Abstract
The American Heart Association (AHA) guidelines assess heart failure (HF) via comorbidities, laboratory markers, and echocardiography, while the New York Heart Association (NYHA) classification evaluates functional capacity. The primary objective of this study was to investigate the correlation between objectified HF-related symptoms and cardiac and muscular exercise capacity in Stage B HF patients with non-reduced ejection fraction. As secondary endpoints, we stratified this analysis for subgroups of NYHA classes to evaluate the primary endpoint for different levels of impairment and for sex to address for differences between men and women. Sixty-two Stage B HF patients with non-reduced EF were screened from an HF-risk cohort. Assessments included medical history, HF-related symptoms (Kansas City Cardiomyopathy Questionnaire, KCCQ), physical examination, laboratory tests, echocardiography, and cardiopulmonary exercise testing (CPET) with cardiac output monitoring. Correlations were analyzed between KCCQ score and exercise capacity markers: maximal oxygen uptake (VO2max), arterio-venous oxygen difference (avDO2), cardiac power output (CPO), mean arterial pressure (MAP), and respiratory efficiency (Ve/VO2). Subgroup analyses were performed by sex and NYHA class determined by VO2max or KCCQ functional scores. Our HF patient cohort showed reduced KCCQ scores (78.3) and VO₂max (22.9 ml/kg/min), and a progressed reduction in avDO₂. In the total cohort, KCCQ scores showed moderate correlations with Ve/VO₂ (r = -0.39) and MAP (r = 0.27). NYHA stratification by VO₂max revealed differences in avDO₂ and cardiac output but not KCCQ scores, while KCCQ-functional stratification only showed differences in Ve/VO₂. Sex-specific analysis showed KCCQ scores correlated with CPO in men (r = 0.65) and Ve/VO2 in women (r = -0.68). Our identified Stage B HFpEF cohort showed already alterations in total, cardiac and muscular exercise limitation. The HF symptom severity was weakly associated to the higher blood pressure and ventilatory inefficiency and, but moderately to strongly correlated CPO in men and Ve/VO2 in women in sex-specific analyses.
Similar content being viewed by others
Introduction
Heart failure (HF) is a syndrome characterized by various symptoms, including shortness of breath, edema, and increased fatigue during daily activities1. In the early stages of HF, patients typically exhibit mild symptoms and functional limitations2. As HF progresses, chronic myocardial pressure-volume overload and reduced cardiovascular exercise capacity lead to severe health outcomes, including increased mortality risk, hospitalization, and healthcare burdens3.
The New York Heart Association (NYHA) classification is based on subjective patient symptoms and corresponding functional status4. This classification accounts for cardiac, pulmonary, and muscular performance impairments, thereby reflecting HF as a syndrome5,6. The current guidelines from the European Society of Cardiology (ESC) and the American Heart Association (AHA) primarily propose a heart-specific classification and staging, focusing on predisposing factors, diagnostic assessments, the presence of structural heart disease or laboratory assessments of cardiac enzymes7,8. So, the NYHA functional classes and ESC/AHA staging co-exist as parallel assessments.
For early stages of HF, according to AHA HF stages A and B7, there is limited evidence about cardiac and muscular capacity alterations and their specific contribution to functional capacity classification (NYHA class) and heart failure related symptoms. Particularly in patients with heart failure with preserved ejection fraction (HFpEF), the multifactorial genesis of cardiac overload and associated limitations in other exercise capacity, such as skeletal muscle exercise capacity, have not been studied in detail.
Hawwa et al. demonstrated a correlation between NYHA class, maximal oxygen consumption, and patient-reported outcomes assessed via the Kansas City Cardiomyopathy Questionnaire (KCCQ) in patients with chronic heart failure but did not differentiate for exact quantification of cardiac and skeletal muscle capacity9.
Cardiopulmonary exercise testing (CPET) can reveal objectified limitations in functional capacities in HF patients10,11,12. Reduced exercise capacity is not solely due to cardiac dysfunction13,14 as HF as a syndromic disease is also influenced by the reduced exercise capacity of the peripheral skeletal muscles15, increased afterload due to arterial hypertension or chronotropic incompetence16. Specifically, the left ventricular cardiac power output and stroke work determine heart functional capacity during exercise stress tests17,18, particularly in HF patients with altered chronotropic variability19.
In the early stages of the disease, it can be hypothetically assumed that there is already an advanced limitation in overall functional capacity. This exercise performance limitation could arise secondary to other diseases and frequently leads to a capacity limitation or dysregulation of peripheral muscles, as well as vascular and pulmonary systems. Recent studies highlight those comorbidities such as hypertension, diabetes, and obesity play significant roles in exacerbating these alterations20. Additionally, poor cardiorespiratory fitness has been linked to higher morbidity and mortality rates in HFpEF patients, underscoring the need for targeted interventions21.
One difficulty in the early detection of HF patients is that NYHA class patients are almost symptom free. Therefore, physiological changes at the onset of HF may not yet be associated with specific symptoms and their severity. Consequently, it is useful to consider patients separately according to NYHA class I and greater I. Gender differences have also been observed in HF patients without reduced ejection fraction22,23. Therefore, it is important to consider sex as a confounding factor or to analyze it separately23, especially in the early stages of the disease.
The purpose of this cross-sectional study was to gain a deeper understanding of the interaction between the functional NYHA classification, heart failure-specific patient-reported outcome measures (PROMs) assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ), and objectified exercise capacity markers in Stage B HF patients with non-reduced EF/preserved EF. Therefore, three main research objectives were addressed: (I) to correlate the KCCQ score with physiological cardiac, muscular and ventilatory exercise markers; (II) to asses and compare these exercise performance parameters in NYHA Class I and II patients classified by (a) maximum oxygen uptake and (b) KCCQ functional score; and (III) to analyses exercise markers and KCCQ scores stratified by sex.
