Abstract
Target cells trigger Vγ9Vδ2 T cell activation by signaling the intracellular accumulation of phospho-antigen metabolites (pAgs) through Butyrophilin (BTN)-3A1 and BTN2A1 to the Vγ9Vδ2 T cell receptor (TCR). An incomplete understanding of the molecular dynamics in this signaling complex hampers Vγ9Vδ2 T cell immunotherapeutic efficacy. A panel of engineered α-BTN3A1 and α-BTN2A1 antibody (mAb) reagents was used to probe the roles of BTN3A1 and BTN2A1 in pAg signaling. Modified α-BTN3A1 mAbs with increased inter-Fab distances establish that tight clustering of BTN3A1 is not necessary to stimulate Vγ9Vδ2 T cell activation, and that antagonism may occur through occlusion of a critical binding interaction between BTN3A1 and a yet unknown co-receptor. Finally, a panel of additional α-BTN2A1 antagonists utilize different biophysical mechanisms to compete with Vγ9Vδ2 TCRs for BTN2A1 binding. The complex structures of BTN2A1 ectodomain and Fabs from three antagonist mAbs provide molecular insights into BTN2A1 epitopes critical for pAg-signaling.
Similar content being viewed by others
Introduction
Vγ9Vδ2 T cells (Vγ9Vδ2s) are a unique, innate-like subset of the adaptive immune system with the potential to be developed into an off-the-shelf immunotherapy1,2. Vγ9Vδ2s were first discovered to respond to tuberculosis infection3 and proliferate polyclonally4,5,6 in cases of bacterial, parasitic, and viral infection including malaria7, listeria8, leishmaniosis9, and tularemia10. Vγ9Vδ2s are also capable of recognizing cells undergoing malignant transformation11 and are the subject of early-stage clinical trials aimed at treating a range of solid and liquid tumors including acute myeloid leukemia, renal cell carcinoma, breast, lung, and liver cancers12,13,14. While Vγ9Vδ2 immunotherapies have good safety profiles, an incomplete understanding of the molecular underpinnings of Vγ9Vδ2 activation precludes realization of their immunotherapeutic potential.
Vγ9Vδ2s are triggered by intracellular increases in the concentration of phosphoantigen metabolite (pAg) byproducts of isoprenoid biosynthesis in target cells15. The potent microbial pAg (E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP) is thought to accumulate in infection via production through the microbial methylerythritol 4-phosphate (MEP) pathway. In malignancy, mutations in pAg-metabolizing enzymes in the mevalonate (MVA) pathway3,11 cause accumulation of pAgs such as isoprenyl pyrophosphate (IPP) above healthy levels.
Vγ9Vδ2s do not employ canonical T cell antigen-recognition as target cells do not directly present pAgs to Vγ9Vδ2s via the major histocompatibility complex (MHC)16,17. Rather, pAg-accumulation is indirectly signaled to Vγ9Vδ2s through heterodimers of Butyrophilin (BTN)3A1 and its isoforms BTN3A2 and BTN3A318, and related BTN2A119,20 through a mechanism that involves multiple necessary events. First, the accumulation of pAg to sufficient intracellular concentrations promotes complex formation between the pAg and the BTN3A1 B30.2 domain21. Binding to pAg induces a conformational change in the BTN3A1 B30.2 domain22 which drives the association of the BTN3A1 and BTN2A1 intracellular domains23,24,25,26. The formation of this intracellular complex is transduced extracellularly through the BTN3A1 juxtamembrane region (JM)22,23,24,26,27,28 to the JM of either BTN3A2 or BTN3A323 to influence the conformation and subsequent binding of BTN3A extracellular IgV-like domains23 to BTN2A124,29,30 or the Vγ9Vδ2 T cell receptor (TCR)19,20,24,30. Substantial efforts by multiple groups have culminated in structural elucidation of the composition and orientation of the activating pAg-signaling complex which includes the pAg, a BTN3A1/BTN3A2 heterodimer, BTN2A1 and the Vγ9Vδ2 TCR19,20,24,25,26,29,30. Structural and functional studies show that BTN2A1 binds the Vγ9 chain of the TCR via residues in the CDR2 and germline-encoded HV4 loops19,20,24 while BTN3A2 contacts TCR residues in the Vδ2 CDR and HV4 regions24. Though our molecular understanding of the pAg-signaling complex has improved, there is conflicting evidence as to whether BTN2A1 and BTN3A alone are sufficient to stimulate Vγ9Vδ2 activation19,20,26,31. Importantly, the cellular and intra-molecular conformational dynamics of BTN3A and BTN2A1 during pAg-signaling remain to be clarified.
Antibody reagents have been critical to probing the cellular and molecular intricacies of Vγ9Vδ2 activation. BTN3A was first implicated in Vγ9Vδ2 activation when an α-BTN3A antibody (mAb), 20.1 mAb, was found to induce dramatic proliferation of γδ T cells18. The 20.1 mAb binds BTN3A ectodomains and stimulates Vγ9Vδ2 activation when incubated with healthy target cells18,32. 20.1 has been explored as an immunotherapy adjuvant13 but a lack of understanding about the mechanism by which it mimics pAg-induced Vγ9Vδ2 activation hinders its development. Structural and cellular studies conducted by our lab and collaborators suggested a model whereby the 20.1 mAb multimerizes BTN3A1 due to its binding orientation32. It has been suggested that this multimerization may mimic pAg-induced Vγ9Vδ2 activation by exerting changes in BTN3A clustering18,32 and BTN3A immobilization18, however this has not been experimentally tested. Another α-BTN3A antibody, 103.2 mAb, has antagonistic effects on Vγ9Vδ2 activation18. Structural and cellular studies in our lab have suggested that 103.2 binds to BTN3A monovalently and may antagonize Vγ9Vδ2 activation either by sterically hindering a critical interaction between BTN3A and a T cell ligand or locking BTN3A dimers in an inactive dimer conformation32. Tailored studies are required to tease these two mechanisms apart. For BTN2A1, the available antibody reagents are limited and their access challenging, restricting the ability to fully probe the role of BTN2A1 in Vγ9Vδ2 biology. To address these challenges, we engineered existing ɑ-BTN3A mAb reagents and developed new ɑ-BTN2A1 mAb binders to modulate and gain deeper insight into the cellular organization and molecular architecture of the pAg-signaling complex.
