Abstract
Plant based natural products could be used as new alternative of chemical insecticides due to their co-friendly, safety to non-target organisms, and low-level resistance properties. In this study, the contact toxicity and sublethal effects of Acroptilon repens L., Russian knap weed, extracts (aqueous and methanolic) were evaluated on the biological, population traits and population projection of greenhouse whitefly, Trialeurodes vaporariorum. The essential oil was extracted from the plant by steam distillation using a Clevenger apparatus and the chemical compounds were identified by gas chromatography-mass spectrometry. Twenty-three chemical components of A. repens were characterized, in which caryophyllene oxide (30.67%) and α-copaene (15.05%), were identified as the predominant compounds of Russian knapweed. Moreover, the results illustrated that some secondary metabolites (e.g. flavonoids, alkaloids and polyphenols) were rich in methanolic extract compared to the control. The leaf dipping method was used for the bioassay tests against whiteflies. According our findings, the aqueous extract (LC50: 1802.59 ppm) was more toxic than the methnolic treatment (LC50: 3849.15 ppm) on the T. vaporariorum adults. The age-stage, two-sex life table theory was used to analyze the life table data. The sublethal concentration of either aqueous or mehanolic extracts of A. repens significantly affected the biological and population growth parameters of T. vaporariorum compared to the control by prolongation the developmental period, adult longevity, reducing the survival rate, fecundity and decreasing the net reproductive rate (R0), intrinsic rate of increase (r), and finite rate of increase (λ) of adults, as well. The overall results demonstrated that both of the Russian knapweed extracts could be considered in management programs of greenhouse whitefly. However, the cost-effective property of aqueous extract should not be neglected.
Similar content being viewed by others
Introduction
Whiteflies are the most common and problematic insect pests on vegetable and ornamental crops1. Among them, the greenhouse whitefly, Trialeurodes vaporariorum, Westwood (Hemiptera: Aleyrodidae), is a common pest and well established in the greenhouse ecosystem, and it is one of the most important economic pests of various greenhouse vegetables, particularly in tomatoes, cherry tomatoes, and cucumber crops2,3,4. Either adults or nymphs cause substantial damages to plants directly, through ingestion of phloem sap, and indirectly, by secretion large amounts of honeydew which supports the growth of sooty mold2. Furthermore, it is known as a carrier of some plant viruses’ pathogens including, Bean Golden Mosaic Virus (BGMV), Tomato Yellow Leaf Virus (TYLCV), and Beat Pseudo Yellow Virus (BPYV), which resulted in losses to tomato and cucurbit crops3,5.
Control of whiteflies is primarily based on chemical insecticides in Iran3,4,5,6. The overuse of insecticides developed the resistance of whiteflies population to numerous conventional insecticides especially pyrethroids7, neonicotinoids8 and ketoenols9. Additionally, the extensive usage of chemical insecticides led to the reduction of non-target insects’ population (e.g., natural enemies, pollinators), environmental pollution and increasing the toxicological risks for farmers and consumers6,10. That is why, the worker safety and attempt to produce organic and healthy products has gained importance in enclosed system such as greenhouse than outdoor or field spaces, therefore, it is essential to develop alternative strategies for pest management in greenhouse system11.
Given the resistance potential of the greenhouse whitefly to conventional insecticides, much effort has been focused on the use of biopesticides with plant-based origin phytochemicals as potential sources of commercial insect control agents in integrated pest management (IPM) program2,3. Over the past two decades, sources for remedies and control of insect pests were shifted to the natural resources and numerous extracts or essential oils from indigenous plants and have evaluated against pests. The use of plant compounds, such as plant extracts, is an increasing interest due to their fewer side effects on non-target organisms and low-risk to environment compared to conventional insecticides and are easily decomposed in soil and are not stored in plants or animals12,13.
Acroptilon repens, Russian knapweed, (Asteraceae) belongs to the Asteraceae family. A. repens not only known as a persistent weed but also it could be used as an herbal insecticide. It has been widely grown and reported from northwestern of Iran such as West Azarbayjan, Zanjan, and Tehran14,15. The aerial tissues of A. repens possess aromatic amines, and sterols16, as well as sesquiterpene lactones17,18. The potential of A. repens refers to the existence of polyphenolic and triterpene constituents, which in turn enhance its biological features, including repellency, contact, fumigant toxicity, anti-oviposition, and antifeedant activity against insect pests19,20,21,22, by altering the insect behavior and promote physiological changes, in addition to causing mortality in insect pest populations.
All of the previous reports considered the insecticidal activity of medicine plants or edible plants. There are a few studies on the insecticidal activity of A. repens extracts, as a weed, against agricultural and medical pests. For instance, the insecticidal properties of A. repens extract were investigated in China against some species of Lepidopteran larvae20. In the other study, Toolabi et al.21 surveyed on the larvicidal activity of methanolic extract of A. repens against third instar larvae of some medical pests (e.g., Anopheles stephensi Liston, Culex pipiens Linnaeus and C. quinquefaciatus Say), in which An. stephensi, was more susceptible than other mosquitoes.
