Abstract
Data on the involvement of proprotein convertase subtilisin/kexin type 9 (PCSK9) and adiponectin in prediabetes progression to type 2 diabetes mellitus (T2DM) remains inconclusive. Therefore, this study investigated the roles of PCSK9 and adiponectin in this process. This study included 1,528 participants with prediabetes and T2DM and conducted correlation analyses to investigate the relationship between PCSK9 and adiponectin levels, pancreatic beta-cell function, and insulin levels. Logistic regression analysis and receiver operating characteristic (ROC) curves were used to determine whether PCSK9 and adiponectin play protective roles in the progression from prediabetes to T2DM and their potential as diagnostic biomarkers for T2DM. In prediabetic patients, the levels of PCSK9 [573.00 (412.35) ng/mL vs. 924.20 (673.38) ng/mL, p < 0.001] and adiponectin [4.50 (2.80) mg/mL vs. 6.22 (4.51) mg/mL, p < 0.001] were significantly higher than those in patients with T2DM. PCSK9 (r = 0.167, p < 0.001) and adiponectin (r = 0.113, p < 0.001) levels were positively correlated with pancreatic cell homeostasis and had protective effects against progression from prediabetes to T2DM (PCSK9: OR = 0.274, 95% CI 0.121–0.621, p = 0.002; adiponectin: OR = 0.135, 95% CI 0.057–0.320, p < 0.001). The combined diagnostic value of PCSK9 and adiponectin for T2DM showed an area under the curve of 0.751 (95% CI 0.727–0.775). Prediabetes and T2DM patients showed significant differences in the PCSK9 and adiponectin levels. PCSK9 and adiponectin have protective effects against the progression of prediabetes to T2DM, and their combined use is a potential biomarker for the transition from prediabetes to T2DM.
Similar content being viewed by others
Introduction
Prediabetes, which precedes type 2 diabetes mellitus (T2DM), represents a high risk for the development of diabetes. Blood glucose levels are elevated above normal yet not high enough to be classified as diabetes. Prediabetes is a heterogeneous subclinical state of diabetes. The total number of reported cases of T2DM by 2021 was approximately 540 million globally, which is expected to increase to 780 million by 20451. The number of patients with prediabetes is closely related to the number of patients with T2DM. It is predicted that by 2030, there will be 460 million prediabetic patients worldwide, over 70% of whom will develop T2DM2,3.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a serine protease derived from the liver and intestines; however, the primary source of circulating PCSK9 is the liver4,5. PCSK9 influences the blood levels of low-density lipoprotein cholesterol (LDL-C) by regulating the degradation of low-density lipoprotein receptors (LDLR) on the surface of hepatocytes through the endosome/lysosome pathway, highlighting new challenges in low-density cholesterol homeostasis6. In addition to the traditional pathway, PCSK9 contributes to the progression of atherosclerosis through alternative mechanisms, including inflammation, apoptosis, and immune responses7,8,9.
Adiponectin, a 30-kDa monomeric glycoprotein secreted by adipocytes, is primarily regulates lipid and glucose metabolism10, enhances insulin sensitivity, exerts anti-inflammatory effects, and protects the cardiovascular system11. The blood concentration of adiponectin typically ranges from 3 to 30 µg/mL. Nielsen et al. indicated that higher plasma adiponectin levels are associated with a high risk of heart failure, atrial fibrillation, aortic valve stenosis, and myocardial infarction12. A study involving a Japanese population showed that low l adiponectin levels increased the risk of T2DM13.
PCSK9 and adiponectin have been extensively studied in T2DM14, although the results have been inconsistent. For example, some studies have shown that high levels of PCSK9 are associated with T2DM15, while others reported no correlation16. Additionally, animal experiments have indicated that low PCSK9 levels are associated with diabetes6. Adiponectin also plays a major role in the progression of healthy individuals to T2DM. However, the underlying mechanisms remain unclear.
Some studies have suggested insulin resistance as a mechanism, whereas others indicated an association with pancreatic cell stability17,18. However, current research on the changes and correlations between PCSK9 and adiponectin from the prediabetic to the diabetic stage remains limited.