Results
Rational engineering of ɑ-BTN3A mAb 20.1 provides insights into the role of BTN3A clustering in pAg-signaling
We have hypothesized that the 20.1 mAb must multimerize BTN3A dimers when binding BTN3A bivalently due to its lateral binding orientation32 (Fig. 1a). This model suggests that the 20.1 mAb mimics a mechanism by which pAg binding to the intracellular B30.2 domain of BTN3A1 may drive tight clustering of BTN3A, seeding early immunological synapses that recruit BTN2A1 and potentially other binding partners to engage with the Vγ9Vδ2 TCR and stimulate activation.
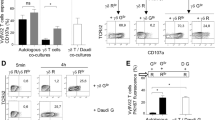
Engineering of 20.1 mAb reveals lack of BTN3A1 clustering in Vγ9Vδ2 activation. (a) Schematic representing the putative effects of increasing 20.1 inter-Fab distance on BTN3A1 membrane organization. (b) Schematic representing key features of modified mAb constructs with hinge-regions lengthened using Glycine-Serine linkers. (c) Activation of G115-TCR expressing Jurkat Jrt-3.5 cells after co-incubation with DaudiΔAAVS1 cells pre-incubated with 20.1 hinge variants at increasing concentrations or control (Buffer). CD69 expression as a marker of T cell activation was assessed by flow-cytometry (gated on isotype antibody). Means + SD and non-linear regression with variable slope—four parameters (n = 3). (d) EC50 of agonism and 95% confidence intervals (CI) for 20.1 hinge variants was calculated by non-linear regression with variable slope—four parameters (R2 = 0.9706, 0.9920, 0.9744, 0.8868, 0.9843, 0.9857, respectively). # = significant difference in EC50 value determined by non-overlapping CI.
To test the role of pAg-induced BTN3A1 clustering in pAg signaling, we engineered the 20.1 agonist mAb with increasing hinge region lengths (Fig. 1b). We hypothesized that these 20.1 mAb hinge variants would increase inter-BTN3A membrane-mobility and clustering radius within BTN3A multimers (Fig. 1a) thereby affecting the potency of Vγ9Vδ2 agonism. The wildtype (WT) 20.1 mAb is a murine IgG1 antibody in which 1 glycine separates the heavy chain cysteine residues forming the final disulfide bond with the Fab light chain and the first of 3 inter-heavy chain disulfide bonds. Glycine-serine (GS) linkers ranging from 4 to 29 amino acids were added to this glycine in the 20.1 heavy chain hinge region to generate 20.1 mAb hinge variants (20.15, 20.110, 20.115, 20.130) (Fig. 1b). Each additional hinge-linker amino acid added ~ 3.5 Å to the radius of diffusion between two 20.1 Fab:BTN3A complexes. This incrementally increased the radius of diffusion in multimerized BTN3A, from ~ 155 Å with the WT 20.1 mAb to ~ 360 Å with the 20.130 mAb hinge variant, by a maximum factor of ~ 2.3X (Fig. 1b). GS linkers did not affect protein stability nor affinity for biotinylated BTN3A1 ectodomain as assessed by SDS-PAGE gel electrophoresis and Bio-Layer Interferometry (BLI), respectively (Supplementary Fig. 1a,b).
Increasing concentrations of the WT 20.1 mAb, the 20.1 mAb hinge variants or the 20.1 Fab were added to Daudi-Cas9-ΔAAVS1 (DaudiΔAAVS1), a B-cell lymphoma target-cell line that readily activates Vγ9Vδ2 T cells, or Daudi-Cas9-ΔBTN3A1 (DaudiΔ3A1)33. Jurkat JRT3.5 T cells expressing the G115 Vγ9Vδ2 TCR34 (G115 Jurkats) were then co-incubated with these mAb-pulsed target-cells overnight and assayed for activation by analysis of CD69 expression via flow cytometry. There was no obvious trend between the 20.1 mAb hinge length and Vγ9Vδ2 activation (Fig. 1c) though increasing the 20.1 mAb hinge length had minor effects on Vγ9Vδ2 activation at different concentrations (Supplementary Fig. 1c) and on the EC50 of Vγ9Vδ2 agonism (Fig. 1d). This indicated that tight clustering and multimerization of BTN3A is likely not the driving factor in 20.1 mAb agonism of Vγ9Vδ2 activation though the existence of another binding partner driving multimerization cannot be excluded. The 20.1 Fab has an infinite radius of diffusion and, as previously reported32, retains the ability to activate Vγ9Vδ2 T cells with reduced potency (Fig. 1c,d, Supplementary Fig. 1d,e). The WT 20.1 mAb, 20.1 mAb hinge variants and the 20.1 Fab were mildly agonistic when incubated with DaudiΔ3A1 target cells (Supplementary Fig. 1d,e), possibly due to interactions with BTN3A2 and BTN3A323, expression of which were retained in this cell line. These data suggest that 20.1 agonism of Vγ9Vδ2 activation is driven by the molecular or steric effects of 20.1 contact with BTN3A. Indeed, BTN3A1 residues bound by both the 20.1 agonist mAb and ɑ-BTN3A agonist mAb CTX2026 are directly adjacent to a recently reported pAg-signaling hotspot29 (Supplementary Fig. 2a).
