Abstract
Public health is seriously threatened by the rise of antibiotic-resistant strains of bacteria, especially methicillin resistant Staphylococcus aureus (MRSA). This study investigated the phytochemical compounds, and possible antibacterial effects of a methanolic extract of the cactus species Opuntia monacantha Haw. against MRSA. The powdered substance was extracted with methanol and filtered, and the filtrate was partitioned with n-hexane, chloroform and ethyl acetate. The fractions with higher percentage yields such as the n-hexane and chloroform fractions went through GC-MS analysis. They consist of various compounds that are known to have strong antioxidant and antimicrobial activities. The antimicrobial activity of the plant was measured via a diffusion assay. On the basis of these results, the Opuntia monacantha crude methanolic extract, and the n-hexane and chloroform fractions significantly inhibited all the MRSA strains used in the test at 100, 75 and 50 mg/mL. Furthermore, the minimum inhibitory concentrations of various fractions of the methanolic extract of Opuntia monacantha were also examined. Therefore, Opuntia could be a promising candidate for strengthening the existing formulations in pharmaceutical science for the treatment of MRSA. However, additional research is necessary before its therapeutic use is recommended. The results are intended to provide important new information for the creation of sustainable and alternative antimicrobial agents.
Similar content being viewed by others
Introduction
The World Health Organization (WHO) lists methicillin-resistant Staphylococcus aureus (MRSA) as a high-priority organism for the development of novel antibiotics because it exhibits resistance to multiple drug classes, including β-lactams, tetracyclines, macrolides, and fluoroquinolones1. MRSA continues to be a critical pathogen in healthcare and community settings, with significant implications for morbidity, mortality, and healthcare costs, despite some regions seeing a decrease in hospital prevalence due to improved infection control2.
Direct contact is the main method of transmission, which is made easier in healthcare settings by contaminated surfaces, medical equipment, or work clothes3. Tens of thousands of fatalities occur each year as a result of the rapid spread of resistant genes and inappropriate antibiotic usage, which has increased the worldwide burden4,5. If resistance trends continue, mortality is expected to rise. Emerging resistance to existing medicines, including vancomycin, highlights the pressing need for novel antimicrobial approaches6,7,8.
The hunt for synthetic antibiotic substitutes has rekindled interest in medicinal plants, which have been used for ages to treat inflammatory and infectious illnesses. Plant-derived compounds have promised antibacterial qualities and a lower risk of side effects than conventional treatments. They are also frequently more affordable and accessible. When it comes to finding bioactive substances that can solve resistance issues and enhance existing therapies, folk medicine is a useful and little-studied resource9,10,11,12.
The prickly pear, Opuntia monacantha, is unique among therapeutic plants because of its phytochemical diversity and capacity to thrive in arid environments13,14. This plant, which belongs to the Cactaceae family, has antibacterial, hepatoprotective, and antioxidant qualities due to its phenolics, terpenoids, vitamins, and alkaloids15,16,17,18. Opuntia monacantha is positioned as a possible option for the development of novel antimicrobial drugs due to studies on its cladodes and stem extracts showing antibacterial activity, including actions against S. aureus.19,20,21,22 From literature, we have seen that in comparison to gram-negative bacteria (P. multocida and E. coli), OM has demonstrated more consistently articulated antibacterial action against the gram-positive bacteria (S. aureus and B. subtilis). With the most notable zone of inhibition (9 mm and 21.5 mm, respectively) and the smallest inhibitory concentration value (90.03 mg/mL and 52.02 mg/mL, respectively), the n-butanol and chloroform fractions showed high activity against S. aureus in light of these data. The highest activity of the chloroform fraction and methanolic extract against B. subtilis was also shown, with reduced MIC values (45.15 and 70 mg/mL) and maximal zones of inhibition (19.3 and 22.8 mm). The structure of the cell membrane and the phytochemical characteristics of the plant are the reasons for showing more resistance towards gram-negative bacteria in comparison to gram-positive bacteria23,24. Medicinal plants produce an infinite number of secondary metabolites with strong antibacterial action, as demonstrated by in vitro tests25,26,27. These low molecular weight antibiotics derived from plants are divided into two categories: phytoanticipins, which have microbial inhibitory effects, and phytoalexins, which are primarily anti-oxidative and are created anew by plants in reaction to microbial infection28,29. Plant antimicrobial secondary metabolites can be broadly categorized into following groups: alkaloids, terpenes, and phenolic chemicals. Several studies have demonstrated that the plant extracts’ and their active compounds’ antimicrobial activity can potentially induce the production of the reactive oxygen species, promote disruption of cell wall and lysis, prevent the formation of biofilms, prevent the construction of cell walls, prevent microbial DNA replication, prevent energy synthesis, and prevent bacterial toxins from harming the host30.
In this study, we investigated the antibacterial potential of the methanolic extract of Opuntia monacantha Haw. particularly against MRSA strains obtained from Ittefaq Trust Hospital, Lahore, Pakistan.
Results
Total percentage yield of extraction and fractionation
The total yield of OM along with its subsequent fractions is shown in Table 1 which represents CM-OM 5% however the yields of nH-OM, Chl-OM, and EA-OM were 16.1%, 9.5%, and 8.5% respectively. The yield of nH-OM was 16.1% greater than that of the other fractions.
Phytochemical screening
In accordance with the USP standards, qualitative phytochemical screening was performed to investigate the presence of secondary metabolites in the crude methanolic extract and fractions31,32. The outcomes are presented in Table 2.
Screening of primary and secondary metabolites
Total phenolic content
With the help of linear regression curves, the TPC was calculated via equation which was y = 0.0109x + 0.022 (R2 = 0.9995), using gallic acid was used to create standard curve, as shown in Fig. 1. The TPC values were expressed as milligrams of gallic acid equivalent for each gram of extract. (mg GAE/g extract) and is presented in Table 3.
Total flavonoid contents (TFC)
As illustrated in Fig. 2, the TFC was determined using the equation y = 0.0078x + 0.0018 (R2 = 0.9993) derived from the linear regression curve created using quercetin as a reference. Table 4 displays the TFC results as milligrams of quercetin equivalent per gram of extract (mg QE/g extract).