Therefore, this cross-sectional study aimed to explore the differences and connections between PCSK9 and adiponectin in prediabetic and T2DM patients to better understand the roles of these two indicators during the progression from prediabetes to T2DM.
Methods
Study population
This study recruited 1,528 participants from Peking University People’s Hospital and Xiangya Hospital of Central South University between October 2022 and September 2023, including 768 patients with T2DM (388 males and 380 females) and 760 patients with prediabetes (349 males and 411 females) (Fig. 1). This study adhered to the ethical principles outlined in the Declaration of Helsinki, and was approved the Peking University People’s Hospital Research Ethics Committee (No. 2022PHB349-01). All enrolled patients provided written informed consent before participation. Medication and physical activity details for all participants can be found in Supplementary Table 1.
Based on the criteria of the American Diabetes Association19, the diagnosis of T2DM includes individuals who meet any of the following conditions: (1) currently receiving treatment with oral hypoglycemic medications or insulin, (2) have a history of T2DM confirmed by a clinician, (3) fasting blood glucose (FBG) ≥ 7.0 mmol/L or 2-h blood glucose (2-h BG) ≥ 11.1 during the oral glucose tolerance test (OGTT), or (4) glycated hemoglobin A1c (HbA1c) levels of 6.5% or higher. The criteria for prediabetes include: (1) FPG levels of 5.6–6.9 mmol/L, (2) blood glucose levels of 7.8–11.0 mmol/L two hours after a 75 g OGTT, or (3) HbA1c levels of 5.7–6.4%.
The exclusion criteria were as follows: (1) severe liver or kidney dysfunction, (2) any known inflammatory or infectious diseases, (3) confirmed or suspected cancer, and (4) pregnancy or lactating patients.
Conventional clinical and laboratory indicator tests
Morning blood samples were collected after a 12-h fast. Serum was separated via centrifuging at 3500 rpm for 10 min at 10–15 °C and stored at − 80 °C.
An ELISA kit (Duduo Biotechnology Co., Ltd. China) was used to measure circulating levels of PCSK9 according to the manufacturer’s instructions. Adiponectin levels were measured using latex-enhanced immunoturbidimetry (Guangdong United Biotechnology Co., Ltd. China). Lipoprotein (a) [Lp(a)] levels were measured using a method similar to that used for the Lp(a) kit (Roche Inc., Switzerland). Insulin levels were measured using an electrochemiluminescence immunoassay (Roche Inc., Switzerland). To detect small dense LDL-C (sdLDL-C), we employed direct quantitative analysis kits (Denka Seiken Co., Ltd., Japan). HbA1c levels were determined using high-performance liquid chromatography (Trinity Biotech Inc., USA). A Beckman AU5832 analyzer (Beckman Coulter Inc., USA) was used to determine FBG, hypersensitive C-reactive protein (hs-CRP), homocysteine (HCY), and serum lipid profiles. These profiles included triglyceride (TG), total cholesterol (TC), LDL-C, high-density lipoprotein cholesterol (HDL-C), Apolipoprotein A-1 (apoA1), and Apolipoprotein B (apoB) levels.
The relevant indicators related to insulin were calculated as follows: the homeostasis model assessment of beta-cell function (HOMA-β) = 20 * insulin (μU/mL)/[FBG (mmol/L) –3.5] for evaluating the beta cell function; the homeostasis model assessment of insulin resistance (HOMA-IR) = insulin (μU/mL) * FBG (mmol/L)/22.5; the homeostasis model assessment of insulin sensitivity (HOMA-IS) was 22.5/[insulin (μU/mL) * FBG (mmol/L)]20.
Statistical analyses
The one-sample Kolmogorov–Smirnov test was used to analyze the distribution of all quantitative variables. Normally distributed data are presented as mean ± standard deviation, and Student’s t-test and analysis of variance were used to compare differences between the groups. Continuous data with non-normal distribution were reported as medians (interquartile ranges), and differences between various groups were compared using the Mann–Whitney U and Kruskal–Wallis tests. Categorical data are presented as percentages (%) and compared using the chi-squared test. Spearman’s correlation analysis was used to calculate correlation coefficients between circulating PCSK9 and adiponectin levels. Univariate and multivariate logistic analyses were used to analyze the association between PCSK9 and adiponectin levels and T2DM. Receiver operating characteristic (ROC) curves, particularly the area under the curve (AUC), were used to evaluate the ability of PCSK9 and adiponectin to discriminate between T2DM and prediabetes. p-value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 22.0 for Windows (SPSS Inc., USA) and GraphPad Prism 7 (GraphPad Software Inc., USA).