ɑ-BTN3A mAb 103.2 inhibits Vγ9Vδ2 activation through bi-valent engagement of BTN3A ectodomains
The engineering of the 20.1 mAb provided insights into the role of cellular reorganization of BTN3A during pAg signaling. We wanted to then investigate the role of BTN3A conformational change in pAg signaling by similarly engineering the antagonist 103.2 mAb. Our original analysis of our complex structure between the 103.2 scFv and BTN3A (PDB ID: 4F9P) used a model derived from IgGs that had long, flexible linkers between the Fab and Fc domains32. Based on this analysis, we concluded that the 103.2 mAb would be able to bind one BTN3A dimer, with each Fab engaging with one BTN3A monomer in an overall 1:1 stoichiometry (mAb to BTN3A dimer). We have since re-analyzed this complex in the context of the functional 103.2 IgG isotype (muIgG1), which has a more restricted length and flexibility of 7 Å in the hinge region between the Fabs connected by the Fc domain (Fig. 2a). To do this, we aligned the 103.2 scFv with 5 full-length murine IgG1 Fabs (Supplementary Fig. 2b). We observed that the distance between the heavy chain cysteines that form the final inter-chain disulfide bond of the Fab are between 79–113 Å apart (Fig. 2a, Supplementary Fig. 2b), a distance that the hinge region must bridge in a bi-valent mAb. Therefore, there is either significant flexibility in the BTN3A monomers between their IgV and IgC domains, or the 103.2 mAb must engage the BTN3A dimer with a 1:2 stoichiometry (one 103.2 mAb to 2 BTN3A dimers). In the first scenario, we have previously shown that while the IgC domains of BTN3A have negligible structural flexibility when dimerized, the IgV domains can rotate up to ~ 20 Å at the point of the linker between the IgV and IgC domains32 (Fig. 2a). Including the general flexibility of ~ 20 Å between Fab IgV and IgC domains (Fig. 2a, Supplementary Fig. 2b), our model positions the 103.2 mAb just within the calculated threshold of monovalent binding to 1 mAb to 1 BTN3A dimer with a limited overall flexibility of ~ 8.5 Å. If monovalent binding between 103.2 and BTN3A occurs in physiological contexts, substantial torsional force on the BTN3A IgV domains would be required, potentially locking them in an inactive conformation. Alternatively, bivalent 103.2 binding to BTN3A may also influence the conformational flexibility of BTN3A dimers, preventing the propagation of intracellular pAg-binding information through JMs to BTN3A extracellular domains, ultimately influencing BTN3A interactions between heterodimers or with other proteins such as BTN2A1.
Engineering of 103.2 mAb reveals lack of BTN3A1 conformational constraint in Vγ9Vδ2 antagonism. (a) Structural alignment of BTN3A1 in complex with the 103.2 single-chain-variable-fragment (scFv) (PDB ID: 4F9P) with 2 mouse IgG1 Fragment antibodies (Fab) (PDB ID: 1BAF) and ColabFold models of the murine IgG1 Hinge regionREF. Distance between heavy chain cysteine residues forming each terminal Fab disulfide bond (yellow) is denoted by *. First hinge-region inter heavy chain cysteine residues shown in blue. Range of orientations of BTN3A1 IgV in relation to BTN3A1 IgC and Fab IgV in relation to Fab IgC determined by structural alignment are indicated. (b) Schematic representing key features of modified mAb constructs with hinge-regions between Fab and hinge disulfide bonds lengthened using Glycine-Serine linkers. (c) Inhibition of activation of G115-TCR expressing Jurkat Jrt-3.5 cells after co-incubation with DaudiΔAAVS1 cells pre-incubated with 5 μM HMBPP and 103.2 hinge variants at increasing concentrations or controls (Buffer + HMBPP). CD69 expression as a marker of T cell activation was assessed by flow-cytometry (gated on isotype antibody). Means + SD and non-linear regression with variable slope—four parameters (n = 3). (d) IC50 of inhibition and 95% confidence intervals for 103.2 hinge variants was calculated by non-linear regression with variable slope—four parameters (R2 = 0.9501, 0.9508, 0.9273, 0.9396, 0.8086, 0.3131 respectively). # = significant difference in IC50 value determined by non-overlapping CI.
To test these models, we generated 103.2 variants with longer hinge regions (103.25, 103.210, 103.215, 103.230) to reduce conformational torsion on BTN3A extracellular domains (Fig. 2b), potentially altering the potency of 103.2 antagonism of Vγ9Vδ2 T cell activation. As with the 20.1 mAb hinge variants, GS linkers in the 103.2 mAb hinge variants did not affect protein stability as assessed by SDS-PAGE gel electrophoresis although we noted a minor increase in their relative affinity for biotinylated BTN3A1 ectodomain as assessed by BLI (Supplementary Fig. 3a,b).
