Abstract
Shea oil, a globally consumed commodity, is intricately linked to the labor of women in Sub-Saharan Africa. This study examines local perceptions and adoption of a traditional shea nut fermentation method using subterranean pits in rural West African communities, and the chemical profiles of the kernels processed through different traditional methods. Key findings are that 1) local processors prefer the pit method due to its convenience and efficiency, and 2) lipid analyses indicate that fermenting shea nuts for three to six months results in an optimal chemical profile, characterized by lower free fatty acid (more than threefold) and polar lipid (more than 15-fold) content compared to boiled kernels. This enhances quality and mechanical processability, both criteria desirable for industrial applications. This study fills an important gap by chemically characterizing a traditional shea processing practice that has received little scientific attention. The results imply that the pit method holds potential for industrial shea oil extraction and for reducing firewood and water use in producing communities. However, this potential depends on fair benefit-sharing, local communities’ willingness to adopt the new practice, and overcoming the challenges for scaling up.
Similar content being viewed by others
Introduction
Shea oil has experienced a significant rise in global demand since being recognized as a Cocoa Butter Equivalent in 19901,2. The oil is derived from shea fruit harvested in the Sudano-Sahelian Savannah of Africa3,4, for which the primary sourcing countries include Burkina Faso, Ghana, Mali, Nigeria, and the Ivory Coast5. Although obtaining precise data on shea kernel production and export is challenging, estimates indicate that annual kernel production in West Africa has increased from 217,000 tons in 20045 to 1.63 million tons in 20156, with 43% being exported7. In 2019, Burkina Faso, the main producing country, had an estimated production potential of 0.85 million tons. Major importing regions and countries include Western Europe, particularly Denmark, France, and the Netherlands, as well as the United States, Singapore, and West African countries such as Ghana and Togo. In importing countries, kernels are processed to extract shea oil for use in confectionary and the cosmetic industry5,8. It is estimated that 2.4 million women collect shea for the export value chain in West Africa, and an additional 4.8 million women collect shea for self-consumption without market linkage7.
Shea nut collectors, referred to as processors in this paper, are crucial in preparing shea kernels for trade. Processing techniques aim to prevent germination and kernel degradation, ensuring high-quality kernels without producing bitter butter9. This paper examines a traditional method used in Burkina Faso and other West African countries, which involves fermenting shea fruits in excavated pits (called “fosse” in French). In this method, local processors deposit their shea fruits into pits with capacities ranging from 300 to 500 kg. They then gently stomp the fruits, cover the pits with soil or other materials, and allow them to ferment for varying durations. Studies have reported that the optimal fermentation period ranges from a few days to several months9,10,11. After emptying the pit, the nuts are either boiled or smoked9,10,12 and then sun-dried and deshelled, or directly sun-dried without further heat treatment10,13. Scholars have portrayed the pit method both as a storage practice for depulped nuts14, resulting in low-quality butter11, and as a fermentation process that depulps the nuts, prevents germination, and yields both high9 and low-quality12,13 butter. However, none of those studies analyzed the chemical composition of the extracted butter, and the characterization of low- or high-quality butter was largely subjective. Thus, despite the pit method apparently being well described in the literature, reports on the method are typically brief and contradictory.
The pit method differs from other traditional shea processing methods, such as boiling, parboiling, or roasting13,15, in terms of water and/or firewood usage. The parboiling method requires approximately 48 kg of firewood to process an 80 kg bag of kernels16, while boiling the nuts over open fires consumes about 114 kg of firewood to obtain the same amount of dried kernels17. Additionally, roasting the nuts, although not requiring water, consumes even more firewood than boiling18. While some processors still boil or roast the nuts after excavating the pits, this step might be omitted if the nuts are intended for industrial purposes. Omitting the boiling or roasting steps would save water and firewood, potentially offering significant environmental benefits if widely adopted across sub-Saharan Africa. However, to evaluate the potential adoption of this method, it is essential to understand why experienced users apply heat treatment to pit-processed nuts and how shea processors unfamiliar with this method perceive it.
The pit-processed nuts might also differ chemically from other traditional methods. However, the literature on this is scarce compared to studies on how storage, boiling, parboiling, roasting and smoking affect the physicochemical properties of shea kernels and butter15,19,20,21,22. The physicochemical properties relevant to assess shea nuts and butter for industrial purposes are: Free fatty acid (FFA) levels (e.g.15,21,23,24,25,26,27), triglyceride content (e.g.26,28,29,30), the presence of undesirable co-extractives15,31, and kernel mechanical processability. FFA content limits for shea butter should be < 1% for cosmetics and < 3% for food32. Industrial processing often requires FFA removal via neutralization, but this leads to triglyceride loss due to deprotonated FFA acting as soaps. Roughly, for every 1% FFA removed, 1% triglyceride is lost33. This unavoidable loss of the desirable triglycerides34 lowers economic returns. With respect to the easy mechanical processability, brittle, porous kernels are preferred to soft, rubbery kernels, for efficient extraction. Total extractables (also ‘total fat’, ‘crude fat’ or ‘fat content’) serve as a quality indicator for mechanical processability34,35. However, while total extractables may be high (67%), the corresponding FFA content can reach 23% of that fraction31. Thus, while it only indirectly measures desirable fat-content, it directly assesses mechanical processability by quantifying apolar extractable solids.
Detailed chemical analyses have additionally explored compounds like triterpene alcohols (e.g.36), -acetates, -cinnamates (e.g.29,30,37), tocopherols (e.g.15,26,38,39), phytosterols (e.g.26,40,41,42), phenolics (e.g.26,43),and volatiles (e.g.22,25,44). However, chemical analyses are seldom linked to different kernel pretreatment methods. Surprisingly, polar lipids in shea kernels remain poorly characterized42, except for total lecithin measures40. Despite the diversity of methods for chemical analysis of shea kernels, research on the pit method’s impact on shea kernel chemistry and quality is scarce.
This study combines social science and chemistry to examine the use and chemical impact of the pit method. The study focuses on Burkina Faso due to its significance as a shea sourcing country and the traditional use of the pit method in the region. The study was guided by the following research questions: 1) How and why is the traditional pit method used in Burkina Faso? 2) What are the pros and cons of the pit method for the local processors, and what drives the adoption among new processors? 3) How does the pit method affect the chemical profile of the kernels? And 4) What are the pros and cons of the pit method for the shea industry?
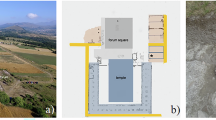
During the investigation, 130 shea processors were interviewed, and 28 shea kernel samples (fresh, boiled, and pit-processed) were chemically characterized (Fig. 1). Further details can be found under the Materials and Methods section.
Map of Burkina Faso with interview locations (purple for experienced pit-processors, orange for new pit processors) and kernel sample collection sites. Top-left: Traditional empty pit from an experienced processor. Bottom: New user with a full pit built for this study. Map generated using QGIS v3.40.1 (https://www.qgis.org/). Photos by Mustaphà Traorè.