Materials and methods
This was a cross-sectional, observational study. The data reported were part of routine medical assessments at the Sports Medicine Outpatient Clinic of the University of Leipzig, prior to trial-specific interventions and randomization for the HITS-trial (Heart failure, Individualized training, Telemonitoring and Self-Management). Methods and data were parts of the protocol of a prospective, randomized, controlled trial „HITS-Trial “, approved by the Ethics Committee of the University of Leipzig (479/19-ek). The study was registered in the German Clinical Trial register DRKS00019022 (28.05.2020) and the study reporting follows the STROBE guideline for reporting of cross-sectional studies24.
Subjects
Patients with a risk constellation for heart failure (AHA HF stage A)7,8 at the outpatient clinic for sport medicine were screened for heart failure according to the AHA and ESC guidelines. Patients with manifest comorbidities related for elevated NTproBNP levels, like chronic kidney diseases, or HF symptoms, like COPD, were excluded from the screening procedure for the observational cohort. All patients were in a stable cardiovascular clinical situation.
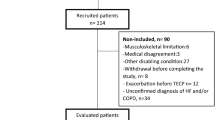
A total of n= 62 patients met the criteria8 for stage B HFpEF diagnosis, including elevated NTproBNP levels, specific risk factors, comorbidities and HF-related symptoms, and had a full evaluable diagnostic data set. Not included for further analysis were 23 patients assessed with reduced EF. All participants of the identified stage B HFpEF/non-rEF were verbally informed and gave their written consent. The cohort selection process is illustrated in Fig. 1.
The Stage B non-rEF patient cohort underwent comprehensively analysis, with additional stratification into sub cohorts based on NYHA classification. Two approaches were used for stratification: (a) maximal oxygen consumption measured during cardiopulmonary exercise testing (NYHA CPET), and (b) symptomatic assessment using the Kansas City Cardiomyopathy Questionnaire (KCCQ) functional status score (NYHA KCCQ). The KCCQ functional score reflects the anamnestic assessment of NYHA class25. This approach was chosen because both maximal oxygen consumption and KCCQ scores are correlated with NYHA class and important clinical outcomes, such as mortality and hospitalization25. To identify potential similarities and differences, we performed stratification based on both assessments. Cut-off values of 21 ml/kg/min for maximal oxygen consumption and 80 for the KCCQ functional status score were applied to distinguish between NYHA Class I and Class II patients. These cut-off values were effectively established for stable HF patients in cohort assessments26,27,28,29, and we used the arithmetic mean difference between NYHA I and II for selecting cut-off values. Stratification by sex was also performed to identify specific characteristics in the correlation between KCCQ total score and exercise parameters.
Patient identification and cohort selection; EF: ejection fraction, mrEF: moderate reduced ejection fraction; non-rEF: non-reduced ejection fraction; NYHA: New York Heart Association; pEF: preserved ejection fraction; rEF: reduced ejection fraction, HF: Heart failure; VO2max: maximum oxygen consumption.
Procedures
Patient examinations and data collection
Patients had two examination days in the outpatient clinic. Day 1 consisted of collection of medical history by medical records, assessment of NYHA class8 via using the functional scale KCCQ (Kansas City Cardiomyopathy Questionnaire) and patient-reported outcome measures (KCCQ), a physical examination and blood sampling. The KCCQ provides an overall score ranging from 0 to 100 and evaluates health outcomes across three subscales: symptoms, clinical status, and functional capacity25,30. Out of medical history, we assessed comorbidities of special interest predisposing heart failure in our data set: ischemic heart diseases (clustered using ICD-10-WHO for: I25 coronary disease and ischemia, I21 heart attack and I70 coronary sclerosis), diabetes mellitus type II, hypertension and atrial fibrillation.
All procedures and diagnostics were done in accordance to our standardized operation procedures (SOP) which are based on guideline recommendations.
A lung function assessment was done to rule out moderate or severe chronical lung diseases. We reported clinical values for lung function assessments: forced vital capacity (FVC), one second vital capacity (FEV1) ratio (FEV1/FVC) and forced expiratory flow 25–75% of FVC (FEF 25–75).
Day 2 included the measurements of anthropometry and body composition (by bioimpedance analysis, Bodycorpus RX4004 SMT Medical), spirometry (Easy One Pro, NDD Medizintechnik AG, Switzerland), echocardiography (Vivid E95, GE Health Care) and a cardiopulmonary exercise test.
Cardio pulmonary exercise tests (CPET) were performed according to ergometry guidelines for cardiac patients5. Patients were examined on a semi-recumbent bicycle ergometer (ErgoSelect 10, Ergoline GmbH, Germany). After finishing of the patient preparation for the CPET, resting values were recorded over two minutes. The CPET began with an initial load of 30 watts and increased by 10 watts per minute. The test was performed at 60 to 75 revolutions per minute by the patients until voluntary exhaustion or occurrence of clinical reasons31. Blood pressure (Riva-Rocci-method)32 was measured at rest, every 3 min and at maximum load during the CPET and the first, third and fifth minute afterwards. This method is our standard measurement for clinical safety reasons. Beginning at rest, impedance cardiography (Manatec Physioflow, France) was recorded beat-by-beat and spiroergometry (Dynostics, Sicada GmbH, Germany) was recorded breath-by-breath until the fifth minute after the end of CPET. For further analysis, all measurements of continuous recorded values were averaged over 30nsecond intervals. The following calculations were used for physiological variables:
Arterio-venous oxygen difference (avDO2):
Left ventricular stroke work (SW):
Left ventricular cardiac power output (CPO):
Mean arterial pressure was calculated by heart rate-corrected calculation as described by Rogers and Oosthuyse33. The respiratory equivalent for oxygen (REVO2) was calculated:
We combined echocardiographic and thoracic impedance cardiographic assessment to incorporate the strengths of both methods34. Both, echocardiography and thorax impedance cardiography are able to measure the stroke volume by the ventricular outflow or the corresponding aortic flow35. The flow-based measuring method corresponding to thoracic impedance cardiography in echocardiographic assessments is the stroke volume measurement based on the systolic velocity time integral in the left ventricular outflow tract (VTI)36. Echocardiography is well-established as a valid and reliable method to determine the stroke volume at rest37. Stress echocardiography did not provide optimal conditions for CPET in terms of continuous measuring in patients and need a special positioning on the bicycle ergometer. Therefore we choose the, impedance cardiography as a reliable method for assessing continuous beat-by-beat hemodynamics during exercise38,39 and is also sensitive for changes in cardiac function in patients39,40 and therefore recommend as guideline-recommended monitoring assessment during CPET5. Calculating the stroke volume of impedance cardiography by relying on aortic flow and body surface area may overestimate the stroke volume in subjects with more body fat, as validation studies for exercise-related CO assessments included mostly healthy subjects41,42. Also, in our cohort of HF patients, chronical maladaptation of the heart and arterial blood vessels could affect the measuring accuracy. Therefore, we ran a cohort specific reliability analysis among the included patients to rule out systematic errors. Echocardiographic VTI-based stroke volume measurements for comparison methods were available in 28 patients.