Increasing concentrations of the 103.2 mAb hinge variants or the 103.2 Fab were added to DaudiΔAAVS1 or DaudiΔ3A1 in the presence of 5 μM HMBPP. While DaudiΔAAVS1 are capable of activating Vγ9Vδ2s without exogenous pAg1, addition of exogenous pAg boosts the potency with which DaudiΔAAVS1 stimulate Vγ9Vδ2 activation thus increasing the dynamic range of inhibition assays. G115 Jurkats were then co-incubated with mAb + pAg-pulsed target-cells overnight and assayed for activation by analysis of CD69 expression via flow cytometry. Increasing the 103.2 hinge-length had no significant effect on the maximum (Fig. 2c) or potency (Fig. 2d) of Vγ9Vδ2 activation though we observed minor effects on Vγ9Vδ2 inhibition at different concentrations (Supplementary Fig. 3c). The 103.2 Fab has an infinite radius of diffusion and did not antagonize Vγ9Vδ2 activation (Fig. 2c,d) and DaudiΔ3A1 cells did not stimulate Vγ9Vδ2 activation in the presence of the 103.2 reagents tested (Supplementary Fig. 3d). Our data suggest that the mechanism of 103.2 antagonism does not involve releasing conformational constraint on BTN3A ectodomains or multimerizing BTN3A though it is possible that our linkers were not long enough to modulate conformational constraint. We therefore posit that the importance of the 103.2 mAb bivalent engagement of BTN3A in Vγ9Vδ2 antagonism may be due to its higher avidity or the added steric bulk of a mAb over Fab or scFv reagents. Higher avidity or steric hindrance may enable the 103.2 mAb to successfully compete with a yet unknown ligand of BTN3A.
ɑ-BTN2A1 mAbs antagonize Vγ9Vδ2 activation with varying potency
The 20.1 and 103.2 mAbs have provided valuable insight into the cellular and molecular changes in BTN3A during pAg-signaling. To better map immunogenic and inhibitory hotspots for Vγ9Vδ2 activation on BTN2A1, we generated a panel of 15 ɑ-BTN2A1 Fabs using phage display selection35,36 with the BTN2A1 ectodomain as the target antigen. Negative selection was carried out using the BTN3A1 ectodomain as the target antigen and Fab selectivity was verified using phage-enzyme linked immunosorbent assay (ELISA) (Supplementary Fig. 4a). ɑ-BTN2A1 Fabs bound the biotinylated, monomeric BTN2A1 ectodomain with varying biophysical affinities in the pico-nanomolar range as assessed by Surface Plasmon Resonance (SPR) (Supplementary Table 1, Supplementary Fig. 4b). Full-length mAb constructs bound endogenous BTN2A1 on DaudiΔAAVS1 with a range of cellular affinities measured by EC50 of binding (Supplementary Fig. 5a,b) and had negligible background staining on Daudi-Cas9 cells where BTN2A1 expression had been knocked out (DaudiΔ2A1) (Supplementary Fig. 5c).
To test the effect of these ɑ-BTN2A1 mAbs on Vγ9Vδ2 activation, ɑ-BTN2A1 mAbs were incubated with DaudiΔAAVS1 or DaudiΔ2A1 at a concentration of 6.8 nM (1 µg/mL) in the presence and absence of 5 μM HMBPP. G115 Jurkats were then co-incubated with these mAb-pulsed target-cells overnight and assessed for CD69 expression via flow-cytometry (Supplementary Fig. 6a). CD69 expression was assessed relative to a no mAb control group. Both in the presence and absence of exogenous pAg, ɑ-BTN2A1 mAbs antagonized Vγ9Vδ2 activation with varying strength (Fig. 3a, Supplementary Fig. 6b), whereas no mAb had agonist properties (Fig. 3a, Supplementary Fig. 6b). DaudiΔ3A1 cells co-incubated with ɑ-BTN2A1 mAbs did not stimulate Jurkat Vγ9Vδ2 T cell activation (Supplementary Fig. 6c,d). 2A1.9 was as strong an antagonist of Vγ9Vδ2 activation as the ɑ-BTN3A1 mAb 103.2 (Fig. 3a) whereas other mAbs like 2A1.4 and 2A1.12 were weak antagonists, and some including 2A1.11 had negligible effects on Vγ9Vδ2 activation (Fig. 3a).
