Abstract
Previous studies have demonstrated that silica can activate the NLRP3 inflammasome, and that macrophage phagocytosis is an essential step in this process. Although carbon is the primary component of coal dust particles, it also contains other impurities such as free silica, clay, sulfides, and carbonate minerals. Additional research is still necessary to discover if NLRP3 can be triggered due to the low silica content of coal dust particles. The purpose of this study is to investigate whether coal dust particles can induce the translation and transcription of NLRP3 inflammasome components in rat alveolar macrophages (NR8383) and whether Cytochalasin B may inhibit this process. According to the findings of our research, coal dust particles can upregulate the NLRP3 inflammasome components and IL-1β, a downstream inflammatory component. Furthermore, LPS and coal dust particles work in concert to raise the NLRP3 inflammasome components protein and transcript level in macrophages. Interestingly, the protein and transcript level of NLRP3 inflammasome components dramatically dropped when cells were concomitantly exposed to the actin polymerization inhibitor Cytochalasin B. This suggests that cellular uptake is required for coal dust particles to exert their pro-inflammatory effect.
Similar content being viewed by others
Introduction
The pathogenesis of coal workers’ pneumoconiosis (CWP) is complex, with no unified explanation currently available. Yet one important stage in the formation of fibrosis is thought to be the overexpression of inflammatory factors that results in long-lasting immune-inflammatory responses. However, the precise chemical pathways in this process remain unclear. The process of engulfing and clearing coal dust particles is facilitated by alveolar macrophages1. The primary mechanisms involving macrophages in CWP and silicosis may involve the release of mediators by activated alveolar macrophages and epithelial cells, secretion of growth factors by alveolar macrophages and epithelial cells, activation of oxidant production by pulmonary phagocytes, and direct cytotoxicity of coal dust particles or silica2. Studies have demonstrated that inhaled silica can result in lysosomal rupture and phagolysosome instability in macrophages3, subsequently causing the release of inflammatory mediators, damage-associated molecular patterns (DAMPs), tissue proteases, and reactive oxygen species (ROS). The NLRP3 inflammasome may get activated as a result of the release of these substances4.
The NLRP3 inflammasome, a complex composed of proteins such as Caspase-1, ASC, and NLRP3, plays a crucial role in the body’s innate immune response. NLRP3 can sense various pathological stimuli both intracellularly and extracellularly, such as microbial infections, cellular damage signals, and environmental stressors. Upon stimuli sensing, NLRP3 assembles an inflammasome complex that mediates the maturation and release of pro-inflammatory cytokines such as interleukin-18 (IL-18) and interleukin-1β (IL-1β)5,6. NLRP3 inflammasome is pivotal in inflammatory responses. Research has demonstrated that silica can stimulate innate immune receptors, which in turn can transfer immune response signals and activate the NLRP3 inflammasome. This can result in the activation of Caspase-1, the maturation and production of IL-1β and IL-18, as well as the pyroptosis of macrophages. IL-1β further stimulates the secretion of numerous inflammatory factors, sustaining an inflammatory response. Additionally, it can cause fibroblast chemotaxis and the epithelial-mesenchymal transition, which can lead to pulmonary fibrosis and excessive extracellular matrix production7,8,9.
Even though it is still unknown whether coal dust particles cause the NLRP3 inflammasome to become activated in CWP, research has revealed that patients with CWP have significantly higher peripheral blood levels of downstream inflammatory factors, such as IL-1β and IL-18, than healthy dust-exposed controls10,11,12. In addition, studies have demonstrated a substantial correlation between the incidence of CWP and single nucleotide polymorphisms at three NLRP3-related loci13. Therefore, the NLRP3 inflammasome is probably triggered by coal dust particles. Furthermore, although the cytoskeleton of actin is essential for macrophage-mediated inflammatory responses14, its precise function in triggering the NLRP3 inflammasome is still unclear. As a result, we first examined the expression of NLRP3 inflammasome components in macrophages exposed to coal dust particles, and then utilized Cytochalasin B to inhibit macrophage phagocytosis to find if macrophage phagocytosis could affect the expression of NLRP3 inflammasome components induced by coal dust particles.