Results
Traditional variations of the pit method
All 130 processors interviewed were women. Most used a single round pit (1.8 m3 ± 0.7 SD, n = 34), but some used up to four pits. Pits, owned individually (75%, n = 24 out of the 32 who responded to this question) or shared (25%, n = 8 out of 32), were used for several years. Filling a pit took around 1.5 months on average (± 0.8 SD, n = 33), sometimes extending to two or four months. Processors deposited whole fruits in the pits, occasionally adding plastic, sacks, or leaves at the pit’s bottom while filling it. Pits were gradually filled and compacted with no added water. Materials used to cover the pits varied: soil and leaves (34%, n = 23 out of 67), only leaves (30%, n = 20), tarpaulin (25%, n = 17), a combination of those (6%, n = 4), or other materials (3%, n = 3). Nuts were typically not stirred in the pit (94%, n = 32 out of 34). Pit location was influenced by proximity to homes or farmhouses for theft deterrence and ease of nut transportation. A processor who dug the pit near her home stated, “It’s close to me to avoid thefts.” Another mentioned, “I monitor the nuts to prevent animals from eating them.” A third processor added, “I can take some nuts when I want to go and sell.” A processor who dug the pit on her farmland mentioned, “During the winter months [the farming season] I sleep in the field.” Another complemented this by saying, “It’s difficult to transport the nuts home, as the field work is not finished.” Most processors stated their method mirrored practices of their elders (82%, n = 30 out of 37), others were unsure about historical practices. A processor stated, “I use the same method today, after digging the pit I have to put the leaves in first and then put the nuts on top and gradually put the leaves around the pit each time when filling.” While another mentioned, “The practice of my elders as far as the pit practice is concerned is the same as I do today. In this practice, after digging the pit, you have to put the nuts progressively while pressing the ones you put before to make the nuts go deeper. I don’t put leaves in my pit because they blacken the nuts.” Another processor used a different practice, “The practice is the same in my opinion as I have seen them do. I put a plastic sheet in the pit before putting the nuts in to prevent the nuts from sprouting, then progressively fill the pit and adjust the level until it is the right level.”
Burial periods for nuts varied: some were buried for less than two weeks, others for one to four months, and a few for up to six months (Fig. 2a). When immediate cash was needed, kernels were retrieved and sold (73%, n = 27 out of 37), while a third kept a reserve at home to boil for quick sales (30%, n = 11 out of 37). Pits were used only when processors had sufficient nuts and were not constrained financially.
(a) Summary of the reported time that experienced processors keep their nuts buried in pits. (b) Experienced pit-processors (answer per respondent = 2.3 ± 1.3, SD) and new pit-processors (answer per respondent = 4.4 ± 2.0, SD) reported benefits of the pit method to the question: “What do you like about the pit method?” Both groups appreciated its convenience and time efficiency. Experienced processors valued its ability to prevent nut germination, especially with large quantities of nuts. New processors found it less demanding in terms of time, natural resources use, and effort, as nuts did not require post-excavation boiling and they did not perform the excavation themselves. The number next to the bar indicates the percentage of processors mentioning that specific benefit.
After the pit process, 73% of processors boiled the nuts (n = 64 out of 88), considering it crucial for high-quality butter and marketability. As a processor said, “[After boiling] the weight increases, making good shea butter.” Another 23% sun-dried and deshelled the nuts without further treatment (n = 20 out of 88), due to factors like wood scarcity, time constraints, or because the nuts were deemed market-ready post pit-extraction (after sun-drying and deshelling). A minority roasted the nuts for butter (4%, n = 4 out of 88).
Despite its benefits for shea processors, new adoption of the pit method relies on financial considerations
Experienced processors highlighted benefits of the pit method (Fig. 2b): (1) Prevents nut germination, crucial for quality. As a processor said, “the nuts sprout quickly if I don’t put them in the pit.” (2) Speeds up pulp putrefaction, aiding depulping. (3) Offers flexibility in processing times, e.g. the nuts are safe during the rainy season and can be dried during the dry season. (4) Allows processing close to gathering sites, reducing the need for transportation. (5) Protects nuts from animals. (6) Produces quality kernels, heavy and bright, for butter-making or selling. In their view, “[the nuts] gain weight and are more beautiful to look at.” 7) Provides storage space, especially for processors lacking warehouses.
New processors valued the pit method differently from experienced ones (Fig. 2b): (1) Required less effort. (2) Convenient for multitasking and choosing processing times. (3) Used less wood and water. They also appreciated 4) resulting kernel weight and quality, (5) avoiding fruit transportation, requiring less strength, and (6) no need to sort nuts. Some mentioned (7) storing nuts for future use. New processors, having not engaged in pit digging and emptying, were unable to assess the overall effort involved compared to other methods. With this caveat in mind, the majority of both experienced and new pit-processors did not mention any disadvantages with the pit method. A few experienced processors mentioned the unpleasant odor emitted by the kernels as the sole drawback, but most did not mind it, noting it dissipates after sun exposure. Some new processors cited challenges such as emptying the pits coinciding with animals roaming freely and difficulties monitoring the condition of the pits, including concerns about rain washing away the covering soil. As one processor explained, “The only problem is that after extracting the kernels from the pit after 6 months and exposing them to the sun, this period coincides with the dry season, so the animals are roaming freely, not tied up so they disturb us by eating the kernels. But what is most interesting or pleasant is that the money from the sale of the nuts is very profitable for us and comes at a time of year when we really need it.”
Most of the processors who started using the pit method in 2022 also continued their usual methods with other nuts, primarily smoking (65%, n = 24 out of 37) or boiling (51%, n = 19 out of 37). They did this to meet buyer demands, make butter, and address financial needs. For example, a processor explained, “A project had given me a loan so I had to provide boiled kernels in exchange.” Smoking or boiling the nuts post-extraction was deemed crucial for transforming them into shea butter for consumption and sale. Some respondents (24%, n = 9 out of 37) did not smoke or boil nuts because they were all in the pit experiment.
Encouraging future use of this method requires considering the following points: 1) Price incentive: Higher prices for pit-processed kernels motivate processors to continue using this method and compensates for the time during which kernels are fermenting in pits and cannot be sold. 2) Savings and financial planning: Fermenting kernels in pits allows effective work and financial management, acting as a form of savings. 3) Positive experience and ease of practice: Processors find the pit method simpler and less cumbersome, excluding considerations of pit digging and emptying. As a processor explained, “If I have to continue this practice for the next time, the price might encourage me to do it or to do it again as long as there are nuts. For example, if the hawkers come by with their little tomato cans, which are their measuring tools, and the price is better than the price per kilogram of what I am going to sell after six months of waiting, I will have no choice but to stop processing the nuts in this way”. Another processor added “The money from the pit nuts helps us because it allows us to hope for a sum at a certain time of the year. If the nuts were not in the pit, we would sell them according to the expenses, so the nuts end up very quickly and then we can find ourselves without money”. However others suggest, “the payment should be done simultaneously to avoid a very long wait before paying.”
Pit kernels exhibit a distinct chemical composition compared to fresh and boiled kernels
Chemical compositions of boiled, fresh and pit kernels were analyzed to determine the characteristic chemical profiles, and to assess variability. The investigated compound classes included FFAs, mono-, di- and triglycerides (MGs, DGs, TGs), fatty acid methyl esters (FAMEs) and polar lipids, as they were all expected to impact kernel and butter quality, with implications for the shea industry. Quality control plots for individual compounds are given in Supplementary Information, Figure SI-1.