Plant based insecticides cause toxic effects at both lethal and sublethal levels which refer to the existence of secondary plant metabolites. They are involved in the cellular and physiological processes by disruption in redox balance, hormonal regulation, neuronal signalization or reproduction in exposed individuals of insect pest population, which in turn led to prolonged lifespan, reduced the fecundity, survival rate and deformities in parental and offspring generations23. Demographic parameters estimation through life table analysis is an essential approach for ecological predictions of population growth, which are valuable for pest management24,25,26. However, there were previous reports about the contact activity of essential oils, such as Thymus vulgaris, Mentha piperita1,2, Trachyspermum ammi, Withania coagulans and Murraya koenigii27, Piper marginatum Jacq. and Manosa alliaceae Miers10 against the different whitefly species. Insecticidal activity of 53 essential oils was assessed against on developmental stage of T. vaporariorum (eggs, nymphs, and adults) by Choi et al.28. They found that the oil type, usage dose, and developmental stage of the insect pests are involved in their susceptibility to essential oils. Mahmoodi et al.29 stated that Petroselinum crispum L. (Apiaceae) essential oil has fumigant toxicity on T. vaporariorum adults under greenhouse conditions. A survey on the anti-oviposition and repellency activities of essential oils and aqueous extracts from five aromatic plants against greenhouse whitefly30 illustrated that the most significant repellency effect occurred 3 and 6 days after infestation with aqueous extracts of Cuminum cyminum L. and Tymus vulgaris.
Due to the specific biological properties of greenhouse whitefly (high dispersion potential, competitive ability, and resistance to conventional insecticides), it is necessary to study alternative tactics to control this pest7,8,9. Our literature review show that the effects of A. repens has already been studied in terms of lethal toxicity for T. vaporariorum but not for sublethal effects. In light of this, this study aimed to assess the biological features of Russian knapweed for on the biological and population growth parameters of greenhouse whitefly.
Results
Chemical constituents of A. repens
The chemical constituents, Kovats index, retention time, and percentage of A. repens are shown in Table 1. The results indicated about 23 compounds in which, caryophyllene oxide (30.67%) and α-copaene (15.05%) were two predominant abundant constituents of A. repens (Table 1).
The flavonoid, phenol, and alkaloid contents of the aerial parts of aqueous and methanolic extracts of A. repens
The total flavonoids, phenol, and alkaloid contents in the aerial parts of the methanolic extract of A. repens are shown in Table 2. The results showed that methanolic extract of Russian knapweed affected the amount of total flavonoids, phenol, and alkaloid. Based on the results of the t-test (α = 5%), these differences were significant (Table 2). The average flavonoid concentration in methanolic extract of A. repens (11.16 ± 0.13 mg/g DW), was significantly more than control treatment (6.21 ± 0.01 mg/g DW). Similarly, Comparison of showed a significant difference based on the t-test (α = 5%) between the control and methanolic extract of A. repens. The lowest amount of the phenol and alkaloid contents were obtained in the control group.
Insecticidal activity of the aqueous and methanolic extracts of A. repens against greenhouse whitefly
The median lethal (LC50) and sublethal (LC25) concentrations of the aqueous and methanolic extracts of A. repens against T. vaporariorum adults are shown in Table 3. Probit analysis revealed that the median lethal (LC50) and sublethal (LC25) concentrations of the aqueous and methanolic extracts of A. repens against T. vaporariorum adults were obtained (1802.60 and 904.360 ppm) and (3849.15 and 1221.78 ppm), respectively. The aqueous extract was more toxic against greenhouse whitefly than the methanolic extract (Table 3).
Sublethal effects of the aqueous and methanolic extracts of A. repens on the biological traits of F1 generation of the greenhouse whitefly
The sublethal effects of A. repens on the biological traits of greenhouse whitefly exposed to the aqueous and methanolic extracts of A. repens are illustrated in Table 4. The shortest egg incubation period in the greenhouse whitefly’s F1 generation observed in control group. However, there were no significant differences between extracts of A. repens in terms of egg incubation period. Exposure to sublethal concentration of A. repens extracts resulted in prolonged developmental time of the greenhouse whitefly and it was shorter in the control group than those exposed to the both extracts of A. repens. The longest female (7.36 ± 0.32) and male (4.41 ± 0.39 days) longevity were recorded in the control group, while the sublethal concentrations of either aqueous or methanolic extracts led to shortened adult longevity of greenhouse white fly. The shortest and longest TPRP were obtained by control (22.68 ± 0.29 days) and methanolic extract of A. repens (27.18 ± 0.76 days) populations, respectively (Table 4). Additionally, the highest reproduction rate (35.52 ± 2.47 eggs/female) was obtained in control groups compared to both treatments.
Sublethal effects of A. repens extracts on the population growth parameters of T. vaporariorum
Our results indicated that exposure to sublethal concentration of aqueous and methanolic extracts of A. repens decreased the population growth parameters including the net reproductive rate, the intrinsic rate of increase, and the finite rate of increase of the greenhouse whitefly. However, the treatment with methanolic extract led to prolonged mean generation time of greenhouse whiteflies (Table 5).