Materials and methods
Materials
The coal dust particles used in this study was taken from coal mines where workers of Coal Group in Datong City, Shanxi Province, with a silica dioxide content of less than 10%. For the tests, rat alveolar macrophages (NR8383 cells, Wuhan Procell Life Science & Technology Co., Ltd.) were used. Cytochalasin B, an inhibitor of macrophage actin polymerization, was obtained from Sigma-Aldrich in the United States.
Cell culture
Cells were cultured in T25 culture flasks in F-12 K supplemented with 15% fetal bovine serum (FBS) and 1% penicillin-streptomycin. The cultivation was carried out at 37 °C in a 5% CO2 atmosphere.
Preparation of dust stock solution
Coal dust particles was subjected to high-temperature, high-pressure sterilization (101 kPa, 121℃) for 4 h15, followed by 6 h of ultrasonic disintegration. After that, a 300 µg/ml dust stock solution was made by combining the dust with an F-12 K basal medium.
Cell stimulation and grouping
In the exposed to coal dust particles group, NR8383 cells were seeded at a minimum cell density of 10^6cells on a 12-well plate using full F-12 K media, with three replicates per concentration. The original medium was taken out, and the dust stock solution was introduced after two PBS washes. The solution was adjusted to a coal dust particles concentration of 200 µg/ml by diluting with F-12 K medium16, with three replicates per concentration and a volume of 1 mL per well. Cell samples were obtained for Real-time fluorescence quantitative PCR, western blot, and enzyme-linked immunosorbent assay testing (ELISA) of the cell lysates following a 12-hour exposure to coal dust particles. Based on previous research and cell viability testing (Cell Counting Kit-8, please refer to the Supplementary Table S1 for details), the chosen concentration and duration for the coal dust particles stimulation described above were established.
Rat alveolar macrophages were stimulated with lipopolysaccharide (LPS) (1 µg/mL) for 6 h followed by medium for 12 h after NR8383 cells were grown to the proper density in the LPS group17. For the LPS + Coal dust particles group, cells were pre-treated with LPS for 6 h before adding the dust stock solution. By diluting with F-12 K medium, the solution was brought to a concentration of 200 µg/mL of coal dust particles. Thereafter, the cells were grown for a further 12 h. The phagocytosis inhibitor CB (1 μg/ml, MedChemExpress, USA) was applied to the cells in the CB treatment group two hours before their exposure to coal dust particles14.
Real-time fluorescence quantitative PCR
The mRNA relative expression levels of NLRP3, ASC, caspase-1, and IL-1β were determined using Real-time fluorescence quantitative PCR. After the treatments for cell grouping, the culture media was withdrawn, and the cells were washed with PBS 1–2 times. After adding 500 µL of Trizol (Beyotime, Shanghai, China), the cells were separated from the wells and moved into centrifuge tubes. Chloroform, isopropanol, and 75% ethanol were used in consecutive order to extract total RNA following vigorous mixing and a 10-minute incubation. After adding 25 µL of DEPC water to each tube, the RNA was dissolved and then mixed. The purity and concentration of the extracted total RNA samples were assessed, and reverse transcription was carried out according to the instructions of the reverse transcription kit (Thermo Fisher Scientific, USA). The reverse transcription reaction was conducted using a PCR thermal cycler (Thermo Fisher Scientific, USA). As an internal reference gene, GAPDH was employed. The target gene and the reference gene were amplified under the same conditions, and the relative expression of the target gene mRNA was analyzed using the 2−ΔΔ Ct method. The primer sequence information is shown in Table 1.
Western blotting
The protein expression levels of the caspase-1, ASC, and NLRP3 were determined by Western Blotting. The culture media is removed once the cells have been grouped and processed, and they are then rinsed with PBS 1–2 times before being placed in a centrifuge tube. Subsequently, each well receives 150 µL of RIPA lysis buffer with a protease inhibitor. The cells are then gently lysed on ice for 30 min at 4 °C with gentle rotation. The BCA protein assay kit’s (Beyotime, Shanghai, China) instructions are followed for quantifying proteins. The proteins are then separated by PAGE gel ( Epizyme, Shanghai, China) electrophoresis, transferred onto a PVDF (Merck Millipore, Germany) membrane, blocked with 5% skim milk at room temperature for 1 h, and washed with TBST containing 0.05% Tween20 three times for 10 min each. Following application, the primary antibodies are diluted 1:1000 and incubated for the entire night at 4 °C: NLRP3 (1:1000, Absin, shanghai, China)、ASC (1:1000, Proteintech, shanghai, China)、caspase-1 (1:1000, Proteintech, shanghai, China). After three TBST washes of the membrane the next day, the secondary antibody (1: 600, Abmart, shanghai, China) is applied and allowed to sit at room temperature for 1 h. The enhanced chemiluminescence (ECL) approach is used to detect and visualize protein signals following three TBST washes. Visualize the fluorescence images using the digital fluorescence scanner Panoramic P250 (Clinx, Shanghai, China). Image J 1.52 software is used for quantitative analysis of the relative protein expression levels.