High FFA levels indicate poor quality in locally produced butter and the industrially extracted TG fraction. A significantly systematic difference between boiled and pit kernels is observed for FFAs, which are primarily oleic and stearic acids, consistent with several recent studies (e.g.25,26,27). FFA levels in pit kernels are at least three times lower and less variable than those in boiled kernels (Table 1).
Total FFA peak heights correlated well with measured FFA levels (in oleic acid equivalents) in the industrial crude extract. FFA levels in pit kernels were typically less than 3.5%, except for one 6-month sample reaching 6.4%. Some boiled samples, like one from site 3, had FFA levels as high as 10.9% (Figure SI-2).
The relative abundances in boiled kernels, fresh kernels, and 3- and 6-month pit kernels of 60 compounds across 7 different compound classes are summarized in a heatmap (Fig. 3), and the key findings are described in the following text.
A heatmap showing the relative intensities of 60 compounds grouped in 7 different compound classes in 28 samples of shea kernels. The shea kernel samples, with individual sample IDs at the bottom of the figure, are shown as vertical columns and are grouped from left to right as fresh kernels from site 4, followed by 3- and 6-month pit kernels from site 4 together with a 6-month pit kernel sample obtained the previous year (2020) from site 5. The boiled kernels from sites 1, 2 and 3 are all shown to the right hand side of the heatmap. Sixty lipids were consistently identified in the samples, and are here shown as horizontal lines, and grouped into compound classes: (a) FFA, (b) FAME, (c) MG/DG, (d) TG, (e) sulfoquinovosyldiacylglycerols (SQDG), f) glycosphingolipids (HexCer), and g) other polar lipids including lysophosphatidylcholines (LPC), glycosyldiacylglycerols (MGDG and DGDG), phosphatidylethanolamines (PE), phosphatidylglycerols (PG), phosphatidylinositols (PI) and phosphatidylcholines (PC). The relative intensities of the compounds between samples were visualized as autoscaled peak heights. The coloring of the heatmap shows the relative intensities (red – high; blue – low) of the individual compounds between samples, but it is important to note that the relative abundance of each compound within an individual sample cannot be directly compared from these data. A similar heatmap with the same data is given in Supplementary Information Figure SI-3, but in this case clustering has been applied to the samples to emphasize the similarities and differences in the chemical profile of the samples, irrespective of their origin and treatment procedures.
For statistical testing of variations between the sample types, samples were grouped into fresh kernels (n = 5) and pit kernels (n = 11), as well as well-preserved boiled kernels (Boiled_2-1, 3, and 4, and Boiled_3-2, n = 4) and poorly preserved boiled kernels (Boiled_1-1 to 4, Boiled_2-2, Boiled_3-1, 3, and 4, n = 8) based on the primary quality indicator, namely FFA content. This stratification of boiled kernels into well-preserved and poorly preserved categories was necessary due to the substantial variation in the lipid profiles of boiled kernel samples (Fig. 3). This stratification makes subsequent comparisons to the less variable pit kernels, and the associated statistical testing, more meaningful.
FFA levels increase as kernels are stored in pits, with levels in pit kernels comparable to well-preserved boiled kernels, but significantly lower than levels in poorly-preserved boiled kernels (p-value < 10–9 from Table SI-1; Fig. 3a and Fig. 4a).
(a–e) Box-plots of relative abundance for FFAs, FAMEs, MGs and DGs and various classes of polar lipids (MGDG, DGDG, LPC, PC, PE, PG, PI) are depicted across fresh kernels, poorly preserved and well-preserved boiled kernels, and pit kernels. All peak heights (or areas for the FAMEs and MGs measured by GC–MS) were normalised to the corresponding mean of the boiled kernels. f) A summarized overview illustrating the pattern of TG degradation products and polar lipids across the four different kernel categories. The relative abundance of each chemical class of compounds is indicated by symbols: low (−), medium (0), and high levels ( +). An extended version of the box-plots, including the more stable TGs and the polar lipids, SQDG and HexCer, are shown in Figure SI-4.
Formation of MGs and DGs indicates early degradation and is not desirable, although they do not decrease the quality of the extracted TG fraction as much as FFAs. Figure 3c and Fig. 4c-d show that MG and DG levels are low in fresh and well-preserved boiled kernels (p-values < 0.023, Tables SI-2 and SI-3). The levels are not significantly different for the pit kernels and poorly preserved boiled kernels, but are higher than for the fresh and well-preserved boiled kernels, and exhibit higher variation. Thus, early degradation of TGs to MGs and DGs occurs to the same extent in poorly preserved boiled kernels and in pit kernels.
Despite increasing levels of degradation products, TG levels did not significantly vary between processing methods. However, well-preserved boiled kernels had slightly higher mean TG levels (9–16% higher) compared to fresh and poorly preserved kernels (Fig. 3d, Table SI-4). The main TGs detected in these studies are consistent with those reported recently (e.g.26).
FAMEs are transformation products derived from e.g. FFAs. Their impact on kernel quality remains uncertain. FAMEs are more abundant in pit kernels, with the lowest levels observed in well-preserved boiled kernels (p-values < 2.0 × 10–4, Table SI-5, Figs. 3b and 4b). This suggests that FAME abundance does not necessarily reflect FFA levels; in boiled kernels FFAs and FAMEs are positively correlated. In contrast, fresh and pit kernels show low FFA but relatively high FAME levels. Thus, the proportion of FAMEs is significantly lower in boiled kernels compared to fresh and pit kernels (approximately 8 times lower).
Polar lipids are integral to plant membranes, and their composition likely varies with shea-processing methods. However, their influence on kernel properties remains poorly understood, although phospholipid class distributions in shea kernels and pulp has been reported with highest levels for PE (61 mg/100 g), PC (33 mg/100 g) and PI (4 mg/100 g)42. Here, relative peak heights of 38 polar lipids45 representing various phospholipid classes were examined (Fig. 3e-g). Polar lipids detected with highest frequency and peak height in this study, i.e. compounds belonging to the PE, PC and PI class, aligned well with those reported by Aremu et al.42. Pit kernels consistently showed lower levels of most polar lipid classes compared to boiled and fresh kernels (p-values from 0.039 to < 10–9). Poorly preserved boiled kernels also exhibited lower levels compared to well-preserved boiled kernels and fresh kernels (Fig. 3g, Table SI-6 to Table SI-12). However, glycosphingolipids (HexCer) lipids showed consistent abundance across all samples (Fig. 3f, Table SI-13). While sulfoquinovosyldiacylglycerols (SQDGs) followed a similar trend as other polar lipids, the difference in magnitude was less (Fig. 3e, Table SI-14).
In summary, the various processing methods yielded kernels that fell into four distinct categories based on their chemical characteristics: (1) fresh kernels; (2) poorly preserved boiled kernels; (3) well-preserved boiled kernels; and (4) pit kernels (Fig. 4).
Cluster analysis (see sample-clustered heatmap in Figure SI-3) and principal component analysis (Figure SI-5) further validate the distribution of kernel samples into the described categories. An exception is the second boiled sample from site 3 (Boiled_3-2), classified as well-preserved due to its low FFA content but which also exhibits low levels of polar lipids. Additionally, two fresh samples (Fresh_4-2 and 4) show slightly more polar lipid degradation compared to the other fresh kernel samples.
The pit method increases total extractables compared to boiling.