Results
Baseline characteristics of all patients
First, we assessed the baseline characteristics of all participants. Table 1 presents the clinical characteristics and laboratory parameters of the study population. There were no significant differences between the T2DM and prediabetic groups in terms of sex (male), age, body mass index (BMI), percentage of hypertension, alcohol consumption, smoking, family history of diabetes (MD), ApoB, ApoA1, TC, HDL-C, LDL-C, TG, sdLDL-C, hs-CRP, or HCY. In contrast, the levels of FBG, HbA1c, insulin, and HOMA-IR were higher in the T2DM than those in the prediabetes groups, while the levels of HOMA-β, HOMA-IS, Lp(a), adiponectin [4.50 (2.80) mg/mL vs. 6.22 (4.51) mg/mL, p < 0.001], and PCSK9 [573.00 (412.35) ng/mL vs. 924.20 (673.38) ng/mL, p < 0.001] were lower than those in the prediabetes group.
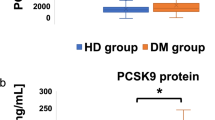
As shown in Supplementary Table 2, PCSK9 levels [710.55 (613.15) ng/mL vs. 607.40 (568.20) ng/mL, p < 0.014] were higher in males than in females, while adiponectin levels [4.79 (3.51) mg/mL vs. 5.81 (4.02) mg/mL, p < 0.001] were lower (Fig. 2A, C). All participants were divided according to sex for statistical analysis (Supplementary Tables 3 and 4). Regardless of the statistics, the adiponectin and PCSK9 levels in the T2DM group were lower than those in the prediabetes group (Fig. 2B, D).
Levels of PCSK9 and adiponectin in different populations. (A) Levels of PCSK9 in different gender populations. (B) PCSK9 levels in T2DM and prediabetes in different genders. (C) Levels of adiponectin in different gender populations. (D) Adiponectin levels in T2DM and prediabetes in different genders. (*p < 0.05, **p < 0.01, ***p < 0.001).
Relationship between PCSK9, adiponectin, pancreatic beta cell function, and insulin
To investigate the relationship between PCSK9 and adiponectin levels in pancreatic beta cells and insulin, we divided all participants into four groups based on the quartiles of PCSK9 and adiponectin levels. As PCSK9 levels increased, the proportion of patients with T2DM, as well as their FBG and HbA1c levels, decreased, while insulin [10.65 (8.18) μU/mL vs. 12.14 (10.12) μU/mL vs. 12.59 (8.28) μU/mL vs. 14.51 (8.51) μU/mL, p < 0.001] and HOMA-β [63.79 (49.73) vs. 75.43 (58.81) vs. 91.41 (67.13) vs. 102.82 (67.31), p < 0.001] levels increased (Table 2). Even when divided into the T2DM group and the prediabetes group and analyzed separately, the results remain consistent with the previous findings (Supplementary Tables 5 and 6). The same result was observed in the adiponectin group, where HOMA-β [68.95 (64.93) vs. 82.84 (67.55) vs. 79.38 (62.79) vs. 98.83 (69.70), p < 0.001] levels increased as adiponectin levels increased (Table 3). After separately analyzing the T2DM group and the prediabetes group, the highest quartile group’s HOMA-β was significantly higher than that of the lowest quartile group (Supplementary Tables 7 and 8, p < 0.05, respectively).