ɑ-BTN2A1 mAbs antagonize Vγ9Vδ2 activation through different mechanisms, including blocking BTN2A1-TCR binding. (a) Inhibition of activation of G115-TCR expressing Jrt-3.5 cells after co-incubation with DaudiΔAAVS1 cells pre-incubated with 5 μM HMBPP and 6.8 nM (1 μg/mL) ɑ-BTN2A1 mAb or controls (MOPC isotype, 20.1 mAb, 103.2 mAb or Buffer + 5 μM HMBPP). CD69 expression was assessed by flow-cytometry (gated on isotype antibody) and normalized to Buffer + 5 μM HMBPP condition (100*Value-Baseline/Baseline). Mean + SD (n = 6, 2 independent experiments). Dunnett test pairwise comparison to – mAb control: ****p < 0.0001, ***p < 0.0002. (b) Inhibition of activation of Vγ9Vδ2 T cells expanded from primary PBMCs after co-incubation with DaudiΔAAVS1 cells pre-incubated with 5 μM HMBPP and increasing concentrations of ɑ-BTN2A1 mAb or controls (MOPC isotype, 103.2 mAb or Buffer + HMBPP). CD25 (top), CD107a (middle) and CD69 (bottom) expression were assessed by flow-cytometry (gated on isotype antibody). Mean + SD (n = 3). (c) Competition between ɑ-BTN2A1 mAbs and the Vγ9Vδ2 TCR for binding to BTN2A1 ectodomain assessed in High-Five cells expressing full-length BTN2A1 stained with 150 nM ɑ-BTN2A1 mAbs or controls (no TCR tetramer, MOPC isotype or TCR tetramer + Buffer followed by 120 nM fluorescently-tagged Vγ9Vδ2-TCR tetramers (G115 clone). TCR tetramer staining was assessed by flow-cytometry (gated on no TCR) and normalized to TCR tetramer, − mAb condition (100*Value-Baseline/Baseline). Mean + SD (n = 6, 2 independent experiments). Dunnett test pairwise comparison to Buffer control: ****p < 0.0001. (d) Correlation between ɑ-BTN2A1 mAb Vγ9Vδ2 antagonism (Baseline-corrected CD69 expression) and Vγ9Vδ2 TCR-competition (Baseline-corrected TCR tetramer staining) as calculated by simple linear regression (R2 = 0.136, p = 0.1944). Strong and weak TCR blocking designated as lower and upper half of the range of TCR tetramer staining, respectively. TCR inhibition classified based on p values from (a). (e) Vγ9Vδ2 TCR competition with ɑ-BTN2A1 Fab or control (20.1 Fab) for binding to BTN2A1 ectodomain assessed by BLI, normalized. Immobilized biotinylated BTN2A1 ectodomain was exposed to 1. 66 μM Fab followed by 2. 66 μM Fab + 80 μM TCR (G115 clone). Step 2 Binding (nm) of TCR to biotinylated BTN2A1 ectodomain with Fab was normalized to Fab alone binding (nm) at the end of Step 1. Bars and circles outlined in black indicate the representative reagent for each class of mAb.
The potency of antagonism for selected mAbs was tested using primary Vγ9Vδ2 T cells expanded from Peripheral Blood Mononuclear Cells (PBMCs) derived from healthy donors, (Fig. 3b) in activation assays (Supplementary Fig. 7a) similar to those previously described. 2A1.9 was the strongest antagonist of primary Vγ9Vδ2 T cell activation, exceeding even 103.2, reducing CD107a, CD25 and CD69 expression below baseline expression levels (Fig. 3b). 2A1.11 and 2A1.12 had no effect on primary Vγ9Vδ2 T cell activation (Fig. 3b). 2A1.12 also had greater variation in antagonism potency than other tested mAbs, suggestive of biochemical differences in its molecular interactions with BTN2A1. DaudiΔ2A1 cells co-incubated with ɑ-BTN2A1 mAbs did not stimulate primary Vγ9Vδ2 T cell activation (Supplementary Fig. 7b).
ɑ-BTN2A1 mAb antagonists block Vγ9Vδ2 TCR engagement with BTN2A1 ectodomain
Linking ɑ-BTN2A1 Fab epitopes on the BTN2A1 ectodomain to mAb effects on Vγ9Vδ2 activation provides potential insights into the molecular architecture of the mature pAg-signaling complex. BTN2A1 is a binding partner of the Vγ9Vδ2 T cell Receptor (TCR) and binding of its CFG-IgV face to the HV4 germline-encoded region of the Vγ9Vδ2 TCR γ-chain is essential but not sufficient for pAg-signaling19,20,23,29. To explore if our antagonistic ɑ-BTN2A1 mAbs block pAg-signaling by competing for the Vγ9Vδ2 TCR epitope on BTN2A1, we tested mAb-TCR competition by using High-Five insect cells expressing full-length BTN2A1 on their cell surface (Supplementary Fig. 8a). These cells were incubated with 150 nM saturating concentrations of our ɑ-BTN2A1 mAbs and were then stained with 120 nM fluorescently tagged tetramers of the G115 Vγ9Vδ2 TCR clone. TCR tetramer staining intensity was assessed relative to a no mAb, full TCR staining control group. G115 tetramer staining of BTN2A1 was significantly reduced to varying degrees in the presence of every ɑ-BTN2A1 mAb (Fig. 3c). Addition of certain mAbs (2A1.9, 2A1.14, 2A1.8, 2A1.7, 2A1.13, 2A1.3 and 2A1.12) had a greater effect on TCR tetramer staining of BTN2A1 (Fig. 3c), potentially indicative of a larger overlapping mAb:TCR epitope on BTN2A1 or a stronger mAb-BTN2A1 binding interface. The G115 tetramer had no background staining on High-five insect cells expressing the control transmembrane protein ADLRG3 (Supplementary Fig. 8b).
To understand the relationship between Vγ9Vδ2 antagonism and TCR competition potency we classified the mAbs based on their potency of Vγ9Vδ2 antagonism and TCR competition (Fig. 3d). A class of strongly antagonistic mAbs that dramatically reduce TCR:BTN2A1 staining emerged (Fig. 3d), represented by the 2A1.9 mAb. Another class of mAbs substantially reduced TCR staining of BTN2A1 but did not reduce Vγ9Vδ2 activation (Fig. 3d) represented by the 2A1.12 mAb. The third class of mAbs weakly blocked TCR binding to BTN2A1 but had neutral effects on Vγ9Vδ2 activation (Fig. 3d) represented by the 2A1.11 mAb. Although no mAbs had strong antagonistic properties without blocking the TCR (Fig. 3d), the 2A1.4 mAb significantly reduced Vγ9Vδ2 activation and was the weakest TCR blocker (Fig. 3d) suggesting that it may have a mechanism of antagonism different from direct TCR competition for BTN2A1 binding.