Sublethal (LC25) effects of A. repens extracts on the age-stage specific survival rate (s xj), fecundity (m x), and maternity (l x m x), life expectancy (e xj) and reproductive value (v xj) of F1 generation of the greenhouse whitefly
The sublethal effects of A. repens aqueous and methanolic extracts on the age-stage specific survival rate (sxj) of greenhouse whiteflies’ F1 generation are presented in Fig. 1. The survival probability of preadult greenhouse whiteflies was considerably lower in the aqueous extract of Russian knapweed and the adult females emerged with delay in both treatments compared to control group. The age-specific survival rate (lx), fecundity (mx) and the maternity (lxmx) of the greenhouse whiteflies are demonstrated in Fig. 2. The appearance of the peaks of the age-specific fecundity (mx) and age-specific maternity (lxmx) occurred with delay in the methanolic group. The age-stage-specific life expectancy (exj) of T. vaporariorum which treated with sublethal concentration of A. repens extracts, was decreased compared to control group (Fig. 3). The life expectancy in the first day of the adult emergence were obtained 10.04, 6.51 and 3 days in the control, methanolic and aqueous populations, respectively. Exposure to the sublethal effects of A. repens aqueous and methanolic extracts affected the age-stage specific reproductive value (vxj) of greenhouse whiteflies’ F1 generation (Fig. 4). The earliest and highest reproductive value peaks were observed in the control group (v21 = 27.81). The peak values obtained for the greenhouse whiteflies populations exposed to extracts of A. repens were close to each other, although occurred on different days (v27 = 7.58, v22 = 7.5 for methanolic and aqueous extracts, respectively).
Population projection
Population projection results illustrated that methanolic and aqueous extracts of A. repens have a notable effect on the predicted population growth of the greenhouse whitefly. Exposing T. vaporariorum to methanolic extract of Russian knapweed resulted in decreasing the population size in the greenhouse whitefly. However, the population size of the greenhouse whitefly was increased in the control group after 60 days (Figs. 5 and 6).
Discussion
According to GC–MS analysis, 23 components were identified in A. repens, in which caryophyllene oxide (30.67%) and α-copaene (15.05%), were characterized as the predominant compounds of Russian knapweed. Previously published studies also identified some of the same main compounds, too (e.g., caryophyllene oxide, α-copaene)17,18. The results reported by Norouzi-Arasi et al.17 were approximately in the same range (36.6%; 15.6%) for caryophyllene oxide and α-copaene, respectively. However, the amount of caryophyllene oxide and α-copaene (6.4%; 22.8%) reported by Tunalier et al.18, were different than those of our findings. The geographical position, may be one of the reasons for these discrepancies31. Plants origin, harvesting time, the procedure of essential oils extraction and geographical position are involved in quantitative differences of A. repens chemicals2,31.
Due to the existence of complex chemical composition in plant-based insecticides, they have multiple modes of action and broad physiological activity, which in turn reduce the probability of developing resistance of polyphagous pests such as greenhouse whitefly to their host plants2,15,32.
Russian knapweed has a various biological features including repellency, contact, fumigant toxicity, anti-oviposition, and antifeedant activity against insect pests19,20,21,22 which related to the abundance of plant secondary metabolites (e.g., alkaloids, glycoalkaloids, terpenoids, organic acids, phenolic compounds, terpenes, essential oils, saponins, flavonoids, and alcohols) in A. repens19,20,21.
There are some documents in terms of insecticidal properties of A. repens against medical pests20,33 and some limited agricultural pests20,22. In the current study, the total content of some secondary metabolites of Russian knapweed (polyphenolics, alkaloids, flavonoids, and terpenes) were the highest in methanolic extract than aqueous extract, but according to obtained results by GC–MS analysis, the sesquiterpenes, class of terpenoids, were more effective in the lethal activity of A. repens, which is consistent with Tunalier et al.18 findings. They stated that the sesquiterpenes, were the most amount (56%) among identified compounds of A. repens. In the current study, the contact toxicity of Russian knapweed may be due to the major amount of caryophyllene oxide as a sesquiterpene. There are previous reports about contact activity of caryophyllene oxides34,35,36,37 against agricultural insects, and the results some of them are consistent with our findings. However, for the contact toxicity of T. vaporariorum, it is not found in previous studies. Ma et al.38 isolated and identified five insecticidal compounds from Cephalotaxus sinensis essential oil, in which caryophyllene oxide was one of the main active constituents exhibited potent contact and fumigant activity against broad spectrum agricultural pests (e.g., Megoura japonica Okamoto & Takahashi, Sitophilus zeamais Motschulsky and Plutella xylostella (Linnaeus). According to our obtained results, it is surprising that the aqueous extract of A. repens was more active and toxic than the methanolic extract of Russian knapweed. It may be due to the chemical structure of sesquiterpenes. Wang et al.20 using immersion method, demonstrated that the existence of ethyl acetate and petroleum ether fraction from the extract of A. repens in florescence stage of whole plant had very strong contact toxicity against fifth instar larva of M. separate.