Statistics
Group differences were tested using a t-test for Gaussian distributions; otherwise, the Wilcoxon rank-sum test was applied. Homogeneity of variance was assessed with Levene’s test, and adjustments were applied if necessary. Correlations between physiological measures and the Kansas City Cardiomyopathy Questionnaire (KCCQ) scores were analyzed using Pearson’s correlation coefficient. Correlations were further examined for potential confounders, and the model was adjusted when significant influences were identified. Maximum oxygen consumption was normalized to body weight. Outliers were identified using the ROUT method (2% false discovery rate) for continuous variables used in group difference analyses and the k-nearest neighbor local outlier clustering method for correlation analyses.
Reliability analyses for stroke volume from echocardiography velocity-time integral (VTI) and impedance cardiography were assessed using the intra-class correlation coefficient (ICC) and a paired t-test. An a priori power calculation (G*Power, University of Düsseldorf, Germany) for the comparison of NYHA I and II heart failure (HF) patients indicated a power of 0.8 at a 0.05 significance level, assuming a clinically relevant mean difference of 10% and a standard deviation of 17%, requiring a sample size of 60 patients. We adopt a conservative mean difference of 10% for maximal oxygen consumption, based on results for NYHA I and II patients by previous studies that analyzed maximal oxygen consumption in NYHA I and II patients5,29. These studies reported a mean difference of 20% between NYHA I and II and a with a one-sided standard deviation of approximating 10%.
Values were presented as means and standard deviation (SD) or as numbers. Statistical analyses were performed using GraphPad Prism 8.0.2 and JASP 0.18.0.0. Graphics for the correlation matrix were created with Matplotlib 3.9.2 for Python. Correlations were considered as negligible below 0.1, weak from 0.1 to 0.39, moderate from 0.4 to 0.69, strong from 0.7 to 0.89 and very strong from 0.90 to 1.0043.
Results
The results part consists of three sections. Patient characteristics and baseline parameters are presented in the first section and the results of the CPET in the second section. The third section provides the results for the correlation of the KCCQ score with physiological parameters.
Patient characteristics and baseline parameters
Patient characteristics and resting values for the total cohort and for the sub cohorts of NYHA class I and class II, as well as gender specific sub cohorts are shown in Table 1. NYHA CPET class I and II patients differed in age, body fat and FEF 25–75%, but not in the prevalence of comorbidities. As expected, men and women differed in height, fat free mass and body fat percentage. NYHA KCCQ class I and II patients showed differences in in ejection fraction (EF) and the KCCQ scores. Lognormal distribution was found for NTproBNP and the Wilcoxon-Test was applied for testing group differences.
Reliability analyses between echocardiography and thoracic impedance cardiography
Sub analyses for reliability of echography and thoracic impedance cardiography revealed a good overall agreement by intra-class coefficient of r = 0.60 and no difference in mean stroke volume (echocardiography: 99.2 (SD: 23.5) ml vs. thoracic impedance cardiography: 99.4 (SD: 15.6) ml, p= 0.475). Our measured increase of 28% stroke volume (103.5 ml to 132.5 ml) during CPET at maximal exercise were in line with observed increase of stroke volumes in elderly of approximately 40%44. Based in the good overall agreement, stroke volumes assessments by thoracic impedance cardiography were considered as valid in our study cohort.
Cardiopulmonary exercise test
The results of CPET of the total cohort and the sub cohorts for NYHA classes at maximum load are presented in Table 2 and gender specific data are presented in Table 3.
NYHA classes, classified via CPET, differed in peak power output, maximum oxygen uptake and carbon dioxide output, ventilation and respiratory equivalent for oxygen, heart rate and cardiac output, as well as avDO2.
Men and Women differed in all variables which are related to the body weight and in body weight independent variables avDO2 and SBP. No differences were found for ventilation parameters of respiratory exchange rate and the respiratory equivalent for oxygen.
KCCQ scores and correlation analysis with physiological parameters
The correlations matrix (Fig. 2) illustrates the correlations between the KCCQ score and physiological parameters. Subscale correlation analysis revealed very strong correlations between the total KCCQ score and its subscales: the KCCQ symptom score (r = 0.95, p < 0.001), the KCCQ functional score (r = 0.97, p < 0.001), and the KCCQ clinical score (r = 0.99, p < 0.001). These correlations did not differ significantly from those observed for the total KCCQ score, confirming that the total score reliably represents all subscales. Consequently, we focused on the total KCCQ score in the correlation analysis with physiological parameters.