The mAb:TCR competition for BTN2A1 binding was validated for representative Fabs using BLI (Fig. 3e, Supplementary Fig. 8c,d). The BTN2A1 ectodomain was immobilized and exposed first (1) to ɑ-BTN2A1 Fab at 66 μM for 150 s (s) followed by (2) 66 μM Fab mixed with 80 μM monomeric G115 Vγ9Vδ2 TCR clone for 150 s. Fabs were included in the TCR association step (2) to minimize Fab dissociation. The 20.1 Fab, as control, was used to determine maximum TCR binding (Supplementary Fig. 8c,d). The results from this biophysical epitope competition tracked with that assessed in High-Five insect cells: 2A1.9 completely blocked Vγ9Vδ2 TCR association with BTN2A1 ectodomain (Fig. 3e, Supplementary Fig. 8c,d), 2A1.12 and 2A1.13 also dramatically reduced Vγ9Vδ2 TCR association with BTN2A1 ectodomain (Fig. 3e, Supplementary Fig. 8c,d), and 2A1.4 and 2A1.11 did not substantially reduce Vγ9Vδ2 TCR association with BTN2A1 ectodomain (Fig. 3e, Supplementary Fig. 8c,d). The largely nonlinear relationship between Vγ9Vδ2 antagonism and TCR competition (Fig. 3d) prompted us to pursue structural determination of Fab:BTN2A1 complexes to assess how mAb epitopes contributed to Vγ9Vδ2 antagonism.
Complex structure shows 2A1.9 Fab sequestering critical residues in the Vγ9Vδ2 TCR epitope on BTN2A1
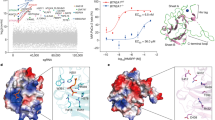
To better understand the molecular underpinnings of mAb antagonism of Vγ9Vδ2 TCR, a 2.8 Å structure of 2A1.9 Fab in complex with monomeric BTN2A1 ectodomain C219S (BTN2A1 ectodomain) was solved by X-ray crystallography (PDB ID: 8VC7, Fig. 4a, Supplementary Table 2). 2A1.9 Fab bound to the IgV-like domain of BTN2A1 ectodomain at a docking angle of 99.6° (Fig. 4a) utilizing all CDR loops except CDRL2 to make molecular contacts with residues located throughout BTN2A1 C”, C’, C, F, and G β strands (CFG-face) (Fig. 4b). The total Buried Surface Area (BSA) on the BTN2A1 ectodomain was 964.6 Å, with the Fab heavy and light chains contributing 658.6 Å and 306.0 Å, respectively. The 2A1.9 Fab formed 15 Hydrogen-bonds (HB) and salt-bridges (SB) with BTN2A1 ectodomain (Supplementary Table 3), 7 of which involved residues in the CDRH3 loop. These stable contacts were strengthened through 154 calculated van der Waals (vdW) interactions between both side- and main-chain atoms of 2A1.9 Fab CDR loops and BTN2A1 ectodomain CFG-face residues (Supplementary Table 3). Due to the abundance of vdW interactions, representative contacts were chosen for each residue pair when multiple atoms formed vdW interactions for visual clarity (Supplementary Table 3).
Structure of 2A1.9 Fab in complex with BTN2A1 ectodomain provides insight into TCR-competition mechanism of Vγ9Vδ2 Antagonism. (a) Structure and surface representation of BTN2A1 ectodomain complexed with antagonistic 2A1.9 Fab as determined by X-ray Crystallography (PDB ID: 8VC7) (b) CFG Face of BTN2A1 ectodomain showing key residues implicated in Vγ9Vδ2 activation20 or TCR binding30 and docking orientation of 2A1.9 Fab CDR loops. (c) Surface representation of BTN2A1 ectodomain with residues contacting 2A1.9 Fab (orange), crucial for Vγ9Vδ2 activation as assessed by mutagenesis20(red) or both (pink) highlighted (Supplementary Table 5). (d) Surface representation of BTN2A1 ectodomain with residues contacting 2A1.9 Fab (orange), Vγ9Vδ2 TCR (PDB ID: 8DFW, green) or both (olive) highlighted (Supplementary Table 5). (e-g) Molecular contacts of 2A1.9 Fab with BTN2A1 residues implicated in Vγ9Vδ2 activation20 or TCR binding30. Orientation and molecular interactions of selected BTN2A1 residues (e) Glu107 and Arg96 (f) Tyr98, Gln100 and Tyr105 (g) Phe43 and Ser44. Highlighted residues of BTN2A1 IgV domain bound to 2A1.9 Fab (light grey) or Vγ9Vδ2 TCR (dark grey) are shown. HB (distance < 3.5 Å) and SB (distance < 4.5 Å) depicted in black, vdW (distance < 4 Å) depicted in yellow. If residue contact involves both HB/SB and vdW only HB/SB is shown.