Secondary plant metabolites cause toxic effects that can be observed at either lethal or sublethal levels, which can ultimately lead to the death of insect pests32. The results of previous investigations evaluated the short-term toxicity of A. repens and there is no documents regarding the sublethal effects of Russian knapweed against any insect pests in long term. Hence, it is essential to obtain a comprehensive perception, regards the ecotoxicological effects of Russian knapweed in the management of insect pests’ population3,25. Our research showed that, both sublethal concentration of aqueous and methanolic extracts of Russian knapweed led to delay in incubation period of eggs, extending the developmental time, prolonged the preadult oviposition period, reduction of survival rate and fecundity compared to control group. It may be due to the antifeedant and repellency effects of phytochemical compounds of A. repens, which in turn cause disruption in cellular and physiological processes in exposed individuals of insect pest by altering the hormonal regulation, neuronal signalization or reproduction3,22,25,32. Similarly, Sharifiyan et al.2 reported the same sublethal biological effects of M. pulegium essential oil and its nanoformulation against greenhouse whitefly.
Our results demonstrated that exposure of the adults to sublethal concentrations of both used extracts had noticeable effects on the population growth parameters by reducing the net reproductive rates (R0), the intrinsic rates of increase (r), the finite rates of increase (λ). However the methanolic extract of A. repens prolonged the mean generation time of the greenhouse whitefly progeny more than the aqueous extract, then followed by the control group.The sublethal effects of A. repens on the aforementioned population growth parameters in this research was more effective than M. pulegium and its nanofrmulation on the adults of T. vaporariorum2. This variation could be due to differences in the bioassay method, the plant species and the chemical structure of the used essential oil, the exposure time of insects, as well2,28. Surprisingly, in the current study, both studied extracts of Russian knapweed were more effective than even those of flonicamide as a chemical insecticide and Sophora flavescens on population dynamics of greenhouse whitefly3, which shows that it could be consider in management of T. vaporariorum.
We also used the obtained life table results to illustrate the dynamics of stage structure and variability of population growth whitefly based on a computer projection program39. The results showed that population growth of the greenhouse whitefly in different treatments was exactly in consistent with the estimated population growth parameters. Therefore, the population projection results would be a good evidence for the population growth rate of the progeny whose parents were exposed to the sublethal concentration of A. repens extracts. For example, insects treated with methanolic extract of Russian knapweed, had slower growth rate and, thereby prolonged mean generation time (T).
In conclusion, the sublethal effects of methanolic and aqueous extracts of Russian knapweed against T. vaporariorum were reported in the current study for the first time. Based on the obtained LC25 and LC50 values, either aqueous or methanolic extracts of A. repens have the potential to be developed as an active plant natural insecticide for the control of greenhouse whitefly. However, due to the cost-effective being of aqueous extract it could be more considered in pest management programs. Further study should be conducted to determine the side effects of A. repens on the greenhouse whiteflies’ enemies and its nanoformulated efficacy against T. vaporariorum in greenhouse and open field, as well.
Material and methods
Sample collection
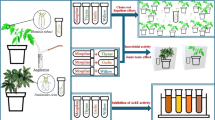
Aerial parts of A. repens was collected from Vaqasluy-e Sofla (45° 1′ E, 37° 39′ N), West Azerbaijan, Iran during the summer season 2021. The identification of A. repens was occurred by Dr. Heydari, and deposited at the herbarium at Agricultural Research, Education and Extension Organization (AREEO), West Azerbaijan, Urmia, Iran with voucher specimen 106606.
Sample preparation for GC–MS analysis and identification of chemical constituents of A. repens
The plants were dried at room temperature until they were crispy. Then, they were powdered entirely by an electric mill and stored in a dark glass container in a refrigerator. Then, they were subjected to hydrodistillation. The eextraction was done using a modified Clevenger-type apparatus. Five hundred fifty grams of the powdered plant was added in 500 ml of distilled water and the extraction processes was continued for 4 h. After extraction, anhydrous sodium sulfate was used to dehydrate the EO. The isolated oils were poured in darkness vials and stored at 4 °C in a refrigerator. Analysis of chemical constituents of A. repens were performed phytochemistry laboratory in Research Institute of Forests and Rangelands, Tehran, Iran. The identification of A. repens was performed on an Agilent 7890A/5975C GC–MS system equipped with a DB-5 fused silica column (30 m × 0.25 mm i.d., film thickness 0.25 μm). The oven temperature was programmed as follows: The initial temperature of 60 °C was increased immediately to 220 °C at a rate of 3 °C/min, subsequently the temperature was increased to 260 °C at 20 °C/min and held in this temperature for 5 min. The injector and transfer line temperatures were 260 °C and 280 °C, respectively. Helium gas was selected as a carrier gas with linear velocity of 30.6 cm/s; split ratio 1:100; ionization energy 70 eV, scan times 1s; and mass range 40–300 a.m.u.
Measurement of some secondary metabolites
To sample preparation of flavonoids and phenols, we used the method of Golizadeh et al.40 with a slight modification. Briefly, 0.5 g of milled sample were dissolved in 5 ml of 80% methanol, then extracted using an ultrasonic device at a temperature of 30 °C for 30 min. After that, they were centrifuged at 10,000 × g for 15 min and 4 °C. The supernatant was used for the measurement of flavonoid and polyphenol content.