Enzyme-linked immunosorbent assay
IL-1β protein expression was measured using the ELISA. Based on the above-mentioned BCA protein quantification results, the cell lysates was diluted to the proper concentration, and the rat IL-1β test kit (NeoBioscience, Shanghai, China) was used to evaluate the protein expression level of IL-1β. The operating steps were strictly in accordance with the manufacturer’s instructions.
Statistical analysis
To ensure the consistency and dependability of the experimental findings, the experiment mentioned above was done three times. Data analysis was performed using SPSS version 23.0. For normally distributed and homoscedastic continuous data, the results are presented as mean ± standard deviation. Differences between the two groups were assessed using the t-test, while one-way analysis of variance (ANOVA) was used for comparisons among multiple groups. Post-hoc multiple comparisons of group means were conducted using the Least Significant Difference test. In cases where the data did not follow a normal distribution, non-parametric tests were applied. A significance level of P < 0.05 was used to indicate statistical differences.
Results
Expression of NLRP3 inflammasome components in rat alveolar macrophages exposed to coal dust particles and treated CB
In order to investigate if coal dust particles may promote the upregulation of NLRP3 inflammasome components, rat alveolar macrophages were exposed to coal dust particles, stimulated with LPS, and treated with both at the same time. Additionally, CB treatment was administered to the coal dust particles group in order to find out whether NLRP3 inflammasome components can be upregulated by coal dust particles uptake (Fig. 1).
Coal dust particles exposure upregulated NLRP3 inflammasome components and IL-1β in rat alveolar macrophages
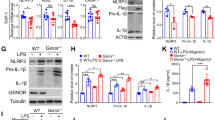
The mRNA of NLRP3, Caspase-1, IL-1β, and ASC in macrophages was markedly upregulated by exposure to coal dust particles, LPS, and LPS + Coal dust particles, as compared to the control group. In addition, the LPS + Coal dust particles group showed higher transcript levels of NLRP3, ASC, Caspase-1, and IL-1β than the LPS group (Fig. 2A). Compared to the control group, coal dust particles exposure, LPS, and LPS + Coal dust particles could increase the protein of NLRP3, ASC, Pro-caspase-1, and IL-1β in macrophages. In addition, the NLRP3, ASC, Pro-aspase-1, and IL-1β protein levels were considerably higher in the LPS + Coal dust particles group than in the LPS group (Fig. 2B, C and D). These findings suggest that coal dust particles and LPS can upregulate the NLRP3 inflammasome components in macrophages and stimulate the release of the downstream inflammatory cytokine IL-1β.
Coal dust particles upregulates the expression of NLRP3 inflammasome components in rat alveolar macrophages (A) Relative mRNA expression levels following coal dust particles exposure and LPS stimulation; (B) Western blotting membrane image following coal dust particles exposure and LPS stimulation; (C) Content of proteins following coal dust particles exposure and LPS stimulation (This is the quantization of the signal in Fig. 2B); (D) IL-1β protein content in cell lysates following coal dust particles exposure and LPS stimulation by ELISA; *P<0.05; * *P<0.01; * * *P<0.001; * * * *P<0.0001.
Coal dust particles can upregulate the expression of NLRP3 inflammasome components through phagocytosis
The results indicate a decrease in the relative mRNA expression levels of NLRP3, ASC, Caspase-1, and IL-1β in coal dust particles exposed macrophages treated with the CB compared with coal dust particles exposure. (Fig. 3A). Following treatment with CB, the protein expression levels of NLRP3, ASC, Pro-caspase-1, and IL-1β decreased compared with coal dust particles exposure. (Figure 3B, C and D). These results imply that NLRP3, ASC, Pro-caspase-1, and IL-1β protein or transcript levels in coal dust particles-exposed macrophages can be upregulated by coal dust particles uptake.