Antioxidant activity
To investigate the properties of free radical scavenging, natural compounds are usually investigated through the DPPH assay. The color of the DPPH solution was compared with the level of antioxidant potential in the tested sample. The percentage inhibition was calculated for the OM crude methanolic extract, different fractions and standards. nH-OM and Chl-OM had the highest percentage of inhibition with low IC50 values. The results are tabulated in Table 5. All the plant extracts had significant antioxidant potential. Triplicate runs of each assay were performed.
GC-MS screening
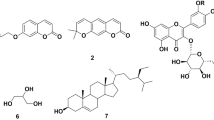
GC–MS of the active fractions n-hexane and chloroform was conducted via National Institute Standard and Technology (NIST) database for further investigation as shown in Figs. 3 and 4. The n-hexane fraction has 32 compounds, and 28 compounds were found in the chloroform fraction. The compounds with the maximum area and reported antioxidant and antimicrobial effects are listed in Tables 6 and 7.
Antibacterial potential of Opuntia monacantha Haw. Cladode
Measurement of antibacterial activity of the extract at 100 mg/ mL
The results in Fig. 5 reveal the clearance zone from the crude methanolic extract of OM and its fractions at 100 mg/ mL. The crude methanolic extract had the maximum inhibitory effect on all the test strains followed by n-hexane, chloroform and ethyl acetate. The negative control did not show any change in condition. Therefore, no data is presented for it. Linezolid and vancomycin which are used as standard drugs significantly inhibited the strains. The zones of inhibition values are given in Table 8 below. All the extracts had a statistically significant effect on every strain of MRSA used in the experiment, as shown in Fig. 6.
Zone of inhibition (ZOI) of the crude methanolic extract of Opuntia monacantha (CM-OM) and its fractions n-hexane (nH-OM), chloroform (Chl-OM), and ethyl acetate (EA-OM) against MRSA strains at 100 mg/ mL along with the negative control (distilled water) and positive control linezolid (LZ) (30 µg) and vancomycin (VAN) (30 µg), (A–D) represents the ZOI of 100 mg of crude methanolic extract and its fractions on MRSA 1, (E–F) represents the ZOI of 100 mg of crude extract and its fractions on MRSA 2, (I–L) represents the ZOI of 100 mg of crude extract and its fractions on MRSA 3 and (M–P) represents the ZOI of 100 mg of the crude extract and its fractions on MRSA 4.
Antibacterial activity of crude methanolic extract of Opuntia monacantha (CM-OM), its fractions nH: n-hexane, Chl: Chloroform EA: ethyl acetate at 100 mg/mL and the commercially available antibiotic discs LZ: Linezolid 30 µg/disk and VAN: vancomycin 30 µg/disk. (a–d) Different strains of methicillin resistant Staphylococcus aureus. The mean zones of inhibition (mm) created by the respective treatments against each bacterial strain were recorded. The data presented are the means of three independent experiments ± SD. *P < 0.05; **P < 0.01, ***P < 0.001 ****P < 0.0001 ns P > 0.05 (for comparisons of all treatments by one-way ANOVA, followed by Tukey’s Test).
The inhibitory zone diameter (mm) includes the well diameter of 6 mm. The values are given as mean ± SDs of three different experiments. Zones of inhibition < 7 mm were considered not to have any activity, Zones between 8 and 11 mm were considered active, and Zones > 11 mm were considered very active.
Measurement of the antibacterial activity of the 75 mg/mL of extracts
The results in Fig. 7 reveal the clearance zone from the crude methanolic extract of OM and its fractions at 75 mg/ml. The crude methanolic extract had the greatest inhibition effect, followed by n-hexane and chloroform. Compared with the standard drugs linezolid and vancomycin, all the extracts significantly inhibited every test strain of MRSA. Unfortunately, ethyl acetate did not exhibit any inhibition at this dose. The negative control also did not show any change in its condition. Therefore, no data are presented for it. The zones of inhibition values are given in Table 9 below. The significant results are shown graphically in Fig. 8.
Zone of inhibition (ZOI) of the crude methanolic extract of Opuntia monacantha (CM-OM) and its fractions n-hexane (nH-OM), chloroform (Chl-OM), and ethyl acetate (EA-OM) against MRSA strains at 75 mg/mL along with negative control (distilled water) and positive control linezolid (LZ) (30 µg) and vancomycin (VAN) (30 µg), (A–D) represents the ZOI of 75 mg of crude extract and its fractions on MRSA 1, (E–F) represents the ZOI of 75 mg of crude extract and its fractions on MRSA 2, (I–L) represents the ZOI of 75 mg of crude extract and its fractions on MRSA 3 and (M–P) represents the ZOI of 75 mg of crude extract and its fractions on MRSA 4.
Antibacterial activity of the crude methanolic extract of Opuntia monacantha (CM-OM), its fractions nH: n-hexane, Chl: chloroform at 75 mg/mL and commercially available antibiotic discs LZ: linezolid 30 µg/disk and VAN: vancomycin 30 µg/disk. (a–d) Different strains of methicillin resistant Staphylococcus aureus. The mean zones of inhibition (mm) created by the respective treatments against each bacterial strain were recorded. The data presented are the means of three independent experiments ± SD. *P < 0.05; **P < 0.01, ***P < 0.001 ****P < 0.0001 ns P > 0.05 (for comparisons of all treatments by one-way ANOVA, followed by Tukey’s Test).
The inhibitory zone diameter (mm) includes the well diameter of 6 mm. The values are given as mean ± SDs of three different experiments. Zones of inhibition < 7 mm were considered not to have any activity, Zones between 8 and 11 mm were considered active, and Zones > 11 mm were considered very active.
Measurement of antibacterial activity of the extracts at 50 mg/ml
The results in Fig. 9 reveal the clearance zone from the crude methanolic extract of OM and its fractions at 50 mg/mL. All the extracts significantly affected every test strain of MRSA except the ethyl acetate fraction. The crude extract and n-hexane fraction showed maximum inhibition at this dose followed by the chloroform fraction. Ethyl acetate and negative control also did not show any change. Therefore, no data are presented for it. The zones of inhibition values are given in Table 10. The significant results in comparison with the standards are shown graphically in Fig. 10.