The flavonoid content of A. repens extracts was determined according to the procedure of Chang et al.41 with some modifications using Quercetin as a standard (0.627–10 mg ml−1). Briefly, 15 µl of extracted sample of A. repens, 500 µl of 10% Aluminium chloride (AlCl3), and 500 µl of Potassium acetate 1M were dissolved in 1.4 ml distilled aqueous and incubated in darkness at room temperature for 30 min, then centrifuged at 10,000 × g for 10 min. The absorbance was read at 415 nm using a spectrophotometer (UV-2100).
Folin-Ciocalteu and Gallic acid (0.627–10 mg ml−1) were used as reagent and standard, respectively to measure polyphenol content of A. repens, as described by Slinkard and Singleton42. Briefly, 5 µl of extracted sample of A. repens, 1200 µl of 10% Folin-Ciocalteu reagent, 960 µl 7% Sodium carbonate (Na2CO3), were dissolved in 180 µl distilled aqueous and incubated in darkness at room temperature for 30 min, then centrifuged at 10,000 × g for 10 min. The absorbance was measured at 760 nm for polyphenol using a spectrophotometer. Three technical replications were carried out for each treatment.
To measure alkaloid content of A. repens, Boromocresole green and Hyoscyamine (0.4–1.2 mg ml−1) were used as reagent and standard, respectively43. Briefly, 1 g of milled sample was dissolved in 20 ml of 80% methanol, then shake on shaker for 24 h. After that, it was filtered with filter paper. The evaporation of methanol was conducted using a rotary at 45 °C in a dark condition. Next, the concentrated sample was dissolved in 1ml Hydrogen chloride (HCl 2N) and filtered. After that, 1 ml of the obtained sample was washed in 10 ml Chloroform (pH = 7, NaOH 0.1N). Finally, 5 ml Boromocresole green reagent, 5ml Phosphate buffer (pH = 4.7) and 4ml Chloroform were added to the aforementioned solution and shake well. The absorbance was measured at 470 nm for alkaloids using a spectrophotometer (Halo-DB-20 UV visible). Three technical replications were carried out for each treatment.
Aqueous and methanolic extraction to study lethal and sublethal
According to Samareh-Fekri22, the immersion method was used to obtain the methanolic and aqueous extracts of A. repens. In this method, 50 g of the powdered plant was soaked in 300 ml of solvents (aqueous and 80% methanol) and placed on a shaker at room temperature for 48 h, then the obtained extracts were passed through filter paper twice and concentrated by a rotary vacuum distillation machine at 40 °C with speed of 100 rpm. The resulting concentrated liquid was spread on a watch glass and placed in a dark place until the solvent was removed entirely, and the extract was obtained as a powder. Concentrated extracts were stored in dark-colored lidded jars inside the refrigerator at 4 °C.
Host plant breeding and insect rearing
The host plant used in this study, was tomato (variety Sun-seed 6189) which obtained from the Plant and Seed Modification Research Institute (Urmia, Iran). Tomato seeds were grown in plastic seedling trays (29 mm in height and 42 mm in diameter) containing a mixture of manure, peat moss, and vermiculite (in a ratio of 1:1:1) in a greenhouse. Finally, the seedlings (6–8 leaflets) were transferred into plastic pots (15 × 20 cm).
The rearing colony of T. vaporariorum was performed by gathering the adults of greenhouse whitefly using aspirator on tomatoes from the greenhouse of Department of Plant Protection, Urmia University, Urmia, Iran. The adults were released into cages (80 × 80 × 100 cm) on tomato pots. To obtain the same-aged greenhouse whitefly, the released adults were removed after 24 h. The colony was monitored until they reached the desired stage for carrying out the bioassays. The insect rearing was done for three generations prior to bioassay tests and demographic studies. The colony was kept in a growth chamber at 25 ± 2 °C and 60 ± 10% RH at a photoperiod of 16: 8 h (L:D).
Dose-mortality response bioassay to obtain the LC50 and LC25 of both aqueous and methanolic extracts of Russian knapweed
To obtain dose-mortality response for adult stage of greenhouse whitefly, we followed the bioassay methods described by Reshadat-Salvanagh et al.3 with some modifications. Five final concentrations in the ranges of (39.06–2500 and 312–7000 ppm) were used for aqueous and methanolic extracts, respectively, which were obtained based on preliminary tests causing the mortality range between 10 and 90%. The leaf dipping method was used in bioassay tests. For this purpose, the tomato leaves were dipped in 500 ml of each aforementioned concentration of aqueous and methanolic extracts of A. repens for 10 s and the treated leaves were air-dried for 10 min at room temperature, then put into the ventilated plastic Petri dishes (8 cm in diameter). Next, 15 adults of greenhouse whiteflies released onto the treated leaves. Distilled aqueous was used as a control. Four replicates were set up for each treatment and control. The number of dead adults were counted 24 h after treatment. The basal ends of the tomato leaflets were wrapped in moistened cotton wicks, to avoid the desiccation of the leaves. The lethal (LC50) and sublethal (LC25) concentrations, and their 95% confidence limits in each treatment were obtained using the Probit analysis44 which conducted by SPSS software (Ver. 20, 2011). The bioassay test was conducted in a growth chamber at 25 ± 2 °C and 60 ± 10% RH at a photoperiod of 16: 8 h (L:D).