The 2A1.9 binding footprint also included critical residues for pAg-signaling (Fig. 4c, Supplementary Table 5) identified through rational mutagenesis based on structural models of the BTN2A1:TCR complex20. We then mapped the binding footprints of the 2A1.9 Fab and the Vγ9Vδ2 TCR (PDB ID: 8DFW)30 on the BTN2A1 ectodomain (Fig. 4d, Supplementary Table 5). Consistent with our cellular and biophysical results, the epitopes of the 2A1.9 Fab and the Vγ9Vδ2 TCR overlapped substantially, with the 2A1.9 Fab sequestering all but 3 residues in the interface between the Vγ9Vδ2 TCR and the BTN2A1 ectodomain (Fig. 4d). To compare the structural and molecular differences in BTN2A1 when bound to either the 2A1.9 Fab or the Vγ9Vδ2 TCR we aligned the BTN2A1 IgV domain in our complex structure with that in the structure of the BTN2A1 ectodomain in complex with the Vγ9Vδ2 TCR (PDB: 8DFW)30. The angle between the BTN2A1 ectodomain IgV and IgC domains was altered 7.3° when bound to the 2A1.9 Fab versus the Vγ9Vδ2 TCR (Fig. 4d). We then analyzed critical BTN2A1 residue side chain orientations and molecular contacts with the 2A1.9 Fab. Residues that formed electrostatic interactions with the Vγ9Vδ2 TCR30 or had been shown by mutagenesis to be critical for pAg-signaling20 were highlighted. The 2A1.9 Fab residue R103 forms a network of HB and vdW interactions with BTN2A1 residues R96 and E107 and alters the position of E107 (Fig. 4e), likely contributing to the high affinity of the 2A1.9 Fab for BTN2A1 as well as its antagonism potency. The 2A1.9 Fab contacts BTN2A1 Y98, Q100, and Y105 residues through G104 and Y105 in its CDRH3 loop via main- and side-chain HB and vdW interactions (Fig. 4f). The 2A1.9 Fab uses side- and main-chain atoms in CDRH3 and CDRH1 residues to sequester BTN2A1 residues F43 and S44 through extensive vdW interactions (Fig. 4g). Altogether these data establish that the mechanism by which 2A1.9 antagonizes Vγ9Vδ2 activation is by direct competition with the Vγ9Vδ2 TCR for BTN2A1 binding.
Complex structure of BTN2A1 with 2A1.12 Fab reveals insight into molecular features governing BTN2A1’s role in pAg-signaling
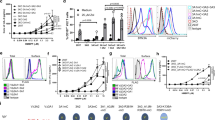
Having verified the importance of the BTN2A1 epitope shared between 2A1.9 and the Vγ9Vδ2 TCR in Vγ9Vδ2 activation, we wished to understand whether other epitopes on BTN2A1 were critical for pAg signaling. Single-particle Cryo-electron microscopy was utilized for high-throughput screening of multiple Fabs in complex with BTN2A1 ectodomain dimer and an anti-Fab nanobody37. We prioritized the 2A1.12 mAb as it is a strong TCR blocker yet has insignificant effects on Vγ9Vδ2 activation (Fig. 3d, Supplementary Fig. 9). A map of the 2A1.12 Fab complexed with BTN2A1 was refined to 3.9 Å (Supplementary Fig. 10, Table 4), and a model was generated using the structures of the BTN2A1 ectodomain (PDB ID: 9DPE) using an AlphaFold2-generated structure of a muIgG1 Fab complexed with a nanobody as a starting model (Fig. 5a,b). The 2A1.12 Fab binds to the BTN2A1 ectodomain CFG-face with a total BSA on the BTN2A1 ectodomain of 1046.3 Å, with the Fab heavy and light chains contributing 687.7 Å and 358.6 Å, respectively. The 2A1.12 Fab formed 3 Hydrogen-bonds (HB) and salt-bridges (SB) with BTN2A1 ectodomain (Supplementary Table 6), 2 of which involved residues in the CDRH3 loop. These stable contacts were strengthened through calculated van der Waals (vdW) interactions between both side- and main-chain atoms of 2A1.12 Fab CDR loops and BTN2A1 ectodomain CFG-face residues (Supplementary Table 6).
Structure of 2A1.12 Fab in complex with BTN2A1 ectodomain shows weaker binding interface with BTN2A1 than the 2A1.9 Fab. (a) Structure and surface representation of BTN2A1 ectodomain complexed with TCR-blocking 2A1.12 Fab as determined by Cryo-EM (PDB ID: 9DPE) (b) CFG Face of BTN2A1 ectodomain showing key residues implicated in Vγ9Vδ2 activation20 or TCR binding30 and docking orientation of 2A1.9 Fab CDR loops. (c) Surface representation of BTN2A1 ectodomain with residues contacting 2A1.12 Fab (purple), 2A1.9 (orange) or both (brown) highlighted (Supplementary Table 5). (d) Surface representation of BTN2A1 ectodomain with residues contacting 2A1.12 Fab (purple), Vγ9Vδ2 TCR (PDB ID: 8DFW, green) or both (olive) highlighted (Supplementary Table 5). (e-g) Orientation and molecular interactions of selected BTN2A1 residues (e) Asp33 (f) Lys51, Arg54, and Arg56 (g) Glu35, Phe39, Phe43, Lys51, Arg96, Tyr98, and Glu107. Highlighted residues of BTN2A1 IgV domain bound to 2A1.12 Fab (light grey) or Vγ9Vδ2 TCR (dark grey) are shown. HB (distance < 3.5 Å) and SB (distance < 4.5 Å) depicted in black, vdW (distance < 4 Å) depicted in yellow. If residue contact involves both HB/SB and vdW only HB/SB is shown. (h) Alignment of ɑ-BTN2A1 mAb CDRH3 loop amino acid sequences.
The 2A1.12 Fab docks to BTN2A1 at an angle of 99.6° (Fig. 5a), equivalent to the docking angle of the 2A1.9 Fab. The binding footprint of 2A1.12 on BTN2A1 substantially overlaps with that of the 2A1.9 Fab (Fig. 5c, Supplementary Table 5,6) and the Vγ9Vδ2 TCR (Fig. 5d, Supplementary Table 5), suggesting that the BTN2A1 CFG-face is antigenic. Specifically, both the 2A1.12 and 2A1.9 Fabs utilize the same CDR residues to form HB with BTN2A1 residues D33 (Fig. 5e, Supplementary Table 3), K51 (Fig. 4e, Supplementary Table 3) and E107 (Figs. 4e, 5g). However, the binding interface between 2A1.9 and BTN2A1 is further stabilized by an additional 12 HB and extensive vdW interactions (Supplementary Table 3). The variable ability of the 2A1.12 and 2A1.9 mAbs to antagonize Vγ9Vδ2 activation and block TCR recognition of BTN2A1 is likely due to the biophysical strength with which their Fab binds BTN2A1.