Zones of inhibition (ZOI) of crude methanolic extract of Opuntia monacantha (CM-OM) and its fractions n-hexane (nH-OM), chloroform (Chl-OM), and ethyl acetate (EA-OM) against MRSA strains at 50 mg/mL along with negative control (distilled water) and positive control linezolid (LZ) (30 µg)and vancomycin (VAN) (30 µg), (A–D) represents the ZOI of 50 mg of crude methanolic extract and its fractions on MRSA 1, (E–F) represents the ZOI of 50 mg of crude extract and its fractions on MRSA 2, (I–L) represents the ZOI of 50 mg of crude extract and its fractions on MRSA 3 and (M–P) represents the ZOI of 50 mg of crude extract and its fractions on MRSA 4.
Antibacterial activity of the crude methanolic extract of Opuntia monacantha (CM-OM), its fractions nH: n-hexane, Chl: chloroform at 50 mg/mL and the commercially available antibiotic discs LZ: linezolid 30 µg/disk and VAN: vancomycin 30 µg/disk. (a–d) Different strains of methicillin resistant Staphylococcus aureus. The mean zones of inhibition (mm) created by the respective treatments against each bacterial strain were recorded. The data presented are the means of three independent experiments ± SD. *P < 0.05; **P < 0.01, ***P < 0.001 ****P < 0.0001 ns = P > 0.05 (for comparisons of all treatments by one-way ANOVA, followed by Tukey’s Test).
The inhibitory zone diameter (mm) includes the well diameter of 6 mm. The values are given as mean ± SDs of three different experiments. Zones of inhibition < 7 mm were considered not to have any activity, Zones between 8 and 11 mm were considered active, and Zones > 11 mm were considered very active.
Measurement of mics of the crude methanolic Opuntia monacantha Haw. and its fractions against MRSA
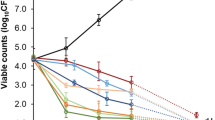
The MICs of the fractions and the crude methanolic extract of OM were investigated via well diffusion assay. The negative control (distilled water) had no inhibitory effect. The crude methanolic acid had the strongest inhibitory effect against all strains with an MIC value of 3.12 mg/mL against MRSA 1, MRSA 2, MRSA 3 and MRSA 4 as shown in Table 11. The n hexane fraction extract had an MIC value of 6.25 mg/mL whereas the chloroform fraction of OM had MIC value of 12.5 mg/mL and the ethyl acetate fraction had an MIC value of 100 mg/mL against MRSA strains.
Discussion
Because of advancements in the medicinal effects of plants and herbs, the importance of natural products has steadily increased in recent decades. Pakistan is home to a wide variety of plants, and the local populace has long used many of these naturally occurring herbs and plants for their medicinal properties. Data show that approximately 25% of various pharmaceutical drugs are generated globally based on the understanding of traditional usage of medicinal plants33. The chemical makeup of the natural source, which is based on the availability of secondary metabolites with significant medicinal value, determines the therapeutic efficacy of these pharmaceutical products. The goal has been to create novel pharmacological entities with the greatest possible therapeutic effects and the least or minimum adverse effects possible. However, as herbal formulations are typically delivered in the form of extracts, teas, juices, and decoctions, use of these goods rationally depends on product quality assurance, which is a prerequisite step in the validation and identification of natural products in any medicinal system. As a result, it is crucial that every component of the plant be genuine and devoid of any adulteration or contamination34. The WHO is consistently highlighting the need for a standardized mechanism for all commercial herbal medicines, given the significant variance between formulation batches35.
With these considerations in mind, this research was conducted to evaluate the antibacterial efficacy of OM to determine its therapeutic importance and establish scientific documentation. The plant was collected and after drying it was subjected to the phytoconstituents extraction via cold maceration involving methanol as a solvent. The fractionation of extracted crude methanolic extract was performed with n-hexane, chloroform and ethyl acetate in the sequence of increasing polarity. The total yield of CM-OM was 5% followed by the subsequent fractions nH-OM, Chl-OM, and EA-OM, which were 16.1%, 9.5%, and 8.5% respectively. The yield of EA was the lowest among all fractions. The nature and volume of solvent mixed during the extraction process determine how much amount can be recovered from a plant, and variance in the extracted components might occur from sample to sample24,36. Pharmacognostic studies are always a crucial for standardizing crude herbal drugs and establishing quality control standards37. According to official guidelines, common standardization methods include physicochemical tests, chromatographic fingerprinting, DNA profiling, macroscopic/microscopic characterizations, chemical tests, quantitative determination of specific compounds or markers or a group of compounds, and tests for microorganisms and chemical contaminants38.
Phytochemical analysis of the crude methanolic extract of OM and its fractions revealed the presence of alkaloids, tannins, saponins and flavonoids whereas steroids were detected only in the crude extract and n-hexane fraction. However anthraquinones were absent39.