In the total cohort, the KCCQ score correlated significantly with the REVO₂ (p < 0.001) and mean MAP (p = 0.01). In the NYHA I CPET cohort, the KCCQ score correlated only with MAP (p = 0.04), whereas in the NYHA II CPET cohort, it was associated with REVO₂ (p = 0.015).
NYHA stratification by the KCCQ functional score revealed significant correlations within the NYHA I subgroup with the KCCQ total score: CO (r = 0.36, p = 0.016), REVO₂ (r = −0.40, p = 0.009), CPO (r = 0.42, p = 0.006), and MAP (r = 0.36, p = 0.016). In contrast, no significant correlations were observed between the KCCQ score and physiological parameters in the NYHA II KCCQ cohort.
The highest correlations were found within sex-specific subgroups. In men, higher KCCQ scores were strongly associated with increased CPO (r = 0.65, p < 0.001), while in women, the KCCQ score was significantly correlated with MAP (r = 0.43, p < 0.001), and inversely with REVO₂ (r = −0.68, p < 0.001). Figure 3 presents the correlations on subject level, demonstrating the different pattern between men and women for CPO and REVO2. In men were other significant correlations for cardiac markers with the KCCQ score: SW r = 0.48 (p = 0.004), max. SV r = 0.43 (p = 0.01), max. CO r = 0.39 (p = 0.017).
No correlations were observed between the KCCQ score and confounders age, height, weight, ejection fraction, lung function parameters, or BMI in any subgroup. Thus, these factors were not included as adjustments in the correlation analysis models. Dichotomous confounders such as beta-blocker use, diabetes mellitus type 2, and ischemic cardiac disease were also analyzed. A significant confounding effect was observed for beta-blocker use in women for REVO2. Inclusion of this confounder changed the correlation between REVO₂ and the KCCQ score from r = −0.58 to −0.68.
Outlier detection in the male subgroup identified six individuals in the CPO-KCCQ correlation. After excluding these individuals, a significant correlation between the KCCQ score and CPO was confirmed (r = 0.65, p < 0.001). There were no further statistical significant correlations.
Correlation matrix for correlation coefficients of KCCQ-score in the total and sub cohorts. Significant correlations are marked with*.; Abbreviations: avDO2: arterio-venous difference for oxygen, CO: cardiac output, CPO: cardiac power output, CPET: cardiopulmonary exercise test, MAP: mean arterial pressure, NYHA: New York Heart Association, SV: stroke volume, SW: stroke work, Ve/VO2: respiratory equivalent for oxygen, VO2max: maximum oxygen consumption.
Discussion
This study explored in detail the relationship between NYHA classification, patient-reported outcomes (KCCQ scores), and objectified exercise capacity markers in Stage B HFpEF patients. By stratifying patients based on NYHA class via CPET or KCCQ functional score and sex, we identified significant patterns for differences in exercise capacity markers correlations to symptomatic outcomes.
Resting values and patient characteristics
Our HFpEF cohort matched the reported characteristics of other heart failure cohorts, both symptomatically and in exercise capacity. Overall, the KCCQ score is consistent with other HF cohorts, which showed KCCQ scores of approximately 85 − 75 for NYHA class I and II patients26,27,28. Whereas mean values were typically found to be just above 80–85 for NYHA I and 75–80 for NYHA II. When NYHA was stratified by VO2max, our cohorts did not confirm this difference between NYHA I and II, neither in total nor in the sub scales of the KCCQ.
In our included patients, typical comorbidities predisposing for HFpEF were found (type 2 diabetes mellitus, hypertension or coronary diseases), consistent with other studies45. Incidences of coronary heart disease and diabetes mellitus type II are balanced between our NYHA groups, so prevalence of comorbidities was no major predictor for differences of NYHA classes in our results. The NYHA I and II cohorts differed in age, percentage of body fat and FEF 25–75%. Our NYHA cohort corresponds to a typical age range for heart failure patients under risk in Germany45. Within the total cohort, we found no significant correlation for age and KCCQ score or relative maximum oxygen consumption. Therefore, we did not adjust our results for age in our analysis. As Cleland et al. found a prevalence for 65% of men in heart failure patients, our cohort consist of a slightly lower 55% proportion of men46, which could be explained by the systematically screening of risk patients in an outpatient setting, whereas HF cohorts typically include symptomatic patients with cardiovascular diseases, which are more common in men. Further gender specific analyses in patient characteristics showed only differences which are related to known sex specific differences in height, weight or lean body mass.
CPET and KCCQ-scores
Our total HFpEF cohort showed a major exercise limitation, expressed as reduced VO2max, in the skeletal muscle capacity (avDO2), which was consistent across all subcohorts. Both NYHA stratifications showed significant differences between NYHA I and II in REVO2, with higher ventilatory drive in NYHA II patients. When stratified by VO2max, the NYHA II group showed additional alterations in the CO and CPO (based on lower HR) and avDO2. Sex-specific differences, not related to a higher body weight in men, were lower relative VO2max, SBP and avDO2. The total KCCQ score showed the highest sex-specific correlations in sex-specific correlations: in men with CPO and in women with Ve/VO2 and MAP.
Objective I: total cohort
Among our total HFpEF cohort, the CPET assessed oxygen uptakes at 22.9 ml/kg/min, reflecting HF typical levels. The relative maximum oxygen consumption in our NYHA class II patients of 19.9 ml/kg/min is in line with typical values of around 20 ml/kg/kg as well as the NYHA class I typically achieve values approximately of 25 ml/kg/min6. A major exercise limitation was the avDO2, which was reduced to almost half of the physiological upper limit47. The KCCQ score in the total cohort was only associated with the Ve/VO2 and the mean arterial pressure. Both physiological parameters are physiologically associated with the symptoms of dyspnea. The Ve/VO2describes a higher ventilator work for a given oxygen demand and workload and a dysregulation is therefore immediately noticeable for the patient as dyspnea under physical load. A higher MAP could be associated with a higher pulmonary arterial pressure48 or higher left ventricular filling pressures49, which also could lead to dyspnea. This underscores the importance of early blood pressure treatment and the need for early identification of patients at risk for heart failure8.