Coal dust particles can upregulate the expression of NLRP3 inflammasome components in rat alveolar macrophages through phagocytosis (A) Relative mRNA expression levels after Cytochalasin B in coal dust particles-exposed macrophages; (B) Western blotting membrane image after Cytochalasin B in coal dust particles-exposed macrophages; (C) Content of proteins after Cytochalasin B in coal dust particles-exposed macrophages (This is the quantization of the signal in Fig. 3B); (D) IL-1β protein content in cell lysates after Cytochalasin B in coal dust particles-exposed macrophages by ELISA; *P<0.05; * *P<0.01; * * *P<0.001; * * * *P<0.0001.
Discussion
Our experiments have shown that both coal dust particles and LPS can upregulate the NLRP3 inflammasome components in macrophages and promote the release of the downstream inflammatory factor IL-1β. When LPS and coal dust particles act together on macrophages, they exhibit a stronger pro-inflammatory effect. To investigate whether phagocytosis is required for the upregulation of NLRP3 components, we treated macrophages with CB, a phagocytosis inhibitor18. The results revealed a decrease in NLRP3 inflammasome components protein or transcript levels in coal dust particles-exposed macrophages after CB treatment, suggesting that coal dust particles uptake is required to promote the transcriptional and protein upregulation of components of the NLRP3 inflammasome. CB, as an actin polymerization inhibition, exerts its function by inhibiting the polymerization of the cell skeleton, particularly by blocking the polymerization of actin19. The actin cytoskeleton not only supports cell structure and shape but also participates in various intracellular and extracellular transport processes, including cell phagocytosis. As a crucial component of their immune defense mechanism, macrophages use phagocytosis to eliminate invasive pathogens and cell debris20. The inhibition of macrophage phagocytic function reduces the engulfment of particles, including coal dust particles21, leading to a subsequent decrease in the release of inflammatory mediators. Since the activation of the NLRP3 inflammasome requires the accumulation and recognition of these factors as one of the signals22,23, CB may limit phagocytosis, which in turn stops the signals from accumulating and inhibits the NLRP3 inflammasome’s production and activation.
According to previous research on Prion diseases、crystalline particle-induced inflammation and liver fibrosis, NLRP3 inflammasome downregulation can be achieved by blocking macrophage phagocytosis24,25,26. This finding is consistent with our findings. How is the NLRP3 inflammasome regulated by macrophage phagocytosis? Previous studies have found that coal dust nanoparticles can stimulate inflammation and epithelial cell transformation using IGF1/ROS-mediated AKT/GSK3β signals to drive the NF-kB/NLRP3 pathway27. Additionally, PM2.5 phagocytosis activates the NLRP3 inflammasome and subsequent release of the pro-inflammatory master cytokine IL-1β28. Thus, we hypothesize that coal dust particles may activate TLR4 after being engulfed by macrophages. The downstream target protein NF-κB is further activated and phosphorylated by TLR4 activation, encouraging the production of several inflammatory factors, including TNF-α, IL-6, IL-1β, and others. NF-κB activation also increases the transcription of NLRP3. Upon activation, NLRP3 will assemble an inflammasome and activate caspase-1, IL-1β, and IL-18. It is necessary to investigate the precise inflammatory pathways further by which coal dust particles upregulate and activate the NLRP3 inflammasome.
Our results showed that the NLRP3 inflammasome components are upregulated by LPS and coal dust particles. The previous findings highlight that LPS-induced inflammation in macrophages involves complex mechanisms and pathways such as membrane fluidization, M1 polarization29,30, pyroptosis, and glycolysis31, all of which may contribute to increased NLRP3 inflammasome levels. Understanding how environmental pollutants like coal dust particles and bacterial components such as LPS interact with the human immune system is crucial. This deeper insight may enhance our understanding of the pathogenesis of chronic respiratory diseases and other health issues triggered by environmental factors, potentially offering insights for developing novel therapeutic approaches.