The total extractable mass, which is indicative of industrial processability of the kernels, increases with the pit method compared to boiling. In Fig. 5, the relative polar lipid levels for selected fresh, boiled and pit kernels (presented as the normalized sum of the peak heights for the 32 compounds depicted in Fig. 3g) are plotted against the measured total extractable mass. Polar lipid levels are typically higher in fresh kernels but decrease to below 20% of the maximum level for the 3- and 6-month pit kernels. In contrast, three out of the four boiled kernels have relatively high polar lipid levels, i.e. from 40–60% of the maximum. The data show an inverse trend, where a decrease in the relative polar lipid levels tends to be associated with a higher fraction of extractable mass. Thus, fresh kernels have less than 56% total extractables (average 52 ± 2% SD, n = 5), while 3- and 6-month pit kernels have more than 53% total extractables (3-month pit average 59 ± 4% SD, maximum 63%, n = 5 and 6-month pit average 63 ± 5% SD, maximum 68%, n = 5) and the boiled kernels have average total extractables of 56 ± 2% (SD, n = 4). Despite the large variability within each class and a low number of boiled kernel samples, the p-value of 0.16 suggests a potential positive effect of pit processing on the total extractable mass (Table SI-15). Interestingly, for two pits (denoted by ● and ■ symbols), the total extractables increase dramatically (by 12–13%) between 3-month and 6-month pit kernels, but for the other three pits, total extractables are highest for 3-month pit kernels but decrease slightly (by 0–4%) for 6-month pit kernels despite a small decrease in polar lipid levels. The industrial implications of these findings are considered in the polar lipids subsection of the discussion.
Polar lipid contents (LPC, MGDG, DGDG, PC, PE, PG, PI) plotted against total kernel extractable mass in weight % for five pit kernel trials from site 4. Kernel types are represented by color: Samples of fresh kernels (black), 3-month pit (red) and 6-month (blue) pit kernels, and two samples of boiled kernels from each of site 1 and 3 (green). Each of the five pits are indicated by a specific symbol. Polar lipid content is the normalized sum of the peak heights for the 32 compounds depicted in Fig. 3g.
Discussion
The traditional pit method may lessen reliance on, and consumption of, natural resources such as firewood and water
The pit method has received limited academic attention in recent years. Through an interdisciplinary approach, we have explored its utilization by rural West African processors and its implications from chemical and industrial perspectives. Our study reveals diverse practices among processors, who use various materials to cover nuts and bury them for varying durations. While some keep nuts underground for less than two weeks (in line with e.g.12 citing Servant et al. (1956)), others extend this period to months, influenced by convenience and time-efficiency, consistent with11 and14. These findings challenge earlier accounts, such as9 who recommended a specific burial duration of five to eight days. The method’s portrayal in literature varies due to its adaptable nature, reflecting the diverse practices of female processors.
Our findings revealed that the majority of processors use the pit method as a pretreatment step before boiling or smoking the nuts, believing it necessary for producing high-quality butter. Nevertheless, processors also boil/smoke the kernels sold at markets. However, our chemical analysis showed that post-excavation processing is not essential to achieve low FFA levels. This suggests that omitting heat treatment could be beneficial and should be promoted to shea processors, highlighting the potential reduction in water and firewood usage associated with the pit method. To truly minimize resource consumption and labor, kernels should be sold directly after excavation and sun-drying, without further heat treatment.
The pit method offers convenience and time-efficiency for new and experienced processors, with few recognizable drawbacks
The benefits of the pit method (i.e., efficient depulping; safeguarding nuts from germination, animals and theft; enabling local storage; releasing time for other tasks during the fermentation period), as stated by experienced processors, are particularly relevant because the shea collection season from April to August coincides with the peak season for agricultural activities46. Key elements to consider in adopting new practices include perceptions of their relative advantages, both economic and non-economic, how these advantages are learned, the decision-making process, and attitudes47,48. The pit method is perceived by processors as convenient and time-efficient, as it allowed them to engage in other tasks while ensuring nut safety and kernel quality. However, the main benefit perceived by new processors, i.e. avoiding the need for boiling or smoking, and thus fetching water and firewood, should be taken with caution, as they were spared from digging pits themselves, a task performed by project officers. Future research should explore perceptions of labor dynamics in the full process, including family involvement in pit digging and emptying.
Regarding economic (dis)advantages, new processors expressed concerns about the selling price when considering whether to adopt and continue using the pit method, posing a risk to adoption47,48. The price of the kernels in the early season (April to June) is significantly lower than in the later season (July to January)49, prompting processors to use the method as a form of savings, but only for those who can afford it. Many of the poorest collectors rely on the early sale of shea kernels to bridge an income gap at the beginning of the agricultural season when other sources of income are scarce50. For these collectors, waiting for fermentation to occur may be less attractive, although still possible, as depulping typically takes place within five to twelve days9,12.
Pit kernels show lower FFA and polar lipid content than boiled kernels but higher FAME levels
FFAs, a key indicator of poor quality, were found to be significantly lower in kernels buried in pits for three months, compared to those buried for six months, and with up to 5–7 times lower levels compared to boiled kernels. The most degraded boiled kernel samples, exhibited FFA content 20 times higher than the most well-preserved pit kernels. However, the most well-preserved boiled kernel samples, showed FFA levels comparable to the most well-preserved pit kernels, with only 9% absolute deviation. This aligns with previous research26,27,51, which found FFA levels in shea products to vary by a factor of 13 (range 1–13% in shea butter), with reported levels of up to 24% in the total extractables fraction e.g.31.
Fresh kernels exhibited low levels of FFA, indicating good quality. However, FFA levels increased in pit kernels the longer they were buried. While traditional methods suggest kernels may be kept buried until commercialization or use10, it is unlikely they can maintain low FFA levels over extended periods. In our study, burial times of three months appeared optimal for maintaining low FFA levels.
The variability in FFA levels was notably higher in boiled kernels, even from the same site, compared to pit kernels. While the FFA levels in the best-preserved boiled kernels were similar to those in the best pit kernels, the quality of boiled kernels was much less consistent and sometimes very poor.
MGs and DGs, early degradation products of TGs, increase as kernels are stored in pits and levels are also elevated in poorly-preserved boiled kernels. This suggests that degradation of TGs to MGs and DGs has commenced in pit kernels, particularly compared to well-preserved boiled kernels. Levels are low in fresh kernels and in well-preserved boiled kernels. In shea butter, DGs have been found to constitute 7–22% of the total content of DGs and TGs52. However, in this study the formed MGs and DGs, does not seem to affect TG content significantly. This suggests that the levels of MGs and DGs could be minor compared to TG levels, but further and quantitative studies are needed.
FAMEs may be formed from a condensation reaction between methanol and FFAs known as esterification. It was observed that the fraction of FAs occurring as FAMEs was low for boiled kernels, and up to eight times higher for fresh and pit kernels. Fermentation of biological material may result in the formation of methanol53. Therefore, a lower conversion of FFAs to FAMEs is expected for boiled and aerated kernels, where less methanol formation occurs. Nonetheless, it is surprising that levels of FAMEs in pit kernels are so high, when the FFA levels are low.