The total phenolic and flavonoid content of the OM extract along with its fractions were analyzed via linear regression equation with gallic acid and catechins used as standards as shown in Figs. 1 and 2 respectively. The results revealed that plant extract contained phenolic compounds, flavonoids and their derivatives40,41. Flavonoids, one of the group of phenolic compounds are also known to have antioxidant and antimicrobial activity42. Antioxidant activity was quantified via DPPH assay which revealed that the percentage inhibition by the n-hexane fraction was the highest (88.43 ± 1.14), with low IC50 value of 79.73 µg/mL. According to the findings, phenolic chemicals made a substantial contribution to medicinal plants’ antioxidant potential43. We can say that plants that are rich in phenolics, have hydroxyl groups that enable them to scavenge free radicals and have antioxidant properties21,44. The various active ingredients found in herbal plants, pharmaceutical drugs or food industry, forensic, and environmental applications, such as, alkaloids, organic acids, alcohols, esters, steroids and long chain hydrocarbons, should be tested. One of the best fast and accurate techniques for the analysis of the obtained extracts is GC-MS45. This method uses gas chromatography to separate the constituents from the mixture, and mass spectrometry to examine each component independently46. The samples were subjected to GC-MS analysis, and the results were compared with public data from the NIST library as shown above in Tables 6 and 7. Chemical studies have shown that OM mainly contains phenols, alkanes, fatty acids, terpenoids, etc., The major compounds identified in the n-hexane fraction are tetradecane, 2,4-di-tert-butylphenol, 14-methylpentadecanoic acid, octacosyl heptafluorobutyrate, stigmasta-3,5-diene and beta-sitosterol. All these compounds have been reported in the literature to possess antimicrobial and antioxidant activities. Studies performed by Sallam and Abed 2022 and Badar and Shabban in 2011 reported tetradecane as an antibacterial compound47,48. In other studies performed by Aissaoui and Mahjoubi in 2018, 2,4-di-tert-butylphenol was characterized as an antibacterial compound. Octacosyl heptaflurobutyrate has been reported as a major compound with high antioxidant capacity done by Elwekeel and Hassan 202349. Other compounds such as 14-methylpentadecanoic acid,, stigmasta-3,5-diene, and beta-sitosterol have also been reported as antimicrobial agents50,51,52. The compounds isolated from the chloroform fraction were 2,4-Di-tert-butylphenol, 3,4, dimethylbezadledehyde, tetracosane, benzenepropanoic acid, 3-(1,1-dimethylethyl)-4-hydroxy-, methyl ester and methyl stearate. 2,4-di-tert-butylphenol is one of the prominent compounds in this study and possesses an anti-MRSA activity, as reported previously53. Polyphenol such as benzenepropanoic acid, 3-(1,1-dimethylethyl)-4-hydroxy-,methyl ester known to have strong antioxidant and antifungal activities54. Other compounds found in our study such as 3,4, dimethyl benzaldehyde, tetracosane, and methyl stearate also possess antibacterial activity55,56,57. However, follow-up experiments is required to isolate and evaluate these compounds individually to determine their MIC values against MRSA.
The main objective of our investigation was to assess the possible antibacterial activity of Opuntia monacantha against MRSA. To our knowledge, it is the first report of antibacterial activity against MRSA by OM species present in Pakistan. The crude methanolic extract of the OM extract had the greatest inhibitory effect on all the MRSA strains. This significant inhibitory activity of the methanolic extract has also been observed against resistant S. aureus by other species of Opuntia21,58,59. Significant inhibitory activity was also observed with the n-hexane fraction, followed by the chloroform fraction as shown in Tables 8 and 9, and 10. These findings are also consistent with the studies of Ennouri et al., 2014 and Elkady et al., 2022 who measured the antibacterial activity of O. inermis hexane extract and O. ficus indica against S. aureus, respectively. They also reported marked significant inhibition of Staphylococcus bacteria by these species60,61. However, the ethyl acetate fraction had a non-significant effect on all the MRSA strains. This finding is not consistent with the literature, where ethyl acetate is known to inhibit S. aureus58,62,63. It is possible that the difference in extraction method and various environmental factors affects the phytoconstituents present in the OM account for their different capacities toward strains of MRSA.
Previous studies have also shown that OM species have a significant effect on the inhibition of S. aureus20,24,64. Our findings are consistent with several earlier studies that demonstrated that phenolic compounds from natural extracts have greater inhibitory effects on gram-positive bacteria than on gram-negative bacteria. The properties of the drug (hydrophobicity or hydrosolubility) and the makeup of the microbial membrane determine how susceptible bacteria are to medications65. Extracts from Opuntia cladodes may have antimicrobial properties because of their high polyphenol content66. A number of polyphenols, including phenolic acids, tannins, lignans, stilbenes, flavonoids (particularly flavonols), and combinations of these in botanical mixtures, have demonstrated strong antibacterial action against both resistant and non-resistant Gram-positive bacteria at low µg/mL range MIC values. Cell walls, lipid membranes, membrane receptors, ion channels, bacterial metabolites, and biofilm formation are all targets of their varied mode of action. Certain combinations of polyphenols and antibiotics were also shown to have synergistic effects23.
The effectiveness of OM and its fractions in reducing MRSA growth was reported in terms of MIC. According to the MIC values as shown in Table 11, CM-OM, even at the lowest measured concentration (3.12 mg/mL), it appears to be effective at preventing the growth of MRSA strains because organisms are absent from all tested concentrations. The n-hexane (nH-OM) and chloroform (Chl-OM) fractions exhibit variable degrees of inhibition and partial effectiveness. nH-OM has shown antibacterial potential between 3.15 and 6.25 mg/mL. For Chl-OM organisms are absent at concentrations above 6.25 mg/mL but present at both 3.12 and 6.25 mg/mL, indicating a MIC slightly above 3.12 mg/mL. However, within the studied concentration range, the ethyl acetate fraction (EA-OM) has no inhibitory effect on all MRSA strains. The results of this examination clearly show that CM-OM is the most promising candidate with high antibacterial activity for additional development in antimicrobials, whereas EA-OM shows little to no activity at the tested dosages.
According to published data, plants with high potential as antimicrobials against bacteria typically have a low minimum inhibitory concentration (MIC), and vice versa. To combat increasing medication resistance in humans, the development of new antimicrobial metabolites from medicinal plants such as OM is crucial. Plant extracts with established antibacterial qualities can play a significant role in medical interventions. Opuntia species been utilized for long to treat various harmful bacterial infections67.
Conclusion & future prospective
Many pharmacognostics studies and kinetics of medicinal plants have demonstrated that plant-derived bioactive chemicals and crude extracts may increase the effectiveness of conventional antimicrobial agents, which may be less expensive, cause fewer side effects, and result in better treatment outcomes. The findings of our research revealed that Opuntia monacantha Haw. methanolic extract and its fractions (n-hexane and chloroform) had antibacterial effects on methicillin-resistant Staphylococcus aureus strains that had been clinically isolated at different concentrations. The activity of crude extract was greater than that of other three extracts because the synergistic properties of other extracts decreased after partitioning. This study validates its historic use in treating bacterial illness. On the basis of these findings, medicinal plants such as Opuntia monacantha Haw. are recommended for use as an alternative therapy for infections caused by bacteria in developing countries. As shown in this study, numerous compounds with antimicrobial and antioxidant activities, the OM extract can be used in pharmaceuticals as an alternative for resistant bacterial strains. However, there are also some study limitations such as limited MRSA strains were tested, potential variability in OM composition from different environments.