The correlations between PCSK9, adiponectin, and pancreatic beta cell homeostasis in patients with prediabetes and T2DM were analyzed to investigate the relationship between PCSK9, adiponectin, and other indicators (Fig. 3). In all patients, as shown in Fig. 3A, PCSK9 was positively correlated with adiponectin (r = 0.289, p < 0.001), insulin (r = 0.132, p < 0.001), HOMA-β (r = 0.167, p < 0.001), and HOMA-IR (r = 0.055, p = 0.030), and negatively with FBG (r = –0.116, p < 0.001), HbA1c (r = − 0.139, p < 0.001), and HOMA-IS (r = − 0.095, p < 0.001). Adiponectin positively correlated with PCSK9 (r = 0.289, p < 0.001), and HOMA-β (r = 0.113, p < 0.001), but negatively with BMI (r = − 0.139, p < 0.001), FBG (r = − 0.175, p < 0.001), HbA1c (r = − 0.199, p < 0.001), and HOMA-IR (r = − 0.069, p = 0.007). Similar results were observed in prediabetic and T2DM patients (Fig. 3B, C).
The correlation between PCSK9 and adiponectin with insulin and pancreatic beta-cell function. (A) The correlation between PCSK9 and adiponectin with insulin and pancreatic beta-cell function in all participants. (B) The correlation between PCSK9 and adiponectin with insulin and pancreatic beta-cell function in prediabetes patients. (C) The correlation between PCSK9 and adiponectin with insulin and pancreatic beta-cell function in T2DM patients.
Serum PCSK9 and adiponectin concentrations are associated with the risk of progression to T2DM in prediabetic patients
We used a multivariate binary logistic regression to better understand the relationship between PCSK9, adiponectin, and T2DM. The patients were divided into four groups based on the quartiles of PCSK9 and adiponectin levels. Using the lowest group as a reference, we analyzed the risk of T2DM in the other three groups according to PCSK9 and adiponectin levels.
Univariate analysis showed that PCSK9 (OR = 0.243, 95% CI 0.180–0.327, p < 0.001) (Fig. 4A) and adiponectin (OR = 0.175, 95% CI 0.128–0.239, p < 0.001) (Fig. 5A) levels were negatively correlated with T2DM. After further adjusting for confounding factors, the results remained consistent (PCSK9: OR = 0.274, 95% CI 0.121–0.621, p = 0.002; adiponectin: OR = 0.135, 95% CI 0.057–0.320, p < 0.001) (Figs. 4B and 5B). To investigate the potential sex differences in PCSK9 and adiponectin levels, we conducted separate analyses in male and female patients. The results remain consistent with previous findings, regardless of univariate (Figs. 4C, E and 5C, E) or multivariate analysis (male patients: PCSK9: OR = 0.244, 95% CI 0.085–0.699, p = 0.009; adiponectin: OR = 0.197, 95% CI 0.061–0.640, p = 0.007; female patients: PCSK9: OR = 0.116, 95% CI 0.017–0.791, p = 0.028; adiponectin: OR = 0.093, 95% CI 0.021–0.417, p = 0.002) (Figs. 4D, F and 5D, F), indicating that PCSK9 and adiponectin have a protective effect against the progression from prediabetes to T2DM.
Logistic regression analysis between PCSK9 and T2DM in different populations. (A) Univariate logistic regression analysis between circulating PCSK9 and T2DM in all patients. (B) Multivariate logistic regression analysis between circulating PCSK9 and T2DM in all patients. (C) Univariate logistic regression analysis between circulating PCSK9 and T2DM in male patients. (D) Multivariate logistic regression analysis between circulating PCSK9 and T2DM in male patients. (E) Univariate logistic regression analysis between circulating PCSK9 and T2DM in female participants. (F) Multivariate logistic regression analysis between circulating PCSK9 and T2DM in female participants.
Logistic regression analysis between adiponectin and T2DM in different populations. (A) Univariate logistic regression analysis between circulating adiponectin and T2DM in all patients. (B) Multivariate logistic regression analysis between circulating adiponectin and T2DM in all patients. (C) Univariate logistic regression analysis between circulating adiponectin and T2DM in male patients. (D) Multivariate logistic regression analysis between circulating adiponectin and T2DM in male patients. (E) Univariate logistic regression analysis between circulating adiponectin and T2DM in female participants. (F) Multivariate logistic regression analysis between circulating adiponectin and T2DM in female participants.
