (2020).
Hopkins, B. S. et al. Machine learning for the prediction of cervical spondylotic myelopathy: A post hoc pilot study of 28 Participants[J]. World Neurosurg. 127, e436–e442. https://doi.org/10.1016/j.wneu.2019.03.165 (2019).
Zhang, J. K. et al. Spinal cord metrics derived from diffusion MRI: improvement in prognostication in cervical spondylotic myelopathy compared with conventional MRI. J. Neurosurg. Spine. 41 (5), 639–647. https://doi.org/10.3171/2024.4.SPINE24107 (2024). Published 2024 Jul 26.
Wang, J., Xu, J. W., Li, H. P. & Zhongguo Gu Shang ;32(10):892–897. doi:https://doi.org/10.3969/j.issn.1003-0034.2019.10.004 (2019).
Lv, B. et al. C7 slope and its association with serum lipid levels and modic changes in patients with cervical spondylotic myelopathy. J. Pain Res. 12, 1767–1776. https://doi.org/10.2147/JPR.S188823 (2019). Published 2019 May 30.
Watanabe Masahiko,Chikuda Hirotaka,Fujiwara Yasushi. Japanese orthopaedic association (JOA) clinical practice guidelines on the management of cervical spondylotic Myelopathy,2020 - Secondary publication[. J] J. Orthop. Sci. 28, 1–45 (2023).
Costa Francesco,Anania Carla Daniela,Agrillo Umberto et al. Cervical Spondylotic Myelopathy: From the World Federation of Neurosurgical Societies (WFNS) to the Italian Neurosurgical Society (SINch) Recommendations.[J].Neurospine et al. 20: 415–429. (2023).
Davies Benjamin, M. et al. Life expectancy in patients with degenerative cervical myelopathy is currently reduced but can be restored with timely treatment.[J].Acta Neurochir (Wien), 165: 1133–1140. (2023).
Hejrati Nader,Pedro Karlo,Alvi Mohammed Ali. Degenerative cervical myelopathy: where have we been? where are we now? where are we going?[J]. Acta Neurochir. (Wien). 165, 1105–1119 (2023).
Yuan, H. Y. H. et al. Liu Risk Factors for Predicting Increased Surgical Drain Output in Patients After Anterior Cervical Decompression and Fusion.[J].World Neurosurg, 164: e980-e991. (2022).
Inoue Takaki,Maki Satoshi,Furuya Takeo. et al. Differences in risk factors for decreased cervical lordosis after Multiple-Segment laminoplasty for cervical spondylotic myelopathy and ossification of the posterior longitudinal ligament: A pilot Study.[J].Asian spine J, 17: 712–720. (2023).
Huang Shengsheng,Sun Xuhua,Chen Liyi et al. MRI T2WI High Signal Is a Risk Factor for Perioperative Complications in Patients with Cervical Spondylosis with Spinal Cord Compression: A Propensity Matching Score Analysis.[J].Biomed Res Int et al. 2022: 8040437. (2022).
Yang Yiyuan,Qu Ruomu,Liu Zexiang. et al. Predicting post-laminoplasty kyphosis in cervical spondylotic myelopathy patients without preoperative kyphosis: a retrospective study.[J].BMC musculoskelet Disord, 24: 526. (2023).
Shlykov Maksim, A. et al. Evaluation of Genetic and Nongenetic Risk Factors for Degenerative Cervical Myelopathy.[J].Spine (Phila Pa 1976), 48: 1117–1126. (2023).
Wang, P. L. et al. Baoge,Rong Is diabetes the risk factor for poor neurological recovery after cervical spine surgery? A review of the literature.[J].Eur J Med Res, 27: 263. (2022).
Cabrera Andrew,Bouterse Alexander,Nelson Michael. Use of random forest machine learning algorithm to predict short term outcomes following posterior cervical decompression with instrumented fusion[. J] J. Clin. Neurosci. 107, 167–171 (2023).