Further exploration is required such as in in-vivo studies, toxicity profile using animal models, and in-silico studies along with molecular docking is essential for the isolation and characterization of the active compounds responsible for the antibacterial activity of the extract to explore the binding potential of the ligands to their target binding sites.
Materials and methods
Plant material collection and pretreatment
The cladodes of Opuntia monacantha Haw. (Cactaceae) as shown in Fig. 11 was collected from the Lahore to Islamabad Motorway (M1-Motorway, Kalarkhar) Punjab, Pakistan in May and June 2023. The identification and authentication of sample was performed by taxonomist Dr. Uzma Hanif, Botany Department of Government College University (GCU), Lahore, Pakistan under the voucher No: GC. Herb. Bot. 4053 for Opuntia monacantha Haw and plant specimen was deposited in the herbarium of Department of Botany, Government College University Lahore, Pakistan with GC. Herb. Bot No.4053. The collection of plants for this study is conducted solely for educational research purposes, adhering strictly to the general publication standards and ethical guidelines of the University of Central Punjab, Lahore, Pakistan. As per these guidelines, no specific permission is required for plant collection in such contexts, provided the research is conducted responsibly and does not involve endangered or protected species or violate local environmental laws. All efforts are made to ensure ethical and sustainable practices during the collection process. This is according to gee After Opuntia monacantha Haw. was collected it was washed carefully with distilled water for surface cleaning and elimination of dust particles as well as contaminants. The plant cladodes were left under shade for air drying at the Pharmacology Research Lab, University of Central Punjab, for 20 days. The dried form was pulverized into fine powder via electrical blender and stored within an airtight container. The study design methodology has been shown in Fig. 12.
Preparation of extract
Using the cold maceration procedure, the powdered plant material (400 g) was precisely weighed and then dipped into 1.3 L of methanol at room temperature (RT) intended for a duration of one whole week. Once the methanolic extract was filtered, the same process was performed three times. The filtrate was subjected to drying via a rotary evaporator set to 40–45 °C at reduced pressure. After that, the residue was collected, weighed and combined with distilled water. Next, it is fractionated by using various solvents (the n-hexane, chloroform, ethyl acetate) that increase in polarity68.
All the fractions were then dried and weighed and calculated the percentage yield of each fraction with the formula given below.
Microorganisms
The microorganisms, used in the study namely methicillin resistant Staphylococcus aureus (MRSA 1, MRSA 2, MRSA 3, MRSA 4) used in the study were confirmed clinical isolate provided by the microbiology department, Ittefaq Trust Hospital, Lahore by Dr. Roman. Phenotype resistance of the strains was maintained throughout the study by keeping control strains with known phenotype resistance as a benchmark to prepare fresh subcultures to avoid the genetic drift.
Media preparation
The bacterial media prepared for the antimicrobial assay. Mueller Hinton agar was used to grow bacteria. In one liter of distilled water, 38 g of powder were dissolved. The mixture was heated until it dissolved completely, and then autoclaved it for 15 min at 121 ˚C (15 Ibs) pressure to sanitize it. Once the mixture had cooled to 45–50˚C, it was transferred into petri dishes.
Well diffusion method
The assay was run to investigate antimicrobial potential. Mueller-Hinton agar media was prepared and sterilized according to standard protocols. The agar plate surface is inoculated by spreading a volume of the microbial inoculum over the entire agar surface. Then a circular holes 6 mm are made aseptically into the agar using a sterile cork borer, and a suitable volume 100 µL of the sample solution at the chosen concentration is applied into the well and then incubated it for 24 h69. The assay was performed in triplicate and zones of inhibition around the wells were calculated at three doses (100, 75 and 50 mg/mL)70,71. Doses were selected with reference to already established anti MRSA activity of another species of Opuntia known as Opuntia ficus indica.21 Compared to manufactured antibiotics, substances produced from plants may have less effective antibacterial action. But with high concentration, molecules with low activity can be evaluated successfully, capturing any possible therapeutic benefit.
Measurement of the minimum inhibitory concentration
The minimum inhibitory concentrations (MICs) of the crude methanolic extract and the n-hexane, chloroform and ethyl acetate fractions were determined via the well diffusion method. This method was selected according to the availability of resources72. Concentrations of 25, 12.5, 6.25, and 3.12 mg/mL of Opuntia monacantha methanolic extract and its fractions were formed. Negative controls for the experiment were formed using distilled water alone. Zones of inhibition diameter were used to calculate the antibacterial activity of each sample73.
Antioxidant activity
Opuntia monacantha and its fractions were interpreted via DPPH assay as explained by74,75 for their antioxidant activity. The stock solutions (1 mg/mL) of OM and the fractions were made in methanol. Various concentrations (50, 100, 150, 200 and 250 µg/mL) of OM, fractions and ascorbic acid (standard compound) were prepared. The standards and samples were incubated for 30 min at room temperature. A UV-Vis spectrophotometer used to measure absorbance at 517 nm. The assay was performed in triplicates and absorbance decrease was recorded. The DPPH activity was calculated with formula given below.
\(\left( \% \right){\text{ Inhibition \, of \, DPPH \, activity }}={\text{ }}[({\text{Ac}} - {\text{As}}) \div {\text{Ac}}] \times {\text{1}}00\)
Where Ac is control absorption and As is sample absorbance respectively.
Gas chromatography-mass spectrometry (GC-MS) analysis
An Agilent 19091-433HP, USA gas chromatograph system and mass spectrophotometer were used to perform the GC-MS analysis. The apparatus was equipped with a column known as HP-5 MS fused silica column (consists of 5% phenyl and 95% methyl siloxane) interfaced with a 5675 C. Mass selective detector (inert) with Triple-Axis detector. Velocity flow of 1.0 milliliters per minute was present in a column, the carrier gas helium was used.
The additional GC-MS parameters included an ion source of 250 °C; Interface with 300 °C for; 16.2 psi; 1.8 mm for the outer time; and an injector of 1 µL of sample in split mode with a split ratio of 1:50 with 300 °C injection temperature. The temperature in the column rose to 150 °V at a rate of 4 °C per minute after five minutes at 36 °C. The temperature was raised to 250 °C at a rate of 20 °C per minute, and it was then held there for five minutes. Elution took 47.5 min in total. The proportional percentage of every component was ascertained in comparison with its average peak area to the total area76.