Prospects of PCSK9 and adiponectin as diagnostic markers for T2DM
Receiver operating characteristic curve analyses were performed to explore the diagnostic prospects of PCSK9 and adiponectin in T2DM. Figure 6 shows that the AUC for PCSK9 was 0.667 (95% CI 0.640–0.694), with an optimal sensitivity of 63.5% and specificity of 66.7%. For adiponectin, the AUC was 0.673 (95% CI 0.646–0.700), with an optimal sensitivity of 69.4% and specificity of 57.4%. However, when the two markers were combined, the AUC increased to 0.751 (95% CI 0.727–0.775). These data suggest that the combination of PCSK9 and adiponectin holds potential value as a biomarker for progression from prediabetes to T2DM.
Discussion
This cross-sectional study, for the first time, revealed differences in PCSK9 and adiponectin levels between patients with prediabetes and those with T2DM in a Chinese population. Our study results showed that patients with prediabetes had significantly higher PCSK9 and adiponectin levels than did those with T2DM. PCSK9 and adiponectin levels were positively correlated with HOMA-β and negatively with FBG and HbA1c. Our data also suggest PCSK9 and adiponectin as protective factors against T2DM.
Research on PCSK9 has primarily focused on cardiovascular diseases7,9,21,22. Previous studies have shown that PCSK9 binds to LDLR, leading to its intracellular degradation, thereby increasing the plasma LDL-C levels and hyperlipidemia. PCSK9 promoted LDLR degradation by enhancing endocytosis and preventing recycling. In addition to the classical pathway, PCSK9 regulates lipid metabolism by modulating ApoB expression and Lp(a) metabolism via endogenous intracellular PCSK9. PCSK9 is associated with endothelial cell apoptosis, macrophage cholesterol efflux, intracellular mitochondrial dysfunction, and inflammation, all of which can lead to metabolic disorders and promote atherosclerosis.
However, the role of PCSK9 in diabetes remains controversial. In a cohort study involving 4,205 patients, Shi et al.23 demonstrated that PCSK9 levels were significantly higher in patients with diabetes than those without and in females than males. However, the authors observed no difference in PCSK9 levels between male patients with and without diabetes. The same results were observed in patients with ST-segment elevation myocardial infarction, with or without diabetes24. Saavedra et al.25 showed that patients with familial hypercholesterolemia carrying the PCSK9-InsLEU genetic variant had a lower risk of coronary artery events but an increased incidence of prediabetes and diabetes. Awan et al.26 found similar results on subjects carrying the apoE3/E2 genotype and the R46L variant. Similarly, PCSK9 gene defects increase the risk of diabetes in mice6,27. This may be related to the abundance of low-density receptors on the surface of pancreatic β-cells. Lower PCSK9 levels allow a large amount of low-density lipoprotein cholesterol to enter cells, leading to intracellular lipid metabolism disorders that ultimately affect insulin synthesis and secretion6,16,28. Our research findings support those of Saavedra et al. and Awan et al. at the levels of prediabetes and T2DM patients. Our study also indicated that PCSK9 levels positively correlated with the homeostasis of pancreatic β-cell function in patients.
Adiponectin, secreted by the adipose tissue, originates from a 30 kDa monomer protein modified into various multimers and then released into the bloodstream29,30,31. The main function of adiponectin is to bind to AdipoR1 and AdipoR2, triggering a series of tissue-specific signal transduction events32,33. This process reduces intracellular ceramide levels and is associated with insulin resistance, apoptosis, inflammation, and atherosclerosis34,35,36. A recent study found that adiponectin acts as a protective factor against the progression of ischemic heart disease in subjects with normal glucose tolerance who undergo percutaneous coronary intervention37,38. Our research showed that prediabetic patients had significantly higher adiponectin levels than did those with T2DM. Additionally, participants with high adiponectin levels had low hs-CRP levels. This indirectly indicated its protective effect on the cardiovascular system.
In recent years, there have been reports on adiponectin, pancreatic cells, and insulin in different populations39,40,41. Szumilas et al. reported that low adiponectin levels are associated with insulin resistance, which can increase the risk of diabetes after organ transplantation39. Similar results were obtained in a previous study involving healthy pregnant women and women with gestational diabetes. Regardless of whether the women were healthy or had gestational diabetes, adiponectin levels were negatively correlated with insulin resistance42. Additionally, in a study involving 232,438 adults with obesity, adiponectin levels negatively correlated with BMI, waist circumference, and FBG43. Our study results indicate that in patients with T2DM and prediabetes, adiponectin levels negatively were correlated with BMI, FBG, HbA1c, insulin, and insulin resistance and positively with pancreatic function issues.