Objective II: NYHA class I vs. NYHA class II patients
The difference between NYHA CPET class I and II patients in exercise performance is based on both higher cardiac output and higher muscular avDO2. With both groups achieving only intermediate maximal avDO2 values, skeletal muscle training status was the main exercise performance limiting factor in NYHA I as well as in NYHA II. As heart rate decreases naturally in age, and is partly compensated by higher stroke volumes, normally the avDO2remains stable50. Differences in NYHA groups in avDO2 are already known. Reddy et al. reported also an avDO2of 10.0 ml/dl in HFpEF patients, but did not separated for NYHA classes51. The peripheral limitation of HFpEF patient is well described in chronical and more progressed patient16,52. So, our patients are newly diagnosed HFpEF patients, and their avDO2 was already diminished on a level of chronical patients, which underlines the complexity in heart failure development by its syndromic frailty characteristic, even in the early diagnostic when patient were actively screened by physicians. This pattern could be confirmed in NYHA stratification by KCCQ functional score. In contrast to the NYHA classification via CPET the classification via KCCQ showed no differences in avDO2, CO or VO2max between the groups. Whereas a classification via CPET lead to a separation of patients via the total power output based on exercise stress reaction the KCCQ functional status includes the subjective perception of the patient. Therefore, the stratification based on the KCCQ functional score in NYHA I or II can be limited by a self-limiting behavior in the daily life and therefore the reduced exercise capacity will not be perceived by the patients. Also, in the beginning of heart failure patients did not recognize all symptoms and therefore the KCCQ functional score and clinical classification is not sensitive enough in newly diagnosed and actively screened HF cohorts. Changes in respiratory regulation are directly related to HF symptoms, therefore the Ve/VO2 may be more attributable to symptom scores.
Both NYHA stratifications showed an increased Ve/VO2, which is in line with recent findings53. Respiratoy equivalents for carbon dioxide > 35 are sensitive for symptomatic HFpEF patients which is in inline of our Ve/VO2 of 37.4 (NYHA II CPET) and 38.1 (NYHA II KCCQ).
By focusing on the cardiac capacity, we found a difference in the cardiac power output and cardiac output between the NYHA CPET classes, which are mainly caused by higher heart rates in NYHA I patients compared to NYHA II patients. Alterations in symptoms and functional capacity seems not only driven by the cardiac capacity and includes skeletal muscle and ventilatory alterations. The NYHA assessment by symptoms and exercise capacity assessment via CPET showed additive insights, but less regularity agreements in the analyzed outcomes.
Objective III: sex-specific analysis
Men and women did not differ in the KCCQ scores in this study. Our demonstrated sex-specific differences in power output, absolute maximum oxygen consumption and cardiac parameters can be mostly attributed to the differences in height and weight between men and women.
The higher avDO2in men is mainly due to genetic differences is in line with demonstrated differences in elderly54. This 10% difference in avDO2 between men and women was the same size as the difference shown between NYHA class I and II patients and highlights the relevance of sex-specific reference values.
An additional main finding between men and women was found in the association of heart failure related symptoms to dedicated exercise capacity markers (Figs. 2 and 3). We firstly demonstrated a correlation in male HFpEF patients between KCCQ total score and the CPO and in female HFpEF with the Ve/VO2and the MAP. Sex-specific differences in HFpEF patients are known and often discussed55,56,57. Pulmonary circulation differences between men and women, especially in the pulmonary capillary wedge pressure, could account for differences in the genesis of symptoms in HFpEF patients58. The lower ventricular contractility reserve in women may explain the correlation of the MAP with the KCCQ58. It can be hypothesized that male HFpEF patients develop more pronounced symptoms as cardiac power output (CPO) declines and their higher contractility reserve diminishes over the course of HF progression.
Our findings the importance of sex-specific clinical exercise diagnostics in HFpEF patients and data analyses interpretations as relevant confounder.
Conclusion
Our results showed that actively screened at-risk heart failure patients were similar to typical chronic heart failure patients in terms of symptoms and exercise capacity. This highlights the importance of early standardized diagnosis or screening for heart failure. Exercise testing is a valid tool to further assess overall work capacity and to differentiate the extent of cardiopulmonary and muscle-related symptom changes. Decreased muscle performance was a major limitation of exercise capacity. Improvements in exercise capacity as a therapeutic focus of exercise therapy in HF patients should be investigated in future studies. Exercise training may reduce this dysregulation by increasing avDO2, thereby reducing cardiac oxygen demand.
Notably, heart failure-related symptoms and patient outcomes showed the strongest correlation to exercise capacity markers in sex-specific sub cohorts. Functional classification by VO2max or KCCQ functional score showed similarities only for altered Ve/VO2 in NYHA II patients. Exercise markers assessed by CPET may reveal individual functional limitations. Our findings underscore the complexity of heart failure development, emphasizing the role of both physiological capacity assessments and patient-reported outcome measures. Depending on the stratification and sub cohort, we found association between heart failure symptoms and blood pressure, CPO, and REVO2. This underlines the need for early blood pressure treatment, exercise training, and gender-specific considerations in the management of heart failure patients at risk. Further research is necessary to reproduce our observations in larger real-world cohorts and to address targeted interventions to improve the quality of life and outcomes for patients at risk of heart failure.