Statistical analysis
The mean ± SEM was used to express the data and analyzed via GraphPad Prism software 5.00 (San Diego, USA), compared via one way ANOVA with Tukey test and plotted. Data was considered significant at P < 0.05.
Data availability
All data is mentioned in the manuscript.
References
Algammal, A. M. et al. Methicillin-resistant Staphylococcus aureus (MRSA): One health perspective approach to the bacterium epidemiology, virulence factors, antibiotic-resistance, and zoonotic impact. Infection and drug resistance, 3255–3265. (2020).
De Oliveira, D. M. et al. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 33 (3). https://doi.org/10.1128/cmr (2020). 00181 – 19.
Lena, P., Ishak, A., Karageorgos, S. A. & Tsioutis, C. Presence of methicillin-resistant Staphylococcus aureus (Mrsa) on healthcare workers’ attire: A systematic review. Trop. Med. Infect. Disease. 6 (2), 42 (2021).
Motamedi, H., Darabpour, E., Gholipour, M. & Seyyed Nejad, S. M. In vitro assay for the anti-brucella activity of medicinal plants against tetracycline-resistant Brucella melitensis. J. Zhejiang Univ. Sci. B. 11, 506–511 (2010).
Baquero, F. Threats of antibiotic resistance: an obliged reappraisal. Int. Microbiol. 24 (4), 499–506 (2021).
Jian, Z. et al. Antibiotic resistance genes in bacteria: occurrence, spread, and control. J. Basic Microbiol. 61 (12), 1049–1070 (2021).
Murray, C. J. et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399 (10325), 629–655 (2022).
Verma, S. K. et al. Sulfur (SVI)-containing heterocyclic hybrids as antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA) and its SAR. Bioorg. Chem., 107241 (2024).
Nicerine, O., Houda, E., Jamila, R. & Atmane, R. Ethnobotanical survey of medicinal plants used in the traditional treatment of genito-urinary diseases in the region of Fez, Morocco. J. Herb. Med., 100861. (2024).
Nigussie, D. et al. Antibacterial activity of methanol extracts of the leaves of three medicinal plants against selected bacteria isolated from wounds of lymphoedema patients. BMC Complement. Med. Ther. 21 (1), 2 (2021).
Chattopadhyay, R. R. et al. A comparative evaluation of antibacterial potential of some plants used in Indian traditional medicine for the treatment of microbial infections. Brazilian Archives Biology Technol. 52, 1123–1128 (2009).
Gerstel, J. et al. Identification of botanicals with potential therapeutic use against methicillin-resistant Staphylococcus aureus (MRSA) infections. Phytother. Res. 32 (12), 2577–2585 (2018).
Rodriguez-Lopez, A. D. et al. Food Industry by-products Valorization and New Ingredients: Cases of Study, in Sustainability of the Food System, 71–99 (Elsevier, 2020).
da Silva, V. M. et al. Bioactive compounds of the opuntia monacantha fruit. Nat. Prod. Res. 37 (11), 1863–1866 (2023).
Aragona, M., Lauriano, E., Pergolizzi, S. & Faggio, C. Opuntia ficus-indica (L.) miller as a source of bioactivity compounds for health and nutrition. Nat. Prod. Res. 32 (17), 2037–2049 (2018).
Veeramani kandan, P. et al. Isolation and characterization of opuntiol from opuntia ficus indica (L. Mill) and its antiproliferative effect in KB oral carcinoma cells. Nat. Prod. Res. 35 (18), 3146–3150 (2021).
Katanić, J. et al. Characterization of bioactivity and phytochemical composition with toxicity studies of different opuntia dillenii extracts from Morocco. Food Bioscience. 30, 100410 (2019).
Surup, F. et al. Opuntisines, 14-membered cyclopeptide alkaloids from fruits of opuntia stricta Var. Dillenii isolated by high-performance countercurrent chromatography. Food Chem. 334, 127552 (2021).
Abid, F. et al. Opuntia monacantha: validation of the anti-inflammatory and anti-arthritic activity of its polyphenolic rich extract in silico and in vivo via assessment of pro-and anti-inflammatory cytokines. J. Ethnopharmacol., 117884. (2024).
El-Hawary, S., El-Tantawy, M., Rabeh, M. & Badr, W. Chemical composition and antimicrobial activity of volatile constituents of cladodes, fruits Peel and fruits pulp from opuntia ficus indica (L.) Mill.(Prickly Pear) growing in Egypt. Egypt. J. Chem. 64 (1), 437–444 (2021).
Malathi, G. & Murugesan, A. K. Phytochemical analysis, antioxidant and antibacterial properties of opuntia ficus-indica (L.) mill. Against the wound infecting bacteria. Int. J. Bot. Stud. 6, 367–373 (2021).
Ben Lataief, S. et al. Chemical composition, antioxidant, antimicrobial and cytotoxic activities of bioactive compounds extracted from opuntia dillenii cladodes. J. Food Meas. Charact. 15, 782–794 (2021).
Álvarez-Martínez, F. J. et al. Antimicrobial capacity of plant polyphenols against gram-positive bacteria: A comprehensive review. Curr. Med. Chem. 27 (15), 2576–2606 (2020).
Bari, M. N. et al. Biological activities of opuntia monacantha cladodes. J. Chem. Soc. Pak., 34 (4). (2012).
Casciaro, B. et al. Nigritanine as a new potential antimicrobial alkaloid for the treatment of Staphylococcus aureus-induced infections. Toxins 11 (9), 511 (2019).
Mickymaray, S. et al. Screening and antibacterial efficacy of selected Indian medicinal plants. Asian Pac. J. Trop. Biomed. 6 (3), 185–191 (2016).
Dewapriya, P. et al. Structure and biosynthesis of extensively N-methylated linear peptides from an Australian marine tunicate-derived talaromyces Sp. Front. Chem. 6, 394 (2018).
Sukalingam, K., Ganesan, K. & Xu, B. Trianthema portulacastrum L.(giant pigweed): Phytochemistry and pharmacological properties. Phytochem. Rev. 16, 461–478 (2017).