Sublethal effects (LC25) of aqueous and methanolic extracts of A. repens on demographic parameters of T. vaporariorum
The sublethal effects of methanolic (1221.788 ppm) and aqueous extraction (904.36 ppm) were studied on the life history and population growth rate of T. vaporariorum, which was carried out according to Reshadat-Salvanagh et al.3 method with some modifications. Briefly, tomato leaflets were dipped in aforementioned sublethal concentrations of treatments for 10 s and transferred into Petri dishes (8 cm diameter), then 150 numbers of the same-aged adults of T. vaporariorum were released on treated leaves for each treatment (50 numbers of adult with three replicates). After 24 h, all alive adults were separated with aspirator and released on untreated tomato-pots and allowed them to laid their eggs for 24 h, then the adults were removed. To demographic and life table studies, 75, 84, and 73 the same-aged of their eggs were used as a cohort for control, aqueous and methanolic extract of A. repens, respectively. Due to the some biological properties of greenhouse whitefly (eggs attachment on the surface of the leaves and slow movement behavior of pre-imaginal stages), and to avoid any hurt to eggs during transferring, the leaves containing one egg were preserved on tomato pots. Then, they were bounded by the clip cages and, the incubation period, developmental period and survival of preadult stages were recorded for each individual daily. Without any manipulating of the old leaf which containing the preimaginal stages of greenhouse whitefly, the new and fresh leaf was placed on the underside of the old leaf so that the insect would move to the lower leaf automatically. After adult emergence, they were differentiated sexually by Gerling and Sinai45 method. Each pair was transferred in a transparent plastic container (5 cm in diameter × 7 cm in height) containing tomato leave. The total pre-reproductive period (TPRP), adult pre-reproductive period (APRP), the number of eggs produced, number of reproductive days, adult longevity (in days), and total lifespan of the progeny of the treated parents were recorded daily. The experimental conditions were used as the same conditions which mentioned earlier.
Data analysis
The comparison between methanolic extract of A. repens and control in terms of flavonoid, phenols, and alkaloid contents was performed by T-test at the 95% probability level using (SPSS, Ver. 20, 2011). The life history data of the greenhouse whitefly were analyzed according to the age-stage, two-sex life table theory46,47 using TWOSEX–MSChart computer program48. According to Efron and Tibshirani49 method, the variance and standard errors of the development time, reproduction time, fecundity, and population parameters were analyzed via a the bootstrap approach with 100,000 replicates. To assign the differences among treatments, we used the paired bootstrap test at a 5% significance level based on the confidence interval of differences. Bootstrap method and paired bootstrap test are included in the TWOSEX–MSChart of the computer program. Also, the life table data was used to project the population growth of T. vaporariorum using the computer program TIMING-MSChart50 based on Chi40 theory. Additionally, the life table’s data based on the 0.025th and 0.975th bootstrap results of the net reproductive rate were used to obtain the uncertainty of population growth of T. vaporariorum51.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Aroiee, H., Mosapoor, S. & Karimzadeh, H. Control of greenhouse whitefly Trialeurodes vaporariorum by thyme and peppermint. J. KMITL Sci. 5(2), 511–514 (2005).
Sharifiyan, M., Mehrkhou, F. & Negahban, M. Sublethal effects of nanoformulated Mentha pulegium L. essential oil on the biological and population growth parameters of the greenhouse whitefly Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Sci. Rep. 14, 27357 (2024).
Reshadat Salvanagh, N., Mehrkhou, F. & Fourozan, M. Lethal and sublethal effects of Flonicamid and BIo2 on life span and population growth parameters of greenhouse whitefly Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Phytoparasitica. 52(90) (2024).
Dadras, S., Mehrkhou, F., Atlihan, R. & Fourouzan, M. The sublethal effects of thiamethoxam-lambda cyhalothrin on the population growth performance of greenhouse whitefly, Trialeurodes vaporariorum, and its parasitoid, Encarsia formosa, (Hymenoptera: Aphelinidae) under laboratory conditions. J. Entomol. Soc. Iran. (2024) (in press).
Safavi, S. A. & Bakhshaei, M. Biological parameters of Trialeurodes vaporariorum (Hemiptera: Aleyrodidae) exposed to lethal and sublethal concentrations of Calypso®. J. Crop Protect. 6(3), 341–351 (2017).
Sohrabi, F., Shishehbor, P., Saber, M. & Mosaddegh, M. S. Lethal and sublethal effects of buprofezin and imidacloprid on Bemisia tabaci (Hemiptera: Aleyrodidae). Crop Protect. 30(9), 1190–1195 (2011).
Karatolos, N., Gorman, K., Williamson, M. S. & Denholm, I. Mutations in the sodium channel associated with pyrethroid resistance in the greenhouse whitefly, Trialeurodes vaporariorum. Pest Manag. Sci. 68(6), 834–838 (2012).
Pappas, M. L., Migkou, F. & Broufas, G. D. Incidence of resistance to neonicotinoid insecticides in greenhouse populations of the whitefly, Trialeurodes vaporariorum (Hemiptera: Aleyrodidae) from Greece. Appl. Entomol. Zool. 48, 373–378 (2013).