Limitations
Our study and interpretation had some limitations. The results of our study cannot be generalized to all patients with HFpEF due to our sample size and the limited sub cohort inclusion, for example, we could not include patients with arrhythmias such as atrial fibrillation in our cohort. Cohorts with larger numbers of patients may reveal more confounders and correlations in the association between PROMs and exercise variables, and thus provide further clinical insights. The impact of our findings on diseases progression and health care burden remains unclear as we did no follow-up assessments. The staging of HF patients is based on the chronic process of HF progression and my not be ideal for our actively screened cohort. We used CPET on a bicycle ergometer for physiological assessments, but these results should be extrapolated with caution to other types of exercise and activities.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to data protection for pseudonymized data but are partially available for aggregated data from the corresponding author on reasonable request.
Abbreviations
- AHA:
-
American Heart Association
- avDO2 :
-
Arterio-venous oxygen difference
- BNP:
-
Brain natriuretic peptide
- BPM:
-
Beats per minute
- CO:
-
Cardiac output
- CPET:
-
Cardio-pulmonary exercise test
- CPO:
-
Cardiac power output
- DBP:
-
Diastolic blood pressure
- ESC:
-
European Society of Cardiology
- FVC:
-
Forced vital capacity
- FEV1:
-
Forced expiratory volume for one second
- HF:
-
Heart failure
- HFpEF:
-
Heart failure with preserved ejection fraction
- HFrEF:
-
Heart failure with reduced ejection fraction
- ICD-10:
-
International classification of diseases, 10th revision
- KCCQ:
-
Kansas City cardiomyopathy questionnaire
- MAP:
-
Mean arterial pressure
- NTproBNP:
-
N-terminal pro brain natriuretic peptid
- NYHA:
-
New York Heart Association
- Non-rEF:
-
Non-reduced ejection fraction
- Pmax:
-
Maximum power output
- SBP:
-
Systolic blood pressure
- SW:
-
Stroke work
- SPO:
-
Stroke power output
- Ve:
-
Ventilation per minute
- Ve/VO2 :
-
Respiratory equivalent for oxygen
- VO2 :
-
Oxygen consumption
- VO2max:
-
Maximum oxygen consumption
- VTI:
-
Velocity time integral
- WHO:
-
World Health Organisation
References
Roger, V. L. Epidemiology of heart failure: A contemporary perspective. Circul. Res. 128, 1421–1434. https://doi.org/10.1161/CIRCRESAHA.121.318172 (2021).
Simonis, G., Stumpf, J., Dörr, R., Kadalie, C. T. & Spitzer, S. G. Dyspnoe Bei linksherzerkrankungen. Der Internist 56, 890–899. https://doi.org/10.1007/s00108-015-3688-x (2015).
Störk, S. et al. Epidemiology of heart failure in Germany: A retrospective database study. Clin. Res. Cardiol.: Off. J. German Cardiac Soc. 106, 913–922. https://doi.org/10.1007/s00392-017-1137-7 (2017).
Yancy, C. W. et al. ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J. Am. College Cardiol. 62, e147-239. https://doi.org/10.1016/j.jacc.2013.05.019 (2013).
Agostoni, P. & Dumitrescu, D. How to perform and report a cardiopulmonary exercise test in patients with chronic heart failure. Int. J. Cardiol. 288, 107–113. https://doi.org/10.1016/j.ijcard.2019.04.053 (2019).
Bredy, C. et al. New York heart association (NYHA) classification in adults with congenital heart disease: relation to objective measures of exercise and outcome. Eur. Heart J. Qual. Care Clin. Outcomes. 4, 51–58. https://doi.org/10.1093/ehjqcco/qcx031 (2018).
Heidenreich, P. A. et al. AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J. Am. College Cardiol. 79, e263-e421. https://doi.org/10.1016/j.jacc.2021.12.012 (2022).
McDonagh, T. A. et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur.Heart j. 42, 3599–3726. https://doi.org/10.1093/eurheartj/ehab368 (2021).
Hawwa, N. et al. Comparison between the Kansas City cardiomyopathy questionnaire and New York heart association in assessing functional capacity and clinical outcomes. J. Card. Fail. 23, 280–285. https://doi.org/10.1016/j.cardfail.2016.12.002 (2017).
Cattadori, G., Salvioni, E., Gondoni, E. & Agostoni, P. Evaluation of noninvasive exercise cardiac output determination in chronic heart failure patients: a proposal of a new diagnostic and prognostic method. J. Cardiovasc. Med. (Hagerstown Md). 12, 19–27. https://doi.org/10.2459/JCM.0b013e3283405c4c (2011).
Fukuda, T. et al. Cardiac output response to exercise in chronic cardiac failure patients. Int. Heart J. 53, 293–298. https://doi.org/10.1536/ihj.53.293 (2012).
Shimiaie, J. et al. Determinants of effort intolerance in patients with heart failure: Combined echocardiography and cardiopulmonary stress protocol. JACC Heart Fail. 3, 803–814. https://doi.org/10.1016/j.jchf.2015.05.010 (2015).
Abudiab, M. M. et al. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 15, 776–785. https://doi.org/10.1093/eurjhf/hft026 (2013).
Nayor, M. et al. Impaired exercise tolerance in heart failure with preserved ejection fraction: quantification of multiorgan system reserve capacity. JACC Heart Fail. 8, 605–617. https://doi.org/10.1016/j.jchf.2020.03.008 (2020).
Kleber, F. X. Reduced arteriovenous oxygen difference on exercise in heart failure. Int. J. Cardiol. 248, 276–277. https://doi.org/10.1016/j.ijcard.2017.08.042 (2017).
Borlaug, B. A. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction. Circul. J.: Official J. Jpn. Circ.. Soc. 78, 20–32. https://doi.org/10.1253/circj.cj-13-1103 (2014).
Bain, R. J., Tan, L. B., Murray, R. G., Davies, M. K. & Littler, W. A. The correlation of cardiac power output to exercise capacity in chronic heart failure. Eur. J. Appl. Physiol. Occup. Physiol. 61, 112–118. https://doi.org/10.1007/BF00236703 (1990).