Inhibiting macrophage phagocytosis is only one of the mechanisms by which CB affects actin. The actin cytoskeleton itself plays an important role in macrophage inflammation. CB may impair inducible nitric oxide synthase production, inhibit enzyme activity, and influence NLRP3 inflammasome activation32. However, more investigation is required to completely understand these systems. The intrinsic chemical characteristics of coal mine dust, such as surface free radicals and iron ions, play a role in the complex pathogenesis of coal mine pollution. When epithelial cells come into contact with the dust or when macrophages engulf coal mine dust, they can produce a lot of reactive oxygen species, which can cause oxidative damage and partial cell death. Phagocytic particles, reactive oxygen species, and numerous inflammatory factors are released by macrophages and epithelial cells. These factors attract granulocytes or monocytes to convert into macrophages for accumulation, carry on engulfing dust particles, release additional inflammatory factors33, and ultimately initiate and worsen the process of pulmonary fibrosis. Lung tissue remodeling results from ongoing coal dust particles deposition and inflammatory reactions, which impair lung function34,35. Determining the mechanisms behind CWP is essential to halting the progression of the disease.
Our study has numerous limitations. Our study did not look into the activation protein or transcript level of NLRP3 inflammasome components, it simply discovered that coal dust particles can increase the levels of NLRP3 inflammasome components and IL-1 β. Furthermore, our results have shown that NLRP3 inflammasome components protein or transcript levels can be decreased by inhibiting macrophage phagocytosis, yet the specific mechanism is still unclear. We should investigate from several cellular levels in our research. At the same time, we did not test whether Cytochalasin B treatment reduces coal dust particles uptake. Furthermore, the makeup of coal dust particles can alter depending on the location. There are still geographical limitations even though we have reduced differences by using a high-temperature sterilizing technique for coal dust particles.
In summary, coal dust particles can upregulate the NLRP3 inflammasome components. We can successfully decrease the protein or transcript level of NLRP3 inflammasome components and IL-1β by inhibiting the phagocytosis. This highlights the critical role that macrophage phagocytosis plays in NLRP3 inflammasome components upregulation by coal dust particles.
Data availability
The data generated in the present study may be requested from the corresponding author.
Abbreviations
- CWP:
-
Coal workers’ pneumoconiosis
- NLRP3:
-
Nod-like receptor binding protein 3
- IL-1β:
-
Interleukin-1β
- IL-18:
-
Interleukin-18
- Caspase-1:
-
Cysteinyl aspartate-specific protease 1
- ASC:
-
Apoptosis-associated speck-like protein
- CB:
-
Cytochalasin B
- DAMPs:
-
Damage-associated molecular patterns
- ROS:
-
Reactive oxygen species
References
Long, J., Stansbury, R. C. & Petsonk, E. L. Small airways involvement in coal mine dust lung disease. Semin Respir Crit. Care Med. 36 (3), 358–365. https://doi.org/10.1055/s-0035-1549451 (2015).
Castranova, V. & Vallyathan, V. Silicosis and coal workers’ pneumoconiosis. Environ. Health Perspect. 108 (Suppl 4), 675–684. https://doi.org/10.1289/ehp.00108s4675 (2000).
Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9 (8), 847–856. https://doi.org/10.1038/ni.1631 (2008).
Weng, S. et al. Effects of the interactions between dust exposure and genetic polymorphisms in Nalp3, Caspase-1, and IL-1β on the risk of silicosis: A Case-Control study. PLoS One. 10 (10), e0140952. https://doi.org/10.1371/journal.pone.0140952 (2015).
Fu, J. & Wu, H. Structural mechanisms of NLRP3 inflammasome assembly and activation. Annu. Rev. Immunol. 41, 301–316. https://doi.org/10.1146/annurev-immunol-081022-021207 (2023).
Kelley, N., Jeltema, D., Duan, Y. & He, Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 20 (13), 3328. https://doi.org/10.3390/ijms20133328 (2019).
Guo, J. et al. Neutralization of interleukin-1 beta attenuates silicainduced lung inflammation and fibrosis in C57BL/6 mice. Arch. Toxicol. 87, 1963–1973 (2013).