Kapantaidaki, D. E. et al. Insecticide resistance in Trialeurodes vaporariorum populations and novel diagnostics for kdr mutations. Pest Manag. Sci. 74(1), 59–69 (2018).
Santana, A. da Silva., Baldin, E. L. L., Lima, A. P. S., dos Santos, T. L. B., Santos , M. C., Vieira, T. M., Crotti, A. E. M. & Takeara, R. New challenges demand new solutions: Selected essential oils as an alternative to control Bemisia tabaci MED in Brazil. Crop Protec. 155, 105909. 1–7 (2022).
İlter, E. Altındişli A Ecological Agriculture and Its Principles 1–6 (Ecological Agriculture, 1996).
Bayramzadeh, N., Mehrkhou, F., Pourmirza, A. A. & Mahmoudian, M. Fumigant toxicity of two nano-capsulated essential oils with sublethal rate of phosphine against three stored-product pests. J. Agric. Sci. Tech. 21(4), 857–872 (2019).
Heydarzade, A., Valizadegan, O., Negahban, M. & Mehrkhou, F. Efficacy of Mentha spicata and Mentha pulegium essential oil nanoformulation on mortality and physiology of Tribolium castaneum (Col.: Tenebrionidae). J. Crop Protect. 8(4), 501–520 (2019).
Razavi, S. M., Narouei, M., Majrohi, A. A. & Mohammaddust Chamanabad, H. R. Chemical constituents and phytotoxic activity of the essential oil of Acroptilon repens (L.) DC from Iran. J. Essent. Oils Bear. Plants. 15(6), 943–948 (2012).
Akhgari, Z., Tanomand, A., Nazari, R. & Zargar, M. Biological and antibacterial features of Acroptilon repens (L.) DC. Cres. J. Med. Biol. Sci. 9(2), 116–122 (2022).
Mallabaev, A., Saitbaeva, I. M. & Sidyakin, G. P. Components of Acroptilon repens. Khim. Prir. Soedin. 1, 123 (1982).
Norouzi-Arasi, H. et al. Chemical constituents and antimicrobial activities of the essential oil of Acroptilon repens (L.) DC. Flavour Fragr. J. 21, 247–249 (2006).
Tunalier, Z., Candan, N. T., Demirci, B. & Baser, K. H. C. The essential oil composition of Acroptilon repens (L.) DC. of Turkish origin. Flavour Fragr. J. 21, 462–464 (2006).
Cis, J., Nowak, G. & Kisiel, W. Antifeedant properties and chemotaxonomic implications of sesquiterpene lacttones and syringing from Rhaponticum pulchrum. Biochem. Syst. Ecol. 34(12), 862–867 (2006).
Wang, X. H. et al. Insecticidal activity of fraction from Acroptilon repens against larvae of Lepidoptera. Southwest China J. Agri. Sci. 28(3), 1124–1129 (2015).
Toolabi, R. et al. Larviciding activity of Acroptilon repens extract against Anopheles stephensi, Culex pipiens and Culex quinquefaciatus under laboratory conditions. Pharmacog. J. 10(3), 453–456 (2018).
Samareh-Fekri, M. Effect of repllency and oviposition deterrence of plant extract and powder of Acroptilon repence (Asteraceae) and Sophora aculeopoides (Fabaceae) on Saw toothed grain beetle (Oryzaelhylus surinamensis) in laboratory conditions. J. Entomol. Res. 12(2), 103–117 (2020).
Stark, J. D. & Banks, J. E. Population-level effects of pesticides and other toxicants on arthropods. Annu. Rev. Entomol. 48(1), 505–519 (2003).
Ramzi, A. et al. Insecticidal effect of wild-grown Mentha pulegium and Rosmarinus officinalis essential oils and their main monoterpenes against Culex pipiens (Diptera: Culicidae). Plants. 11(9), 1193 (2022).
de França, S. M., Breda, M.O., Barbosa, D. R. S., Araujo, A. M. N., & Guedes, C. A. The sublethal effects of insecticides in insects. In: Biological Control Pest Vector Insect (eds. Shields, V. D.C.), 23–39 (2017).
Liang, P. Z. et al. Toxicity and sublethal effects of flupyradifurone, a novel butenolide insecticide, on the development and fecundity of Aphis gossypii (Hemiptera: Aphididae). J. Econ. Entomol. 112(2), 852–858 (2019).
Hyder, M., Li, Y., Wang, M., J. Mao, J., Mari, J. M., Bukero, A., Soomro, H. U., Bukero, A. A. & Zhang, L. Insecticidal activity, Chemical Constituents of Trachyspermum ammi, Withania coagulans and Murraya koenigii ethanloic extracts against Bemisia tabaci, Braz. J. Biol. 84, 260298 (2022).
Choi, W. I., Hoi, E.-H., Lee, H. E. H., Choi, B. R., Park, H. M. & Ahn, A. Y. Toxicity of plant essential oils to Trialeurodes vaporariorum (Homoptera: Aleyrodidae). J. Econ. Entomol. 96(5), 1479–1484 (2003).
Mahmoodi, L., Valizadegan, O. & Mahdavi, V. Fumigant toxicity of Petroselinum crispum L. (Apiaceae) essential oil on Trialeurodes vaporariorum (Westwood) (Hemiptera: Aleyrodidae) adults under greenhouse conditions. J. Plant Protect. Res. 54 (3), (2014).