Lang, C. C., Karlin, P., Haythe, J., Lim, T. K. & Mancini, D. M. Peak cardiac power output, measured noninvasively, is a powerful predictor of outcome in chronic heart failure. Circ. Heart. Fail. 2, 33–38. https://doi.org/10.1161/CIRCHEARTFAILURE.108.798611 (2009).
Snyder, E. M., van Iterson, E. H. & Olson, T. P. Clinical classification of heart failure patients using cardiac function during exercise. Exerc. Sport Sci. Rev. 43, 204–213. https://doi.org/10.1249/JES.0000000000000061 (2015).
Shah, S. et al. Supranormal left ventricular ejection fraction, stroke volume, and cardiovascular risk: Findings from population-based cohort studies. JACC Heart Fail. 10, 583–594. https://doi.org/10.1016/j.jchf.2022.05.007 (2022).
La Gerche, A. et al. Heart failure with preserved ejection fraction as an exercise deficiency syndrome: JACC focus seminar 2/4. J. Am. Coll. Cardiol. 80, 1177–1191. https://doi.org/10.1016/j.jacc.2022.07.011 (2022).
Cao, Y. et al. Sex differences in heart mitochondria regulate diastolic dysfunction. Nat. Commun. 13, 3850. https://doi.org/10.1038/s41467-022-31544-5 (2022).
Kaur, G. & Lau, E. Sex differences in heart failure with preserved ejection fraction: From traditional risk factors to sex-specific risk factors. Women’s Health (Lond. Engl.) 18, 17455057221140209. https://doi.org/10.1177/17455057221140209 (2022).
Elm, E. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ (Clinical Res. ed.) 335, 806–808. https://doi.org/10.1136/bmj.39335.541782.AD (2007).
Spertus, J. A., Jones, P. G., Sandhu, A. T. & Arnold, S. V. Interpreting the Kansas City cardiomyopathy questionnaire in clinical trials and clinical care: JACC state-of-the-art review. J. Am. Coll. Cardiol. 76, 2379–2390. https://doi.org/10.1016/j.jacc.2020.09.542 (2020).
Greene, S. J. et al. Comparison of New York heart association class and patient-reported outcomes for heart failure with reduced ejection fraction. JAMA Cardiol. 6, 522–531. https://doi.org/10.1001/jamacardio.2021.0372 (2021).
Joseph, S. M. et al. Comparable performance of the Kansas City cardiomyopathy questionnaire in patients with heart failure with preserved and reduced ejection fraction. Circ. Heart. Fail. 6, 1139–1146. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000359 (2013).
Spertus, J. A. & Jones, P. G. Development and validation of a short version of the Kansas City cardiomyopathy questionnaire. Circ. Cardiovasc. Qual. Outcomes. 8, 469–476. https://doi.org/10.1161/CIRCOUTCOMES.115.001958 (2015).
Lim, F. Y. et al. Correlation of the new York heart association classification and the cardiopulmonary exercise test: A systematic review. Int. J. Cardiol. 263, 88–93. https://doi.org/10.1016/j.ijcard.2018.04.021 (2018).
Green, C. P., Porter, C. B., Bresnahan, D. R. & Spertus, J. A. Development and evaluation of the Kansas City cardiomyopathy questionnaire: A new health status measure for heart failure. J. Am. Coll. Cardiol. 35, 1245–1255. https://doi.org/10.1016/s0735-1097(00)00531-3 (2000).
Fletcher, G. F. et al. Exercise standards for testing and training: A scientific statement from the American heart association. Circulation 128, 873–934. https://doi.org/10.1161/CIR.0b013e31829b5b44 (2013).
Ros, H. H. & de Jong & Indirect blood pressure measurement using the Riva Rocci Korotkoff method. J. Clin. Monit. 11, 149–150. https://doi.org/10.1007/BF01617741 (1995).
Rogers, G. & Oosthuyse, T. A comparison of the indirect estimate of mean arterial pressure calculated by the conventional equation and calculated to compensate for a change in heart rate. Int. J. Sports Med. 21, 90–95. https://doi.org/10.1055/s-2000-8865 (2000).
Kizakevich, P. N. et al. Comparative measures of systolic ejection during treadmill exercise by impedance cardiography and doppler echocardiography. Biol. Psychol. 36, 51–61. https://doi.org/10.1016/0301-0511(93)90080-r (1993).
Blohm, M. E. et al. Impedance cardiography (electrical velocimetry) and transthoracic echocardiography for non-invasive cardiac output monitoring in pediatric intensive care patients: A prospective single-center observational study. Crit. Care. (Lond., Engl.) 18, 603. https://doi.org/10.1007/s00134-009-1570-9 (2014).
Axler, O., Megarbane, B., Lentschener, C. & Fernandez, H. Comparison of cardiac output measured with echocardiographic volumes and aortic doppler methods during mechanical ventilation. Intensive Care Med. 29, 208–217. https://doi.org/10.1007/s00134-002-1582-1 (2003).
Galderisi, M. et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 18, 1301–1310. https://doi.org/10.1093/ehjci/jex244 (2017).
Woltjer, H. H., Bogaard, H. J. & de Vries, P. M. The intra- and interobserver variability of impedance cardiography in patients at rest and during exercise. Physiol. Meas. 17, 171–178. https://doi.org/10.1088/0967-3334/17/3/003 (1996).
Kurpaska, M. et al. Exercise impedance cardiography reveals impaired hemodynamic responses to exercise in hypertensives with dyspnea. Hypertens. Res.: Off. J. Jpn. Soc. Hypertens. 42, 211–222. https://doi.org/10.1038/s41440-018-0145-y (2019).