This not only supports the aforementioned research in prediabetic populations but also demonstrates the potential value of using PCSK9 and adiponectin for the combined diagnosis of T2DM (AUC = 0.751, 95% CI 0.727–0.775).
Limitations
This study has some limitations. First, the involvement of only two centers might have introduced a potential selection bias, necessitating further validation of our findings through broader multicenter studies. Second, as a cross-sectional study, follow-up information was lacking, possibly limiting our understanding of the roles of PCSK9 and adiponectin in the development of T2DM. Therefore, we are currently addressing these issues by collaborating with more centers and conducting longer patient follow-ups.
Conclusion
Patients with prediabetes exhibited significantly higher levels of PCSK9 and adiponectin than did those with T2DM. Both PCSK9 and adiponectin levels were positively correlated with pancreatic cell function homeostasis. The combined use of PCSK9 and adiponectin is a potential biomarker for the progression from prediabetes to T2DM.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sun, H. et al. IDF diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119. https://doi.org/10.1016/j.diabres.2021.109119 (2022).
Ding, L. et al. The prognostic value of the stress hyperglycemia ratio for all-cause and cardiovascular mortality in patients with diabetes or prediabetes: Insights from NHANES 2005–2018. Cardiovasc. Diabetol. 23, 84. https://doi.org/10.1186/s12933-024-02172-8 (2024).
Strati, M., Moustaki, M., Psaltopoulou, T., Vryonidou, A. & Paschou, S. A. Early onset type 2 diabetes mellitus: An update. Endocrine 85, 965–978. https://doi.org/10.1007/s12020-024-03772-w (2024).
Ding, Z. et al. PCSK9 expression in the ischaemic heart and its relationship to infarct size, cardiac function, and development of autophagy. Cardiovasc. Res. 114, 1738–1751. https://doi.org/10.1093/cvr/cvy128 (2018).
de Carvalho, L. S. F., Campos, A. M. & Sposito, A. C. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and incident type 2 diabetes: A systematic review and meta-analysis with over 96,000 patient-years. Diabetes Care 41, 364–367. https://doi.org/10.2337/dc17-1464 (2018).
Da Dalt, L. et al. PCSK9 deficiency reduces insulin secretion and promotes glucose intolerance: The role of the low-density lipoprotein receptor. Eur. Heart J. 40, 357–368. https://doi.org/10.1093/eurheartj/ehy357 (2019).
Armentaro, G. et al. Serum proprotein convertase subtilisin/Kexin type 9 and vascular disease in type 2 diabetic patients. Eur. J. Clin. Invest. 53, e13900. https://doi.org/10.1111/eci.13900 (2023).
Azar, Y. et al. Circulating PCSK9 linked to dyslipidemia in Lebanese schoolchildren. Metabolites https://doi.org/10.3390/metabo12060504 (2022).
Lupo, M. G. et al. PCSK9 promotes arterial medial calcification. Atherosclerosis 346, 86–97. https://doi.org/10.1016/j.atherosclerosis.2022.01.015 (2022).
Li, C. et al. Adiponectin inhibits the progression of obesity-associated papillary thyroid carcinoma through autophagy. Endocrinology https://doi.org/10.1210/endocr/bqae030 (2024).
Huang, K. et al. The variation and correlation of serum adiponectin, nesfatin-1, IL-6, and TNF-alpha levels in prediabetes. Front. Endocrinol. 13, 774272. https://doi.org/10.3389/fendo.2022.774272 (2022).
Nielsen, M. B. et al. Plasma adiponectin levels and risk of heart failure, atrial fibrillation, aortic valve stenosis, and myocardial infarction: Large-scale observational and Mendelian randomization evidence. Cardiovasc. Res. 120, 95–107. https://doi.org/10.1093/cvr/cvad162 (2024).
Nagao, H. et al. Correlation between plasma glutamate and adiponectin in patients with type 2 diabetes. Endocr. J. 71, 55–63. https://doi.org/10.1507/endocrj.EJ23-0506 (2024).