Sukalingam, K., Ganesan, K. & Xu, B. Protective effect of aqueous extract from the leaves of Justicia tranquebariesis against thioacetamide-induced oxidative stress and hepatic fibrosis in rats. Antioxidants 7 (7), 78 (2018).
Mickymaray, S. Efficacy and mechanism of traditional medicinal plants and bioactive compounds against clinically important pathogens. Antibiotics 8 (4), 257 (2019).
Dubale, S. et al. Phytochemical screening and antimicrobial activity evaluation of selected medicinal plants in Ethiopia. J. Exp. Pharmacol., 51–62. (2023).
Verma, A. K. & Singh, S. Phytochemical analysis and in vitro cytostatic potential of ethnopharmacological important medicinal plants. Toxicol. Rep. 7, 443–452 (2020).
Alghanem, S. M. & El-Amier, Y. A. Phytochemical and biological evaluation of pergularia tomentosa L.(Solanaceae) naturally growing in arid ecosystem. Int. J. Plant. Sci. Ecol. 3 (2), 7–15 (2017).
Wang, H. et al. Advancing herbal medicine: enhancing product quality and safety through robust quality control practices. Front. Pharmacol. 14, 1265178 (2023).
Prabhakar, P. & Mamoni, B. Technical problems, regulatory and market challenges in bringing herbal drug into mainstream of modern medicinal practices. Res. J. Biotechnol. 16, 3 (2021).
Abubakar, A. R. & Haque, M. Preparation of medicinal plants: basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 12 (1), 1–10 (2020).
Rashid, S. et al. Microscopic investigations and pharmacognostic techniques used for the standardization of herbal drug Nigella sativa L. Microsc. Res. Tech. 81 (12), 1443–1450 (2018).
Noviana, E., Indrayanto, G. & Rohman, A. Advances in fingerprint analysis for standardization and quality control of herbal medicines. Front. Pharmacol. 13, 853023 (2022).
Saleem, M., Irshad, I., Baig, M. K. & Naseer, F. Evaluation of hepatoprotective effect of chloroform and methanol extracts of opuntia monacantha in paracetamol-induced hepatotoxicity in rabbits. ||| Bangladesh J. Pharmacol. 10 (1), 16–20 (2015).
Abid, F. et al. Opuntia monacantha: validation of the anti-inflammatory and anti-arthritic activity of its polyphenolic rich extract in Silico and in vivo via assessment of pro-and anti-inflammatory cytokines. J. Ethnopharmacol. 326, 117884 (2024).
Jaios, E. S., Abdullah, M. Q. H. & Wahab, I. R. A. Biological activities and LC-MS/MS profiling of methanolic extract of Opuntia monacantha Haw.(Cactaceae). In AIP Conference Proceedings. (AIP Publishing, 2022).
Abeysinghe, D. et al. Phytochemical screening, total polyphenol, flavonoid content, in vitro antioxidant and antibacterial activities of Sri Lankan varieties of Murraya koenigii and Micromelum minutum leaves. Heliyon, 7 (7). (2021).
Ayele, D. T., Akele, M. & Melese, A. Analysis of total phenolic contents, flavonoids, antioxidant and antibacterial activities of Croton macrostachyus root extracts. BMC Chem. 16 (1), 30 (2022).
Chaves, N., Santiago, A. & Alías, J. C. Quantification of the antioxidant activity of plant extracts: analysis of sensitivity and hierarchization based on the method used. Antioxidants 9 (1), 76 (2020).
Konappa, N. et al. GC–MS analysis of phytoconstituents from amomum Nilgiricum and molecular Docking interactions of bioactive Serverogenin acetate with target proteins. Sci. Rep. 10 (1), 16438 (2020).
Arora, S., Kumar, G. & Meena, S. GC-MS analysis of bioactive compounds from the whole plant hexane extract of cenchrus Setigerus Vahl. Pharma Sci. Monit. 8 (4), 137–146 (2017).
Badr, S. E. et al. Chemical composition and biological activity of ripe pumpkin fruits (Cucurbita Pepo L.) cultivated in Egyptian habitats. Nat. Prod. Res. 25 (16), 1524–1539 (2011).
Sallam, K. M. & Abed, N. Evaluation of antimicrobial potential of tetradecane extracted from pediococcus acidilactici DSM: 20284-CM isolated from curd milk. Egypt. J. Chem. 65 (3), 705–713 (2022).
Elwekeel, A. et al. Anti-inflammatory, anti-oxidant, GC-MS profiling and molecular Docking analyses of non-polar extracts from five Salsola species. Separations 10 (2), 72 (2023).
Onifade, A. K., Akinyemi, D. D. & Ogundare, A. O. Antibacterial Activities of Vernonia Amygdalina (Del.) Stem Bark Extracts on Multiple antibiotic-resistant bacteria Isolated from Wound Samples (Microbes and Infectious Diseases, 2023).
Uttu, A. J., Sallau, M. S., Ibrahim, H. & Iyun, O. R. A. Isolation, characterization, and Docking studies of Campesterol and β-sitosterol from strychnos Innocua (Delile) root bark. J. Taibah Univ. Med. Sci. 18 (3), 566–578 (2023).
Nweze, C., Ibrahim, H. & Ndukwe, G. Beta-sitosterol with antimicrobial property from the stem bark of pomegranate (Punica granatum Linn). J. Appl. Sci. Environ. Manage. 23 (6), 1045–1049 (2019).
Chawawisit, K., Bhoopong, P., Phupong, W. & Lertcanawanichakul, M. 2, 4-Di-tert-butylphenol, the bioactive compound produced by Streptomyces sp. KB1. J. Appl. Pharm. Sci., 5 (3), 007–012. (2015).
Kumar, V., Sharma, A., Thukral, A. K. & Bhardwaj, R. Phytochemical profiling of methanolic extracts of medicinal plants using GC-MS. Int. J. Res. Dev. Pharm. Life Sci. 5 (3), 2153–2158 (2016).
Ramesh, S. et al. Nanorod-like structure of ZnO nanoparticles and Zn8O8 clusters using 4-dimethylamino benzaldehyde liquid to study the physicochemical and antimicrobial properties of pathogenic bacteria. Nanomaterials 13 (1), 166 (2022).