Kemps, H. M. C. et al. Evaluation of two methods for continuous cardiac output assessment during exercise in chronic heart failure patients. J. Appl. Physiol. (Bethesda Md. : 1985) 105, 1822–1829. https://doi.org/10.1152/japplphysiol.90430.2008 (2008).
Charloux, A. et al. A new impedance cardiograph device for the non-invasive evaluation of cardiac output at rest and during exercise: Comparison with the direct Fick method. Eur. J. Appl. Physiol. 82, 313–320. https://doi.org/10.1007/s004210000226 (2000).
Stalter, A. et al. Évaluation à l’effort D’une Nouvelle méthode de mesure du débit cardiaque basée Sur L’analyse de L’onde de Pouls (comparaison EsCCO(®) vs Physioflow(®)). Ann. Cardiol. Angeiol. 65, 1–6. https://doi.org/10.1016/j.ancard.2015.01.001 (2016).
Schober, P., Boer, C. & Schwarte, L. A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 126, 1763–1768. https://doi.org/10.1213/ANE.0000000000002864 (2018).
Sullivan, M. J., Cobb, F. R. & Higginbotham, M. B. Stroke volume increases by similar mechanisms during upright exercise in normal men and women. Am. J. Cardiol. 67, 1405–1412. https://doi.org/10.1016/0002-9149(91)90472-w (1991).
Khatibzadeh, S., Farzadfar, F., Oliver, J., Ezzati, M. & Moran, A. Worldwide risk factors for heart failure: a systematic review and pooled analysis. Int. J. Cardiol. 168, 1186–1194. https://doi.org/10.1016/j.ijcard.2012.11.065 (2013).
Cleland, J. G. F. et al. The EuroHeart failure survey programme–a survey on the quality of care among patients with heart failure in Europe. Part 1: Patient characteristics and diagnosis. Eur. Heart J. 24, 442–463. https://doi.org/10.1016/s0195-668x(02)00823-0 (2003).
Richard, R. et al. Non-invasive cardiac output evaluation during a maximal progressive exercise test, using a new impedance cardiograph device. Eur. J. Appl. Physiol. 85, 202–207. https://doi.org/10.1007/s004210100458 (2001).
Dubé, B. P., Agostoni, P. & Laveneziana, P. Exertional dyspnoea in chronic heart failure: The role of the lung and respiratory mechanical factors. Eur. Respir. Rev.: Off.J. Eur. Respir.Soc. 25, 317–332. https://doi.org/10.1183/16000617.0048-2016 (2016).
Lewis, G. D., Shah, R. V., Pappagianopolas, P. P., Systrom, D. M. & Semigran, M. J. Determinants of ventilatory efficiency in heart failure: the role of right ventricular performance and pulmonary vascular tone. Circ. Heart. Fail. 1, 227–233. https://doi.org/10.1161/CIRCHEARTFAILURE.108.785501 (2008).
Rodeheffer, R. J. et al. Exercise cardiac output is maintained with advancing age in healthy human subjects: Cardiac dilatation and increased stroke volume compensate for a diminished heart rate. Circulation 69, 203–213. https://doi.org/10.1161/01.CIR.69.2.203 (1984).
Reddy, Y. N. V., Olson, T. P., Obokata, M., Melenovsky, V. & Borlaug, B. A. Hemodynamic correlates and diagnostic role of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. JACC Heart Fail. 6, 665–675. https://doi.org/10.1016/j.jchf.2018.03.003 (2018).
Dhakal, B. P. et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ. Heart. Fail. 8, 286–294. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001825 (2015).
Massa, L. et al. The added value of cardiopulmonary exercise testing in the diagnosis of heart failure with preserved ejection fraction. Eur. Heart J. 45 https://doi.org/10.1093/eurheartj/ehae666.957 (2024).
Farinatti, P. T. V. & Soares, P. P. Cardiac output and oxygen uptake relationship during physical effort in men and women over 60 years old. Eur. J. Appl. Physiol. 107, 625–631. https://doi.org/10.1007/s00421-009-1162-y (2009).
Houstis, N. E. & Lewis, G. D. Causes of exercise intolerance in heart failure with preserved ejection fraction: Searching for consensus. J. Card. Fail. 20, 762–778. https://doi.org/10.1016/j.cardfail.2014.07.010 (2014).
Smereka, Y. & Ezekowitz, J. A. HFpEF and sex: Understanding the role of sex differences. Can. J. Physiol. Pharmacol. 102, 465–475. https://doi.org/10.1139/cjpp-2023-0403 (2024).
Sotomi, Y. et al. Sex differences in heart failure with preserved ejection fraction. J. Am. Heart Assoc. 10, e018574. https://doi.org/10.1161/JAHA.120.018574 (2021).
Beale, A. L., Meyer, P., Marwick, T. H., Lam, C. S. P. & Kaye, D. M. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation 138, 198–205. https://doi.org/10.1161/CIRCULATIONAHA.118.034271 (2018).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
S.K.: First Authorship. Data analyses, statistical analyses, literature research and hypothesis development, study development and data acquisition and main manuscript writing. Data acquisition: S.K., J.H., C.P.; A.S., R.F., M.B., S.K.: Development of the study protocol; MAB, T.S.: Proofreading manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
All study procedures are in accordance with the Declaration of Helsinki and have been reviewed by the Ethics Committee of the Faculty of Medicine of the University Leipzig.
Informed consent
All participants gave their written informed consent to participate.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kwast, S., Hoffmann, J., Pökel, C. et al. Cardiopulmonary exercise capacity markers and their link to symptom burden in patients at risk for heart failure with non-reduced ejection fraction. Sci Rep 15, 8940 (2025). https://doi.org/10.1038/s41598-025-94172-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94172-1
Keywords
This article is cited by
-
Altered cardiac contractility and aerobic muscular capacity markers during exercise in patients with obesity and DMT II
BMC Sports Science, Medicine and Rehabilitation (2025)