Guo, J. et al. Effects of silica exposure on the cardiac and renal inflammatory and fibrotic response and the antagonistic role of interleukin-1 beta in C57BL/6 mice. Arch. Toxicol. 90 (2), 247–258 (2016).
Rong, Y. et al. Blocking TGF-rmbeta expression inhibits silica particle-induced epithelialmesenchymal transition in human lung epithelial cells. Environ. Toxicol. Pharmacol. 40 (3), 861–869 (2015).
Vallyathan, V. et al. Changes in Bronchoalveolar lavage indices associated with radiographic classification in coal miners. Am. J. Respir Crit. Care Med. 162 (3 Pt 1), 958–965. https://doi.org/10.1164/ajrccm.162.3.9909074 (2000).
Nadif, R. et al. IL18 and IL18R1 polymorphisms, lung CT and fibrosis: A longitudinal study in coal miners. Eur. Respir J. 28 (6), 1100–1105. https://doi.org/10.1183/09031936.00031506 (2006).
Tang, Y., Duan, J., Wang, Y. & Yuan, L. Associations of HMGB1 gene polymorphisms with risk of coal workers’ pneumoconiosis susceptibility in Chinese Han population. Inhal Toxicol. 32 (4), 170–176. https://doi.org/10.1080/08958378.2020.1764153 (2020).
Ji, X. et al. Polymorphisms in inflammasome genes and risk of coal workers’ pneumoconiosis in a Chinese population. PLoS One. 7 (10), e47949. https://doi.org/10.1371/journal.pone.0047949 (2012).
Kim, M. Y., Kim, J. H. & Cho, J. Y. Cytochalasin B modulates macrophage-mediated inflammatory responses. Biomol. Ther. (Seoul). 22 (4), 295–300. https://doi.org/10.4062/biomolther.2014.055 (2014). PMID: 25143807; PMCID: PMC4131529.
Wang, R. et al. The role of macrophage polarization and related key molecules in pulmonary inflammation and fibrosis induced by coal dust dynamic inhalation exposure in Sprague-Dawley rats. Cytokine 173, 156419. https://doi.org/10.1016/j.cyto.2023.156419 (2024).
Zosky, G. R., Bennett, E. J., Pavez, M. & Beamish, B. B. No association between pyrite content and lung cell responses to coal particles. Sci. Rep. 11 (1), 8193. https://doi.org/10.1038/s41598-021-87517-z (2021).
Nitkin, C. R. & Bonfield, T. L. Balancing anti-inflammatory and anti-oxidant responses in murine bone marrow derived macrophages. PLoS One. 12 (9), e0184469. https://doi.org/10.1371/journal.pone.0184469 (2017).
Okada, A. et al. Active phagocytosis and diachronic processing of calcium oxalate monohydrate crystals in an in vitro macrophage model. Kidney Blood Press. Res. 44 (5), 1014–1025. https://doi.org/10.1159/000501965 (2019).
Kocsis, Á. et al. Dependence of mitochondrial function on the filamentous actin cytoskeleton in cultured mesenchymal stem cells treated with cytochalasin B. J. Biosci. Bioeng. 132 (3), 310–320. https://doi.org/10.1016/j.jbiosc.2021.05.010 (2021).
Brown, G. C. Cell death by phagocytosis. Nat. Rev. Immunol. 24 (2), 91–102. https://doi.org/10.1038/s41577-023-00921-6 (2024). Epub 2023 Aug 21.
Valberg, P. A., Brain, J. D. & Kane, D. Effects of colchicine or cytochalasin B on pulmonary macrophage endocytosis in vivo. J Appl Physiol Respir Environ Exerc Physiol. ;50(3):621-9. (1981). https://doi.org/10.1152/jappl.1981.50.3.621. PMID: 7195895.
Pandian, N., Kanneganti, T. D. & PANoptosis: A unique innate immune inflammatory cell death modality. J. Immunol. 209 (9), 1625–1633 (2022).
Nozaki, K., Li, L. & Miao, E. A. Innate sensors trigger regulated cell death to combat intracellular infection. Annu. Rev. Immunol. 40, 469–498 (2022).
Shi, F. et al. Inhibition of phagocytosis and lysosomal acidification suppresses neurotoxic prion peptide-induced NALP3 inflammasome activation in BV2 microglia. J. Neuroimmunol. 260 (1–2), 121–125. https://doi.org/10.1016/j.jneuroim.2013.04.016 (2013).