Enema, O. et al. Phytochemical and antioxidant studies of leaf of tetrapleura tetraptera (schum and thon) Taubert (mimosaceae). Brifish J. Pharm. Med. Res. 4, 1865–1875 (2019).
Chaidir, Z. et al. Examination of the antibacterial and antifungal properties of fatty acids and fatty acid Methyl ester obtained from Nannochloropsis oculata. Rasayan J. Chem., 13 (2). (2020).
Bargougui, A., Tag, H. M., Bouaziz, M. & Triki, S. Antimicrobial, antioxidant, total phenols and flavonoids content of four cactus (Opuntia ficus-indica) cultivars. Biomedical Pharmacol. J. 12 (2), 1353–1368 (2019).
D’Angeli, F. et al. Antibacterial, Antitumor (Lung Cancer Cell H292) and Antioxidant Properties of Sicilian Prickly Pear Cactus (Opuntia Ficus-Indica) Cladode Extracts, 1943–1960 (Journal of biological regulators and homeostatic agents, 2024).
Ennouri, M., Ammar, I., Khemakhem, B. & Attia, H. Chemical composition and antibacterial activity of opuntia F.cus-indica F. inermis (cactus pear) F.owers. J. Med. Food. 17 (8), 908–914 (2014).
Elkady, W. M. et al. Endophytic fungus from opuntia ficus-indica: a source of potential bioactive antimicrobial compounds against multidrug-resistant bacteria. Plants 11 (8), 1070 (2022).
Alghamdi, A. et al. Research Article Biological Activities and GC-MS Analysis of Aloe vera and Opuntia ficus-indica Extracts. (2023).
Zeshan, M. et al. Efficacy of anti-bacterial compounds and plant extracts against methicillin resistant Staphylococcus aureus isolates from nosocomial infections. (2015).
Das, G. et al. Cactus: chemical, nutraceutical composition and potential bio-pharmacological properties. Phytother. Res. 35 (3), 1248–1283 (2021).
Blando, F. et al. Antimicrobial and antibiofilm activity against Staphylococcus aureus of opuntia ficus-indica (L.) mill. Cladode polyphenolic extracts. Antioxidants 8 (5), 117 (2019).
Bhattacharya, D. et al. Antibacterial activity of polyphenolic fraction of Kombucha against enteric bacterial pathogens. Curr. Microbiol. 73, 885–896 (2016).
Alghamdi, A. et al. Biological activities and GC-MS analysis of Aloe Vera and opuntia ficus-indica extracts. J. Chem. 2023 (1), 6504505 (2023).
Arshad, N., Ishtiaq, S., Khan, F. Z., Analysis, H. P. L. C. G. C. M. S. & Hepatoprotective and antioxidant activities of saussurea hypoleuca spreng. Root. Egypt. J. Chem. 64 (8), 4343–4349 (2021).
Chavez-Esquivel, G. et al. Antimicrobial activity of graphite oxide doped with silver against Bacillus subtilis, Candida albicans, Escherichia coli, and Staphylococcus aureus by agar well diffusion test: synthesis and characterization. Mater. Sci. Engineering: C. 123, 111934 (2021).
Abdullahi, A. et al. Phytochemical profiling and antimicrobial activity of ginger (Zingiber officinale) essential oils against important phytopathogens. Arab. J. Chem. 13 (11), 8012–8025 (2020).
Kassym, L., Kussainova, A., Semenova, Y. & McLoone, P. Antimicrobial effect of honey phenolic compounds against E. coli—An in vitro study. Pharmaceuticals 17 (5), 560 (2024).
Mostafa, A. A. et al. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci. 25 (2), 361–366 (2018).
Shegute, T. & Wasihun, Y. Antibacterial activity and phytochemical components of leaf extracts of Agave Americana. J. Exp. Pharmacol., 447–454. (2020).
Sallam, I. E. et al. Evaluation of antioxidant activity and biotransformation of opuntia ficus fruit: the effect of in vitro and ex vivo gut microbiota metabolism. Molecules 27 (21), 7568 (2022).
Baliyan, S. et al. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of ficus religiosa. Molecules 27 (4), 1326 (2022).
Olivia, N. U., Goodness, U. C. & Obinna, O. M. Phytochemical profiling and GC-MS analysis of aqueous methanol fraction of hibiscus Asper leaves. Future J. Pharm. Sci. 7, 1–5 (2021).
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project, King Saud University, Riyadh, Saudi Arabia for funding this work through the project number (RSP2025R457). Authors also would like to acknowledge the contribution of the microbiology department of Ittefaq hospital, Model Town Lahore, Pakistan, for providing the confirmed resistant strains of MRSA.
Funding
This research is supported by the Researchers Supporting Project Number (RSP2025R457), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
S.J: Conceptualization, Methodology, Formal analysis, Writing - original draft. Data curation, Investigation; M.A.K: Conceptualization, Supervision, Project administration, Investigation; A.A.: Formal analysis, Data curation, Writing - review & editing; H.H.: Methodology, Validation, Visualization, Writing - review & editing; S.U.R: Data curation, Visualization; review & editing, A.I.; Formal analysis, Funding acquisition, Visualization, Writing - review & editing; G.A.S: Data curation; Funding acquisition, Investigation, Writing - review & editing; Y.A.B.J: Funding acquisition, Formal analysis, Visualization; Project administration, Writing - review & editing. All authors reviewed, commented and agreed on the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The collection of plants for this study is conducted solely for educational research purposes, adhering strictly to the general publication standards and ethical guidelines of the University of Central Punjab, Lahore, Pakistan. As per these guidelines, no specific permission is required for plant collection in such contexts, provided the research is conducted responsibly and does not involve endangered or protected species or violate local environmental laws. All efforts are made to ensure ethical and sustainable practices during the collection process.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jameel, S., Khan, M.A., Asif, A. et al. Exploring the antibacterial efficacy of Opuntia monacantha in combatting methicillin-resistant Staphylococcus aureus. Sci Rep 15, 9552 (2025). https://doi.org/10.1038/s41598-025-93939-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-93939-w