Wang, J. Li Haopeng,Yang Baohui,Predictive nomogram for clinical prognosis in cervical spondylotic myelopathy with intramedullary T2-Weighted increased signal intensity: A novel digital tool for patient prognosis Education.[J]. Front. Public. Health. 10, 898242 (2022).
Abedi, F., Zarei, B. & Elyasi, S. Albumin: a comprehensive review and practical guideline for clinical use. Eur. J. Clin. Pharmacol. 80 (8), 1151–1169. https://doi.org/10.1007/s00228-024-03664-y (2024).
Rothschild, M. A., Oratz, M. & Schreiber, S. S. Serum albumin. Am. J. Dig. Dis. 14 (10), 711–744. https://doi.org/10.1007/BF02233577 (1969).
Hülshoff, A., Schricker, T., Elgendy, H., Hatzakorzian, R. & Lattermann, R. Albumin synthesis in surgical patients. Nutrition 29 (5), 703–707. https://doi.org/10.1016/j.nut.2012.10.014 (2013).
Reingardiene, D. Albuminas Ir Jo vartojimas [Albumin and its use]. Med. (Kaunas). 38 (3), 349–353 (2002).
Sookoian, S. & Pirola, C. J. Liver enzymes, metabolomics and genome-wide association studies: from systems biology to the personalized medicine. World J. Gastroenterol. 21 (3), 711–725. https://doi.org/10.3748/wjg.v21.i3.711 (2015).
Wu, W. et al. Xian-Ling-Gu-Bao induced inflammatory stress rat liver injury: inflammatory and oxidative stress playing important roles. J. Ethnopharmacol. 239, 111910. https://doi.org/10.1016/j.jep.2019.111910 (2019).
Huang, G., Li, W., Zhong, Y., Liao, W. & Zhang, Z. Mendelian randomization to evaluate the causal relationship between liver enzymes and the risk of six specific bone and joint-related diseases. Front. Immunol. 14, 1195553. https://doi.org/10.3389/fimmu.2023.1195553 (2023). Published 2023 Aug 16.
Alexandre, J. T. M. et al. Anti-inflammatory and antiresorptive effects of calendula officinalis on inflammatory bone loss in rats. Clin. Oral Investig. 22 (6), 2175–2185. https://doi.org/10.1007/s00784-017-2308-7 (2018).
Du, W., Wang, Z., Dong, Y., Hu, J. & Chen, X. Association between fibrinogen and bone mineral density in postmenopausal women. J Orthop Surg Res. ;18(1):376. Published 2023 May 22. (2023). https://doi.org/10.1186/s13018-023-03785-7
Kim, J. S. et al. Implication of the association of fibrinogen citrullination and osteoclastogenesis in bone destruction in rheumatoid arthritis. Cells 9 (12), 2720. https://doi.org/10.3390/cells9122720 (2020). Published 2020 Dec 20.
Menter, D. G. et al. Platelet first responders in wound response, cancer, and metastasis. Cancer Metastasis Rev. 36 (2), 199–213. https://doi.org/10.1007/s10555-017-9682-0 (2017).
Nurden, A. T. Platelets, inflammation and tissue regeneration. Thromb. Haemost. 105 (Suppl 1), S13–S33. https://doi.org/10.1160/THS10-11-0720 (2011).
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
WCL designed this study. JX is responsible for writing articles, conducting statistical analysis, reviewing articles, and creating images.DGY and HL are responsible for collecting data and conducting statistical analysis.All authors reviewed the manuscript.All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the the First Affiliated Hospital of Guangxi Medical University Ethics Review Committee (2023-E717-01). Due to the retrospective nature of the study, the First Affiliated Hospital of Guangxi Medical University Ethics Review Committee waived the need of obtaining informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xiao, J., Deng, G., Ling, H. et al. Constructing a nomogram model for patients with cervical spondylotic myelopathy. Sci Rep 15, 8901 (2025). https://doi.org/10.1038/s41598-025-93703-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-93703-0