Wu, Z. M. et al. PCSK9 inhibitor added to high-intensity statin therapy to prevent cardiovascular events in patients with acute coronary syndrome after percutaneous coronary intervention: A randomized, double-blind, placebo-controlled, Multicenter SHAWN Study. Am. Heart J. https://doi.org/10.1016/j.ahj.2024.06.004 (2024).
Karapapak, M., Kara, Z. M. Y. & Duzgun, E. The predictive utility of circulating PCSK9 levels on diabetic retinopathy stage. Curr. Eye Res. https://doi.org/10.1080/02713683.2024.2386360 (2024).
Gonzalez-Lleo, A. M. et al. Impact of PCSK9 inhibitors in glycaemic control and new-onset diabetes. Cardiovasc. Diabetol. 23, 4. https://doi.org/10.1186/s12933-023-02077-y (2024).
Onodera, T. et al. Protective roles of adiponectin and molecular signatures of HNF4alpha and PPARalpha as downstream targets of adiponectin in pancreatic beta cells. Mol. Metab. 78, 101821. https://doi.org/10.1016/j.molmet.2023.101821 (2023).
Castela, I. et al. Decreased adiponectin/leptin ratio relates to insulin resistance in adults with obesity. Am. J. Physiol. Endocrinol. Metab. 324, E115–E119. https://doi.org/10.1152/ajpendo.00273.2022 (2023).
American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2022. Diabetes Care 45, S17–S38. https://doi.org/10.2337/dc22-S002 (2022).
Wallace, T. M., Levy, J. C. & Matthews, D. R. Use and abuse of HOMA modeling. Diabetes Care 27, 1487–1495. https://doi.org/10.2337/diacare.27.6.1487 (2004).
Maliglowka, M. et al. Insight into the evolving role of PCSK9. Metabolites https://doi.org/10.3390/metabo12030256 (2022).
Zou, Y. et al. Targeting PCSK9 ameliorates graft vascular disease in mice by inhibiting NLRP3 inflammasome activation in vascular smooth muscle cells. Front. Immunol. 13, 894789. https://doi.org/10.3389/fimmu.2022.894789 (2022).
Shi, J. et al. Association of circulating proprotein convertase subtilisin/kexin type 9 levels and the risk of incident type 2 diabetes in subjects with prediabetes: A population-based cohort study. Cardiovasc. Diabetol. 19, 209. https://doi.org/10.1186/s12933-020-01185-3 (2020).
Song, L. et al. Association of PCSK9 with inflammation and platelet activation markers and recurrent cardiovascular risks in STEMI patients undergoing primary PCI with or without diabetes. Cardiovasc. Diabetol. 21, 80. https://doi.org/10.1186/s12933-022-01519-3 (2022).
Saavedra, Y. G. L., Dufour, R. & Baass, A. Familial hypercholesterolemia: PCSK9 InsLEU genetic variant and prediabetes/diabetes risk. J. Clin. Lipidol. 9, 786–793. https://doi.org/10.1016/j.jacl.2015.08.005 (2015).
Awan, Z. et al. Regional distribution and metabolic effect of PCSK9 insLEU and R46L gene mutations and apoE genotype. Can. J. Cardiol. 29, 927–933. https://doi.org/10.1016/j.cjca.2013.03.004 (2013).
Peyot, M. L. et al. Substantial PCSK9 inactivation in beta-cells does not modify glucose homeostasis or insulin secretion in mice. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 1866, 158968. https://doi.org/10.1016/j.bbalip.2021.158968 (2021).
Carugo, S., Sirtori, C. R., Corsini, A., Tokgozoglu, L. & Ruscica, M. PCSK9 inhibition and risk of diabetes: Should we worry?. Curr. Atheroscler. Rep. 24, 995–1004. https://doi.org/10.1007/s11883-022-01074-y (2022).
Fang, H. & Judd, R. L. Adiponectin regulation and function. Compr. Physiol. 8, 1031–1063. https://doi.org/10.1002/cphy.c170046 (2018).
Hafiane, A. Adiponectin-mediated regulation of the adiponectin cascade in cardiovascular disease: Updates. Biochem. Biophys. Res. Commun. 694, 149406. https://doi.org/10.1016/j.bbrc.2023.149406 (2024).