Honarpisheh, M. et al. Phagocytosis of environmental or metabolic crystalline particles induces cytotoxicity by triggering necroptosis across a broad range of particle size and shape. Sci. Rep. 7 (1), 15523. https://doi.org/10.1038/s41598-017-15804-9 (2017). Published 2017 Nov 14.
Gaul, S. et al. Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J. Hepatol. 74 (1), 156–167. https://doi.org/10.1016/j.jhep.2020.07.041 (2021).
Zhang, Y. et al. Coal dust nanoparticles induced pulmonary fibrosis by promoting inflammation and epithelial-mesenchymal transition via the NF-κB/NLRP3 pathway driven by IGF1/ROS-mediated AKT/GSK3β signals. Cell. Death Discov. 8 (1), 500. https://doi.org/10.1038/s41420-022-01291-z (2022). Published 2022 Dec 29.
Caceres, L. et al. Molecular mechanisms underlying NLRP3 inflammasome activation and IL-1β production in air pollution fine particulate matter (PM2.5)-primed macrophages. Environ. Pollut. 341, 122997. https://doi.org/10.1016/j.envpol.2023.122997 (2024).
de la Haba, C., Morros, A., Martínez, P. & Palacio, J. R. LPS-Induced macrophage activation and plasma membrane fluidity changes are inhibited under oxidative stress. J. Membr. Biol. 249 (6), 789–800. https://doi.org/10.1007/s00232-016-9927-9 (2016).
Huang, X., Li, Y., Fu, M. & Xin, H. B. Polarizing macrophages in vitro. Methods Mol. Biol. 1784, 119–126. https://doi.org/10.1007/978-1-4939-7837-3_12 (2018).
Aki, T., Funakoshi, T., Noritake, K., Unuma, K. & Uemura, K. Extracellular glucose is crucially involved in the fate decision of LPS-stimulated RAW264.7 murine macrophage cells. Sci. Rep. 10 (1), 10581. https://doi.org/10.1038/s41598-020-67396-6 (2020).
Fernandes, P. D., Araujo, H. M., Riveros-Moreno, V. & Assreuy, J. Depolymerization of macrophage microfilaments prevents induction and inhibits activity of nitric oxide synthase. Eur. J. Cell. Biol. 71 (4), 356–362 (1996).
Schins, R. P. & Borm, P. J. Mechanisms and mediators in coal dust induced toxicity: a review. Ann. Occup. Hyg. 43 (1), 7–33. https://doi.org/10.1016/s0003-4878(98)00069-6 (1999).
Vanka, K. S. et al. Understanding the pathogenesis of occupational coal and silica dust-associated lung disease. Eur. Respir Rev. 31 (165), 210250. https://doi.org/10.1183/16000617.0250-2021 (2022).
Zhang, Y. et al. Coal dust nanoparticles induced pulmonary fibrosis by promoting inflammation and epithelial-mesenchymal transition via the NF-κB/NLRP3 pathway driven by IGF1/ROS-mediated AKT/GSK3β signals. Cell. Death Discov. 8 (1), 500. https://doi.org/10.1038/s41420-022-01291-z (2022).
Funding
The study was supported by. Shanxi Province science and technology cooperation and exchange special project (Regional cooperation project) (202204041101031); Shanxi Province scientific and technological achievements transformation guidance project (202304021301063); Research Project Supported by Shanxi Scholarship Council of China (2023 − 190). The funder did not play any role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; nor in the preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
HZ and YZ conceived and designed the study. YZ and JJY analyzed the data. JJY and YR performed statistical analysis. YNC, HQ and YWH confirm the authenticity of all the raw data. All authors contributed to the writing of the manuscript and reviewed, read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Yan, J., Ren, Y. et al. Coal dust particles can upregulate the expression of NLRP3 inflammasome components in rat alveolar macrophages through phagocytosis. Sci Rep 15, 8989 (2025). https://doi.org/10.1038/s41598-025-93946-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-93946-x
Keywords
This article is cited by
-
Role of macrophage ATP metabolism disorder in SiO2‑induced pulmonary fibrosis: a review
Purinergic Signalling (2025)