However, FAMEs can also be formed through the direct reaction between alcohols and acylglycerols, i.e. MGs, DGs and TGs, in a process known as alcoholysis or trans-esterification. In alcoholysis the FFAs are not the precursors for the formation of FAMEs. In biological systems, alcoholysis is catalysed by lipase enzymes54, and it is the major mechanism of ester biosynthesis in dairy lactic acid bacteria and yeast55. In pit fermentation, the precursors acylglycerols and methanol, as well as lipases, are present. The differences in patterns of FFAs, FAMEs, MGs and DGs could originate from different degradation pathways dominating between boiled kernels (hydrolysis from TGs to DGs and MGs and to FFAs, with little conversion to FAMEs through esterification) and pit kernels (alcoholysis of acylglycerols in the presence of methanol to produce DGs, MGs and FAMEs, but not FFAs). In the case that FFAs are produced in pits, they will be quickly transformed to FAMEs by esterification in the presence of methanol54.
The implication of FAME levels on the kernel quality is unknown. However, it is presumed FAMEs could be easily removed by the deodorization process e.g.56 in the industrial refining of the extracted TG fraction. Thus, it is possible that it is an industrial advantage to have FAs converted to the methylated form to a larger degree, as found in the pit kernels. A more detailed study of the factors affecting chemical composition in pit kernels is currently being carried out. Preliminary data shows that quantities of FAMEs constitute less than 3% of FFA quantities57, thus the impact of FAMEs on kernel quality is likely to be negligible. Nonetheless, the formation of FAMEs and potentially other fatty acid esters during pit processing of shea kernels, and their impact on kernel quality warrants further exploration.
Polar lipids are membrane lipids and partly responsible for plant cell integrity585960. Well-preserved boiled kernels often exhibit a rubbery texture due to the presence of these polar lipids. Industrial processing typically requires degumming to ease the mechanical processing of these rubbery kernels and to reduce downtime of mechanical equipment. By degumming, the phospholipids are solidified as a gum, and separated from the TG fraction by centrifugation34. In contrast, poorly preserved boiled kernels have a more porous and brittle structure, making them easier to process mechanically. Our findings indicate that polar lipids undergo efficient degradation in poorly preserved boiled kernels and pit kernels, suggesting breakdown of cell structures. These results suggest that the good mechanical properties (porous brittle structure and a lack of gum-like constituents) are also characteristic of pit kernels. This hypothesis is further supported by the fact that, as well as having lower polar lipid levels, the pit kernels exhibited higher, up to 68%, total extractables (Fig. 5).
The chemical profile of pit kernels indicates high quality with low FFA content and good mechanical processability, both crucial in an industrial context
The pit method yields kernels with low FFA levels akin to well-preserved boiled kernels as well as good mechanical processability and high total extractables, similar to poorly preserved boiled kernels. This method thus combines the advantageous properties of both types of boiled kernels, resulting in kernels of more consistent quality compared to boiled kernels, which exhibited significant variations in chemical composition and processability.
The pit method may offer an advantage over other processing methods by producing low levels of FFAs, and to some degree FAMEs which are considered the less problematic TG degradation products. FAMEs are potentially easier to remove during the industrial processing due to their higher volatility, potentially eliminating the need for additional neutralization steps that decrease TG yield34. However, further research is needed to validate this hypothesis and fully understand the implications of higher FAME levels on kernel quality and industrial processability. Additionally, further research is needed to understand the challenges of industrial upscaling with a new kernel profile, including costs for differentiated storage and logistics, supply chain disruptions, regulatory compliance, market acceptance, waste management, and training requirements616263. Last but not least, intellectual property rights and benefit-sharing agreements to ensure fair compensation for local communities needs to be addressed64,65.
Conclusion
Traditionally, there are numerous variations of the pit method. Some processors use leaves, others use plastic, and some simply use soil to cover and protect the nuts. The pits are located in places convenient for the daily routines of the processors, and the nuts are kept underground until it is convenient to retrieve them. Thus, the pit method varies according to traditional practices passed down through generations, as well as the daily routines and seasonal activities of individual processors. Processors new to the method found the absence of boiling or smoking the nuts both practical and convenient, as it saves water, firewood, and labor, and reduces exposure to heat. In such cases, the pit method may represent a more sustainable alternative to processing methods that include kernel boiling or smoking. However, the adoption of this new method depended on financial considerations and the availability of nuts.
The findings from testing the 3- and 6-month pit processes align with the traditional burial times used by experienced processors, highlighting that the optimal chemical profile for pit kernels is achieved after three months. While our findings provide valuable insights, we want to emphasize that local variations in burying times, post-pit processing, and materials used have been developed through generations of conscious adaptation to their environment, and are therefore valid in their own right.
The pit method yields kernels with low and less variable free fatty acid (FFA) levels, i.e., less than 4% FFA in the industrial crude extract for 3-month- and less than 7% FFA in the industrial crude extract for 6-month pit-processed kernels. In contrast, boiled kernels obtained from three sites across Burkina Faso exhibited significant variability in their lipid profiles, representing both high-quality, well-preserved kernels and low-quality, poorly preserved kernels with FFA content as high as 11% in the industrial crude extract. Furthermore, pit kernels exhibited high mechanical processability, making them suitable for industrial processing. This high mechanical processability was associated with reduced polar lipid content, with pit kernels having less than a quarter of the polar lipid content compared to fresh kernels.
Notably, pit kernels contain higher levels of fatty acid methyl esters (FAMEs) compared to boiled kernels. The generation of FAMEs as a triglyceride (TG) degradation product is associated with the pit fermentation process. The implications of increased FAME levels on shea kernel quality appear to be negligible in an industrial context due to their relatively low levels and their potential to be removed by deodorization.
In conclusion, the traditional pit method, without subsequent boiling or smoking of kernels, has potential as a sustainable kernel preservation method. Further investigations are needed to assess the labor and resources associated with establishing and maintaining pits, to evaluate how large scale adoption may affect the shea supply and market, to identify factors important for pit kernel quality, and to evaluate the industrial processing of pit kernels and the quality of the resulting shea products.
Material and methods
Interviews
The social science data on the use of the pit method is derived from 130 interviews conducted as follows:
Sample A: 56 shea processors were randomly selected from nine communities across Burkina Faso in April 2021, with follow-up interviews in April 2022.
Sample B: 37 shea processors were randomly chosen from a specific community in Burkina Faso known for its frequent use of this method. Each respondent was interviewed twice, in November 2021 and February 2022.
Sample C: 37 interviews were conducted in a community where the pit method was introduced for the purpose of this research. Each respondent received training to perform the anaerobic pit kernel experiment with six months of storage and was interviewed twice: three months after filling the pits in November 2021 and immediately after excavating the pits in February 2022. Five of these processors provided kernel samples for the chemical analyses. All participants obtained help to dig a pit.
All methods were performed in accordance with the relevant guidelines and regulations: informed consent was obtained from all subjects and/or their legal guardian(s), following Free Prior Informed Consent rules, GDPR rules, and ethical considerations for human research, as approved by the Ethical Committee and the Faculty Secretariat of the University of Copenhagen.
The interviews collected information about respondents’ background, including gender, age, ethnicity, level of education, the number of collected bags, and years of experience in collecting and processing shea nuts. Additionally, the data covered during these interviews included:
Sample A: Data related to the traditional pit-method process, such as burying times (in days or months), the number of pits, covering materials, filling process, perceptions of the advantages and disadvantages of the pit method, and post-pit processing (boiling, smoking, and/or direct sun-drying).