Peng, J., Chen, Q. & Wu, C. The role of adiponectin in cardiovascular disease. Cardiovasc. Pathol. 64, 107514. https://doi.org/10.1016/j.carpath.2022.107514 (2023).
Baldelli, S. et al. The role of adipose tissue and nutrition in the regulation of adiponectin. Nutrients https://doi.org/10.3390/nu16152436 (2024).
Gianopoulos, I., Mantzoros, C. S. & Daskalopoulou, S. S. Adiponectin and adiponectin receptors in atherosclerosis. Endocr. Rev. https://doi.org/10.1210/endrev/bnae021 (2024).
Bauza-Thorbrugge, M. et al. Adiponectin stimulates Sca1(+)CD34(-)-adipocyte precursor cells associated with hyperplastic expansion and beiging of brown and white adipose tissue. Metabolism 151, 155716. https://doi.org/10.1016/j.metabol.2023.155716 (2024).
Karimian, J. & Shekarchizadeh-Esfahani, P. Soy supplementation does not affect serum adiponectin levels in adults: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. Res. 13, 130–138. https://doi.org/10.7762/cnr.2024.13.2.130 (2024).
Begum, M. et al. Adiponectin: A promising target for the treatment of diabetes and its complications. Life https://doi.org/10.3390/life13112213 (2023).
Sasso, F. C. et al. Adiponectin and insulin resistance are related to restenosis and overall new PCI in subjects with normal glucose tolerance: The prospective AIRE Study. Cardiovasc. Diabetol. 18, 24. https://doi.org/10.1186/s12933-019-0826-0 (2019).
Bocian-Jastrzebska, A., Malczewska-Herman, A. & Kos-Kudla, B. Role of leptin and adiponectin in carcinogenesis. Cancers https://doi.org/10.3390/cancers15174250 (2023).
Szumilas, K., Wilk, A., Szumilas, P., Dziedziejko, V. & Pawlik, A. Role of leptin and adiponectin in the pathogenesis of post-transplant diabetes mellitus. Prostaglandins Other Lipid Mediat. 174, 106876. https://doi.org/10.1016/j.prostaglandins.2024.106876 (2024).
Baker, J. F. et al. Associations between adiponectin and the development of diabetes in rheumatoid arthritis. J. Clin. Endocrinol. Metab. https://doi.org/10.1210/clinem/dgae010 (2024).
Gao, S. et al. The effect of circulating adiponectin levels on incident gestational diabetes mellitus: Systematic review and meta-analysis. Ann. Med. 55, 2224046. https://doi.org/10.1080/07853890.2023.2224046 (2023).
Muntean, M. et al. Serum levels of adipolin and adiponectin and their correlation with perinatal outcomes in gestational diabetes mellitus. J. Clin. Med. https://doi.org/10.3390/jcm13144082 (2024).
He, L. et al. The role of adiponectin in the association between abdominal obesity and type 2 diabetes: A mediation analysis among 232,438 Chinese participants. Front. Endocrinol. 15, 1327716. https://doi.org/10.3389/fendo.2024.1327716 (2024).
Acknowledgements
Thank you to all team members for their valuable contributions to this research.
Funding
This study was supported by the Beijing Natural Science Foundation (7222194) and the Natural Science Foundation of Hunan Province (2021JJ41023).
Author information
Authors and Affiliations
Contributions
Jun-Xu Gu: study design and planning. Kun Wang, Ai-Min Zhang, Yue Yin, Shanshan Li, Na Zhang, Li Qin, Chun-Yan Wang, and Lin Pei: data collection, review, and interpretation. Kun Wang, Lin Pei, Jun-Xu Gu, and Ai-Min Zhang: manuscript writing. Jia Mei, Ju-Xu Gu, Lin Pei, and Ming Su: final approval for manuscript submission.All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gu, JX., Wang, K., Zhang, AM. et al. The correlation between proprotein convertase subtilisin/kexin type 9 and adiponectin in the progression from prediabetes to type 2 diabetes mellitus. Sci Rep 15, 8517 (2025). https://doi.org/10.1038/s41598-025-93750-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-93750-7