Dehghani, M. & Ahmadi, K. Anti-oviposition and repellence activities of essential oils and aqueous extracts from five aromatic plants against greenhouse whitefly Trialeurodes vaporariorum Westwood (Homoptera: Aleyrodidae). Bulg. J. Agric. Sci. 19(4), 696–701 (2013).
Vasile, C., Sivertsvik, M., Mitelut, A. C., Brebu, M. A., Stoleru, E., Rosnes, J. T., T˘anase, E. E., Khan, W., Pamfil, D., Cornea, C. P., Irimia, A. & Popa, M. E. Comparative analysis of the composition and active property evaluation of certain essential oils to assess their potential applications in active food packaging. Materials. 10, 45 (2017).
Chowa´nski, S., Adamski, Z., Marciniak1, P., Rosi´nski, G., Büyükgüzel, E.,Büyükgüzel, K., Falabella, P., Scrano, L. Ventrella, E. Lelario, F. & Bufo, S. A. review of bioinsecticidal activity of solanaceae alkaloids. Toxins. 8, 60 (2016).
Nararak, J. et al. Excito-repellent activity of β-caryophyllene oxide against Aedes aegypti and Anopheles minimus. Acta Tropica. 197, 105030 (2019).
Guo, S. S. et al. Chemical composition and bioactivities of the essential oil from Etlingera yunnanensis against two stored product insects. Molecules. 20, 15735–15747 (2015).
Oh, M. S., Yang, J. Y., Kim, M. G. & Lee, H. S. Acaricidal activities of β-caryophyllene oxide and structural analogues derived from Psidium cattleianum oil against house dust mites. Pest Manag. Sci. 70, 757–762 (2024).
Cárdenas-Ortega, N. C. et al. Composition of the essential oil of Salvia ballotiflora (Lamiaceae) and its insecticidal activity. Molecules. 20, 8048–8059 (2015).
Bett, P. K. et al. Chemical composition of Cupressus lusitanica and Eucalyptus saligna leaf essential oils and bioactivity against major insect pests of stored food grains. Ind. Crop. Prod. 82, 51–62 (2016).
Ma, S., Jia, R., Guo, M., Qin, K. & Zhang, L. Insecticidal activity of essential oil from Cephalotaxus sinensis and its main components against various agricultural pests. Ind. Crop. Prod. 150, 112403 (2020).
Chi, H. Timing of control based on the stage structure of pest populations: a simulation approach. J. Econ. Entomol. 83, 1143–1150 (1990).
Golizadeh, M., Mehrkhou, F., Atlihan, R. & Güz, N. The biological and physiological responses of Leptinotarsa decemlineata Say (Col. Chrysomelidae) to different potato cultivars. Pest Manag. Sci. 78 (9) (2022).
Chang, C. C., Yang, M. H., Wen, H. M. & Chern, J. C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 10(3), 178–182 (2002).
Slinkard, K. & Singleton, V. L. Total phenol analysis: automation and comparison with manual methods. Am. J. Enol. Vitic. 28(1), 49–55 (1977).
Ajanal, M., Gundkalle, M. B. & Nayak, S. U. Estimation of total alkaloid in Chitrakadivati by UV-spectrophotometer. Anc. Sci. Life. 31(4), 198–201 (2012).
Finney, D. Probit Analysis (Cambridge University Press, 1971).
Gerling, D. & Sinai, P. Buprofezin effects on two parasitoid species of whitefly (Homoptera: Aleyrodidae). J. Econ. Entomol. 87(4), 842–846 (1994).
Chi, H. & Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 24, 225–240 (1985).
Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 17(1), 26–34 (1988).
Chi,H.TWOSEX-MSChart: a computer program for age stage, two-sex life table analysis. National Chung Hsing University, Taichung, Taiwan (2024).
Efron, B. & Tibshirani, R. J. An Introduction to the Bootstrap. Monographs on Statistics and Applied Probability (Chapman & Hall/CRC, 1993).
Chi, H. TIMING-MSChart: A Computer Program for the Population Projection Based on Age-Stage, Two-Sex Life Table (National Chung Hsing University, 2024).
Huang, H. W., Chi, H. & Smith, C. L. Linking demography and consumption of Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae) fed on Solanum photeinocarpum (Solanales: Solanaceae) with a new method to project the uncertainty of population growth and consumption. J. Econ. Entomol. 111(1), 1–9 (2017).
Acknowledgements
We are grateful to Vice president for Research of Urmia University for financial support.
Funding
This project was funded by Urmia University.
Author information
Authors and Affiliations
Contributions
S.S.S. performed the experiments, F.M. designed the experiments and wrote the manuscript, F.M. and M.F. analyzed the bioassay and life table data, and M.F. designed the secondary metabolite measurements.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sarboland, S.S., Mehrkhou, F., Fattahi, M. et al. Biocontrol potential of aqueous and methanolic extracts of Russian knapweed, Acroptilon repens, L. (Asteraceae) against Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Sci Rep 15, 14928 (2025). https://doi.org/10.1038/s41598-025-93885-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-93885-7