Sample B: In addition to the data collected in Sample A), information was gathered about the size of the pits (width, length, and depth in meters), time required to fill the pits (in days or months), the pit’s location, ownership of the pits, the use of other shea nut processing methods, benefits and disadvantages of other traditional processing methods, decision-making concerning the method(s) used, and how respondents manage the early market (between April and July).
Sample C: This sample included data on respondents’ prior knowledge of the method, perceived advantages and disadvantages of the pit method and other traditional methods, decision-making regarding the method(s) used, and incentives for adopting and continuing each practiced method.
Chemical analysis
The sample preparation and chemical analyses of the shea kernel samples are briefly described below.
Shea kernels were collected from Burkina Faso and transported to the University of Copenhagen by courier. On arrival, the kernels were oven-dried at 60 °C for 48 h to improve stability and stored in a dehumidified room with app. 30% humidity until further analysis.
Boiled kernels originating from sites 1, 2 and 3 (Fig. 1) were retrieved at time of evacuation in 2020 and represent the variability of samples at the point of entry to an industrial supply chain. Kernels from pits which had been sun-dried, but not boiled or smoked, were retrieved from five pit-treatments at site 4 at time 0 (n = 5; fresh kernels), and after 3 months (n = 5; 3-month pit kernels) and 6 months (n = 5; 6-month pit kernels) and trailing the season of 2021. An additional kernel sample from site 5 obtained after 6 months of pit treatment trailing the season of 2020 was also included for comparison.
The chemical fingerprints of the shea samples were assessed using both liquid chromatography with electrospray quadrupole-time-of-flight high resolution mass spectrometry detection (LC-HRMS) and gas chromatography with electron ionization quadrupole mass spectrometry detection (GC–MS). Lipid analysis was carried out using an Acquity UPLC BEH C18 column (2.1 mm × 100 mm, 1.7 µm, 130 Å, Waters) maintained at 40 °C and four different LC-HRMS methods that targeted different lipid classes (FFAs, DGs, TGs and various classes of polar lipids). Mobile phases (A) acetonitrile: water (3:2 (v/v)) with 10 mM ammonium acetate and (B) isopropanol: acetonitrile (9:1 (v/v)) with 10 mM ammonium acetate were used for FFAs, and (A) acetonitrile: water (3:2 (v/v)) with 10 mM ammonium formate and (B) isopropanol: acetonitrile (9:1 (v/v)) with 10 mM ammonium formate for DGs, TGs and polar lipids. The HRMS was operated in all-ion-fragmentation mode, to yield information on both precursor and product ions. GC–MS analysis was carried out using a ZB-5HT Inferno™ column (15 m × 0.25 mm × 0.10 µm, Phenomenex) which allowed the temperature gradient to range from 60 °C to 370 °C. Samples were introduced by a 0.2 µL splitless injection. The MS was operated in selected ion monitoring (SIM) mode with 20 SIM ions ranging from m/z 28 to m/z 609 monitored in each of 29 different retention time windows. Data from the GC–MS analysis were used to evaluate levels of FAMEs and MGs.
Before chemical analysis the shea kernels were crushed and milled to a fine powder, and depending on the analysis methods, a variety of sample extraction and clean-up steps were used. All sample preparation was carried out with ice-cold solvents and stored at < 4 °C until analysis, to maintain sample integrity.
For lipid analysis, samples were initially extracted with a liquid–liquid extraction procedure using methanol: methyl tert-butyl ether: chloroform in a 1.33:1:1 (v/v/v) ratio, evaporated to dryness and reconstituted in a 2:1 (v/v) mixture of chloroform: methanol. The reconstituted extract was fractionated using solid phase extraction (SPE) with an NH2-stationary phase and elution solvents (hexane, chloroform, ethyl acetate, methanol, 2% acetic acid in diethyl ether, 0.2 M ammonium acetate in methanol) in various combinations and ratios of increasing polarity, to yield two purified fractions: “neutral lipids” and “polar lipids”. The two fractions were subsequently evaporated to dryness and reconstituted in chloroform: methanol in a 2:1 (v/v) ratio before analysis. The headspace was always replaced by nitrogen to prevent lipid oxidation.
The sample preparation for semi-volatiles analysis involved extraction with 2,6-di-tert-butyl-4-methylphenol (BHT; 5 mg / mL) in hexane. The hexane-extract was cleaned by liquid–liquid partitioning using 0.9% NaCl in water, and residual water removed prior to GC–MS analysis.
Industrial measures were total extractables and FFA content. Total extractables are the fraction of mass extracted from kernels by an apolar solvent, and were measured by Soxhlet extraction of kernel homogenate for 18 h using diethylether. The total free fatty acid content was quantified as weight percent of free fatty acids in oleic acid equivalents and the analysis was performed according to the AOCS Ca 5a-40 procedure ‘Free fatty acids in crude and refined fats and oils’. Instead of hot ethanol, a 1:1 (v/v) solution of 95% ethanol and diethylether was used according to the IUPAC 2.201 procedure ‘Determination of the acid value (a.v.) and acidity’. The quantities of FFAs were correlated to FFA peak heights obtained by MS-Dial (Figure SI-2).
Data analysis and statistics
The qualitative data from the interviews was coded and transformed into quantitative data, and descriptive statistical analyses were conducted using Excel.
Lipid peak heights were extracted using MS-Dial v4.9.266. Peak areas of FAMEs and MGs were extracted using MassHunter Quantitative Analysis v10.2 (Agilent). The p-values quoted indicate the significance assessed by one-way ANOVA with Tukey post hoc testing (OriginPro 2020, OriginLab Corporation). The polar lipids measure from Fig. 5 represents the combined data for a total of 32 compounds or groups of isomers. It was calculated by first normalising each polar lipid to its maximum value, summing the polar lipid levels across each sample, and finally normalising the sum of polar lipids to the maximum value across samples. In that way, each polar lipid influences the sum equally, regardless of its concentration and ionisation efficiency.
Data availability
Data is provided within the manuscript and supplementary information files, and the data that support the findings of this study have been deposited in Figshare (https://doi.org/10.6084/m9.figshare.26202266).
References
Chalfin, B. Shea butter republic: state power, global markets, and the making of an indigenous commodity (Routledge, 2004).
Wardell, A. & Fold, N. Globalization in a Nutshell: historical perspectives on the changing governance of the shea commodity chain in northern Ghana. Int. J. Commons 7(2), 367–405 (2013).
Boffa, J. M. Agroforestry parklands in sub-Saharan Africa (The Food and Agriculture Organization of the United Nations Conservation Guide, 1999).
Hall, J. B., Aebischer, D. P., Tomlinson, H. F., Osei-Amaning, E., Hindle, J. R. Vitellaria paradoxa. A monograph. (School of Agricultural and Forest Sciences Publications. University of Wales, Bangor, UK, 1996).
Lovett, P. N. The shea butter value chain: production, transformation and marketing in West Africa (West Africa Trade Hub, Technical Report No. 2, Accra, Ghana, 2004).
Naughton, C. C., Lovett, P. N. & Mihelcic, J. R. Land suitability modeling of shea (Vitellaria paradoxa) distribution across sub-Saharan Africa. Appl. Geogr. 58, 217–227 (2015).
Bockel, L., Veyrier, M., Gopal, P., Adu, A. & Ouedraogo, A. Shea value chain as a key pro-poor carbon fixing engine in West Africa (The Food and Agriculture Organization of the United Nations and Global Shea Alliance, 2020).
ITC. Stratégie nationale de dévelopment durable de la filière karité du Burkina Faso 2015–2019. Report from Le Centre du Commerce International. [Accessed on 6th March 2024 at https://faolex.fao.org/docs/pdf/Bkf188967.pdf]
Terpend, M. N. La filière Karité: Produit de cueillette, Produit de luxe (Grapp, 1982).
François, M., Niculescu, N., Badini, Z. & Diarra, M. L. beurre de karité au Burkina Faso: Entre marché domestique et filiè res d’exportation. Cahiers Agricultures 18(4), 369–375 (2009).
Sidibé, A. et al. Women, shea, and finance: how institutional practices in a Malian cooperative create development impact. Int. J. Agric. Sustain. 12(3), 263–275 (2014).
Hyman, E. L. A comparison of labor-saving technologies for processing shea nut butter in Mali. World Dev. 19(9), 1247–1268 (1991).
Elias, M. & Carney, J. African Shea Butter: A Feminized Subsidy from Nature. Africa 77(1), 37–62 (2007).
Crèlerot, F. M. Importance of shea nuts for women’s activities and young child nutrition in Burkina Faso: the case of the Lobi [PhD dissertation]. The University of Wisconsin-Madison (1995).
Honfo, F. G., Linnemann, A. R., Akissoe, N., Soumanou, M. M. & van Boekel, M. A. J. S. Characteristics of traditionally processed shea kernels and butter. Inter. J. Food Sci. Technol. 48(8), 1714–1721 (2013).
Jasaw, G. S., Saito, O., Gasparatos, A., Shoyama, K., Boafo, Y. A. & Takeuchi, K. Ecosystem services trade-offs from high fuelwood use for traditional shea butter processing in semi-arid Ghana. Ecosystem Services 27(A), 127–138 (2017).
Noumi, E.S., Dabat, M. H. & Blin, J. Energy efficiency and waste reuse: a solution for sustainability in poor West African countries? Case study of the shea butter supply chain in Burkina Faso. Journal of Renewable and Sustainable Energy 5(5), 053134:1–15 (2013).
Naughton, C. C., Zhang, Q. & Mihelcic, J. R. Modelling energy and environmental impacts of traditional and improved shea butter production in West Africa for food security. Sci. Total Environment 576, 284–291 (2017).
Aculey, P. C., Lowor, S. T., Kumi, W. O. & Assuah, M. K. The effect of traditional primary processing of the Shea fruit on the kernel butter yield and quality. Am. J. Food Technol. 2(7), 73–81 (2012).
Agene, V. N. Effect of different processing methods and periods of storage in different containers on some quality characteristics of kernel and extracted butter of shea nut. (Master Thesis) (2015) [Accessed on 5th February 2022 at https://ir.knust.edu.gh/handle/123456789/8186]
Honfo, F. G., Linnemann, A. R., Soumanou, M. M., Akissoe, N. & van Boekel, M. A. J. S. Effect of storage period and boiling time of fresh shea nuts on physico-chemical characteristics of kernel and butter. Afr. J. Food Sci. 16(6), 160–171 (2022).
Honfo, F. G., Linneman, A. R., Soumanou, M. M., Akissoe, N. & van Boekel, M. A. J. S. Quality characteristics and volatile compounds of shea butter under different storage conditions. Agricult. Food Sci. J. Ghana 15, 1507–1525 (2022).
Goumbri, B. W. F. et al. African shea butter properties related to common extraction technologies: a review. Food Bioprocess Technol. 15, 231–248 (2022).
Ofoegbu-Chibuzo, N. E., Chukwu, U. J. & Okoye, I. P. Physicochemical analysis and fatty acid content of chemical and traditional extracts of shea kernel (Vitellaria paradoxa) from Kwara State Nigeria. Open Access Library J. 9, e8295 (2022).
Oussou, K. F. et al. Comparative elucidation of aroma, key odorants, and fatty acid profiles of Ivorian shea butter prepared by three different extraction methods. Separations 9, 245 (2022).
Abdel-Razek, A. G., Abo-Elwafa, G. A., Al-Amrousi, E. F., Badr, A. N.; Hassanein, M. M. M., Qian, Y., Siger, A., Grygier, A., Radziejewska-Kubzdela, E., Rudzi´nska, M. Effect of refining and fractionation processes on minor components, fatty acids, antioxidant and antimicrobial activities of shea butter. Foods 12, 1626 (2023).
Goumbri, B. W. F., Kouassi, A. K., Djang’eing’a, R. M., Semdé R., Mouithys-Mickalad, A., Sakira A. K., Yaméogo G. B. J., Somé, T. I. & Danthine, S. Quality characteristics and thermal behavior diversity of traditional crude shea (Vitellaria paradoxa Gaertn) butter from Burkina Faso. Food Biophysics 19, 609–626 (2024).
Obibuzor, J. U., Abigor, R. D., Omoriyekemwen, V., Okogbenin, E. A. & Okunwaye, T. Effect of processing germinated shea kernels on the quality parameters of shea (Vitellaria paradoxa) butter. J. Cereals Oilseeds 4(2), 26–31 (2013).
Akihisa, T. et al. Triacylglycerol and Triterpene Ester Composition of Shea Nuts from Seven African Countries. J. Oleo Science 60(8), 385–391 (2011).
di Vincenzo, D. et al. Regional variation in shea butter lipid and triterpene composition in four African countries. J. Agricult. Food Chem. 53(19), 7473–7479 (2005).
Ajala, E. O., Aberuagba, F., Olaniyan, A. M. & Onifade, K. R. Optimization of solvent extraction of shea butter (Vitellaria paradoxa) using response surface methodology and its characterization. J. Food Sci. Technol. 53(1), 730–738 (2016).
USAID/WATH (2005): Guide à l’exportation du beurre de karité. West Africa Trade Hub, Accra, Ghana. [Accessed on 30th January 2024 at http://hubrural.org/IMG/pdf/wath_shea_butter_value_chain_study_synthesis_fr.pdf]
Chumsantea, S., Aryusuk, K., Lilitchan, S., Jeyashoke, N. & Krisnangkura, K. Reducing Oil Loses in Alkali Refining. J. Am. Oil Chemists’ Soc. 89(10), 1913–1919 (2012).
Gunstone, F. D., Oils and fats in the food industry Ch.3 (Wiley-Blackwell, 2008).
Abdul-Hammed, M., Jaji, A. O. & Adegboyega, S. A. Comparative studies of thermophysical and physicochemical properties of shea butter prepared from cold press and solvent extraction methods. Journal of King Saud University – Science 32(4), 2343–2348 (2020).
Akihisa, T. et al. Triterpene Alcohol and Fatty Acid Composition of Shea Nuts from Seven African Countries. J. Oleo Sci. 59(7), 351–360 (2010).
Akihisa, T. et al. Anti-Inflammatory and Chemopreventive Effects of Triterpene Cinnamates and Acetates from Shea Fat. J. Oleo Sci. 59(6), 273–280 (2010).
Allal, F. et al. Fatty acid and tocopherol patterns of variation within the natural range of the shea tree (Vitellaria paradoxa). Agroforestry Syst. 87(5), 1065–1082 (2013).
Maranz, S. & Wiesman, Z. Influence of Climate on the Tocopherol Content of Shea Butter. J. Agricult. Food Chem. 52(10), 2934–2937 (2004).
Njoku, O. U., Eneh, F. U., Ononogbu, I. C. & Adikwu, M. U. Compositional and toxicological studies on shea butter. J. Nutraceut. Funct. Med. Foods 2(3), 33–39 (2000).
Segman, O., Wiesman, Z. & Yarmolinsky, L. Shea butter in chocolate and related products Ch.17 (AOCS Press, 2012).
Aremu, M. O., Andrew, C., Salau, R. B., Atolaiye, B. O., Yebpella, G. G., & Enemali, M. O. Comparative studies on the lipid profile of shea (Vitellaria paradoxa C.F. Gaertn.) fruit kernel and pulp. Journal of Applied Sciences 19: 480–486 (2019).
Maranz, S., Wiesman, Z. & Garti, N. Phenolic constituents of shea (Vitellaria paradoxa) kernels. J. Agricult. Food Chem. 51(21), 6268–6273 (2003).
Honfo, F. et al. Influence of roasting of shea kernels on their fat content and some quality characteristics of shea butter. J. Food Stud. 6(1), 66–80 (2017).
Liebisch, G. et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid Res. 61(12), 1539–1555 (2020).
Carney, J. & Elias, M. Revealing Gendered Landscapes: Indigenous Female Knowledge and Agroforestry of African Shea. Canadian Journal of African Studies / Revue Canadienne des Études Africaines 40(2) (2006).
Alexander, K. S. et al. What is technology adoption? Exploring the agricultural research value chain for smallholder farmers in Lao PDR. Agricult. Hum. Values 37, 17–32 (2020).
de Oca, M., Munguia, O., Pannell, D. J. & Llewellyn, R. Understanding the Adoption of Innovations in Agriculture: A Review of Selected Conceptual Models. Agronomy 11, 139 (2021).
Questiaux, F. Social differentiation and household dynamics associated with early season shea nut collection and trading in Burkina Faso. Forum Devel. Stud. 51(1), 101–120 (2024).
Pouliot, M. Contribution of “Women’s Gold” to West African Livelihoods: The Case of Shea (Vitellaria paradoxa) in Burkina Faso. Econ. Botany 66, 237–248 (2012).
Honfo, F. G., Akissoe, N., Linnemann, A. R., Soumanou, M. & van Boekel, M. A. J. S. Nutritional Composition of Shea Products and Chemical Properties of Shea Butter: A Review. Critical Rev. Food Sci. Nutr. 54(5), 673–686 (2014).
Stübiger, G., Werther, W. & Krist, S. Characterization of shea butter using a combination of MALDI-TOF-MS, SPME-GC-MS and multivariate data analysis. Current Bioactive Compounds. 11 (2015).
Ohimain, E. I. Methanol contamination in traditionally fermented alcoholic beverages: the microbial dimension. SpringerPlus 5(1), 2193–1801 (2016).
Canet, A., Bonet-Ragel, K., Benaiges, M. D. & Valero, F. Lipase-catalysed transesterification: Viewpoint of the mechanism and influence of free fatty acids. Biomass Bioenergy 85, 94–99 (2016).
Liu, S. Q., Holland, R. & Crow, V. L. Esters and their biosynthesis in fermented dairy products: A review. Inter. Dairy J. 14(11), 923–945 (2004).
Gupta, M. K. Practical Guide to Vegetable Oil Processing Ch. 8 (AOCS, 2017)
Toma, P. Identification and quantification of bioactive and semi-volatile compounds in shea by HT-GC-MS – a contribution to the Sheaine project. MSc-thesis from University of Copenhagen, Denmark, 2024. Available upon request to Nielsen, N.J. (njn@plen.ku.dk), (2024).
Yu, L., Zhou, C., Fan, J., Shanklin, J. & Xu, C. Mechanisms and functions of membrane lipid remodeling in plants. Plant J. 107(1), 37–53 (2021).
Reszczyńska, E. & Hanaka, A. Lipid composition in plant membranes. Cell Biochem. Biophys. 78, 401–414 (2020).
Fujii, S., Wada, H. & Kobayashi, K. Role of Galactolipids in Plastid Differentiation Before and after light exposure. Plants 8(10), 357 (2019).
da Cunha Rodrigues, L., Nunes de Carvalho, P.I., Dacanal, G.C., & Lopes de Oliveira, A. Pressurized liquid extraction (PLE) in an intermittent process as a new alternative for production of tincture from medicinal plants: The scale up and economic evaluation for production of passion fruit (Passiflora edulis Sims) leaf hydroalcoholic extract. Food and Bioproducts Processing 149, 272–283 (2025),
Huang, Y., Zheng, B. & Wang, Z. Supplier–remanufacturing and manufacturer–remanufacturing in a closed-loop supply chain with remanufacturing cost disruption. Annals Operations Res. 324, 61–92 (2023).
Ramos, P. R.; da Cunha Rodrigues, L.; Zabot, G. L.; & Lopes de Oliveira, A. Extraction of soybean oil with pressurized ethanol: prospects for a new processing approach with an analysis of the physical properties of crude oil and implementation costs through scale-up in an intermittent process. Processes12, 2224 (2024).
Carroll, S. R., Herczog, E., Hudson, M., Russell, K. & Stall, S. Operationalizing the CARE and FAIR Principles for Indigenous data futures. Sci. Data 8, 108 (2021).
Golan, J., Athayde, S., Olson, E. A. & McAlvay, A. Intellectual Property Rights and Ethnobiology: An Update on Posey’s Call to Action. J. Ethnobiol. 39(1), 90–109 (2019).
Tsugawa, H., Ikeda, K. & Takahashi, M. A lipidome atlas in MS-DIAL 4. Nat. Biotechinol. 38, 1159–1163 (2020).
Acknowledgements
The research was funded by Innovation Fund Denmark through a Grand Solutions Grant (project no. 9067-00030B). AAK Denmark is acknowledged for sharing expertise on industrial processing and for providing the industrial measures. We would like to express our gratitude to François Questiaux and Mustapha Traore for their assistance with data collection, to Peter Christensen and Jette Petersen for their technical assistance, to Eleni Pappa for the contribution to the literature review, and to Romina Forte Nerán for curating references.
Funding
Innovation Fund Denmark #9067-00030B
Author information
Authors and Affiliations
Contributions
N.T.G., N.J.N., J.H.C. and M.P. carried out conceptualization (equal). N.T.G., and N.J.N. co-led data acquisition design, analysis and writing (N.T.G. social science and N.J.N. chemistry). D.I.P. supported data analysis and writing, produced Figs. 2–6 and supplementary materials (chemistry). N.T.G. produced Fig. 1, and supplementary materials (interviews). E.W. and Y.D. supported data acquisition. E.M. supported data analysis (chemistry). All authors reviewed and edited the text. J.H.C. and M.P. led the funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Turreira-García, N., Nielsen, N.J., Pattison, D.I. et al. Fermenting shea nuts using the traditional pit method yields better physicochemical properties with potential environmental benefits. Sci Rep 15, 9502 (2025). https://doi.org/10.1038/s41598-025-93921-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-93921-6