Molecular intricacies in Fab:BTN2A1 interface may drive the potency of mAb Vγ9Vδ2 antagonism
We were curious to further explore how various mAbs had the ability to sequester the Vγ9Vδ2 TCR epitope on BTN2A1 with variable effects on Vγ9Vδ2 activation ranging from strong inhibition to neutral. To probe whether the physiological properties of mAbs could be characterized by differences in biophysical or cellular affinity, Fab KD, kA, kD, and mAb EC50 were plotted as a function of Vγ9Vδ2 TCR blocking potency (Supplementary Fig. 11a) or antagonism (Supplementary Fig. 11b). We observed that 2A1.12 and 2A1.4 have 5.8X and 1.7X faster respective kD properties than 2A1.9 (Supplementary Table 1) and the strongest antagonists tended to have biophysical affinities (KD) below 1 nM (Supplementary Fig. 11b). However, there was no overall correlation between mAb affinity for BTN2A1 and Vγ9Vδ2 antagonism or TCR blocking (Supplementary Fig. 11). We then aligned the CDRH3 loop sequences of ɑ-BTN2A1 mAbs (Fig. 5h) and observed that the R103 and GY motif (Fig. 4e,f) used by the 2A1.9 Fab to sequester critical BTN2A1 residues from the Vγ9Vδ2 TCR were highly conserved in our panel (Fig. 5h), likely forming the core binding interface of our Fab panel with BTN2A1. Molecular differences in the surrounding Fab CDR loop sequences may drive differential binding dynamics with BTN2A1, controlling whether Fab contact with BTN2A1 persists on the time scale necessary to compete with Vγ9Vδ2 TCR binding in a physiological context and antagonize T cell activation. For example, our structural data show that both 2A1.9 and 2A1.12 bind BTN2A1 with the conserved R103 and GY motif yet 2A1.9 does so through 3 HB/SB compared to 1 HB/SB used by 2A1.12 (Supplementary Tables 3, 5). Additionally, 2A1.9 stabilizes this core binding interface through an additional 12 HB/SB compared to the 2 stabilizing HB/SB between 2A1.12 and BTN2A1. Overall, our data highlight the complicated mechanisms by which antibodies bind to and influence the behaviors of their targets with important implications in the development of antibody-based therapeutics and research tools.
Discussion
Upon binding to a pAg metabolite, BTN3A1 is responsible for initiating a series of cellular and molecular events on the target cell membrane involving BTN2A1 to signal distress to Vγ9Vδ2 T cells. In this study, we rationally modified and developed antibodies against these key players in pAg signaling to resolve long-standing questions in our understanding of the Vγ9Vδ2 activation mechanism. The 20.1 mAb or pAg binding to BTN3A1 causes BTN3A immobilization with subsequent puncta formation on the cell membrane and localization to the target cell—T cell interface18,32 potentially creating BTN3A clusters that seed early immunological synapses. Our work suggests that while the 20.1 mAb may indeed cluster and immobilize BTN3A on the cell membrane, contact between 20.1 and the BTN3A ectodomain alone appears to drive 20.1 agonism. This may suggest an allosteric mechanism whereby 20.1 contact with BTN3A generates an epitope for binding to BTN2A1, the Vγ9Vδ2 TCR22,24 or a yet unknown ligand. Alternatively, a mechanism by which the 20.1 mAb occludes or allosterically alters an inhibitory epitope on BTN3A is possible as 20.1 agonism potency decreases as the bulk of the 20.1 reagent decreases from a mAb to a Fab and then to an scFv32. Indeed, an antigenic hotspot on BTN3A1 critical for pAg signaling to the MOP clone of the Vγ9Vδ2 TCR lies directly adjacent to the 20.1 epitope on BTN3A129. Binding of the 20.1 agonist to BTN3A1 may alter or occlude this antigenic hotspot enabling the recruitment of a Vγ9Vδ2 TCR ligand or binding to the Vγ9Vδ2 TCR itself.
In contrast to the 20.1 mAb, the 103.2 mAb loses antagonistic function when modified into a Fab or scFv, both of which do not inhibit Vγ9Vδ2 activation32. We hypothesized that the bivalent 103.2 mAb may conformationally constrain BTN3A ectodomain dimers to inhibit Vγ9Vδ2 activation. We predicted that mAb hinge lengths greater than 10 amino acids would alleviate torsional force of monovalent 103.2 mAb binding to BTN3A dimers, potentially rescuing Vγ9Vδ2 activation. Our experiments show that the 103.2 mAb likely does not antagonize Vγ9Vδ2 activation by locking BTN3A ectodomains in an inactive conformation. The 103.2 mAb may instead sterically occlude access to critical BTN2A1 and Vγ9Vδ2 epitopes19,20,24,29,30 on the rest of the BTN3A ectodomain as it binds to the top of the IgV domain of BTN3A dimers32. While the the Fc domain of the 103.2 mAb may provide this steric hindrance, mAb bi-valency may be key to 103.2 antagonism, allowing the 103.2 mAb to compete with alternate ligands for BTN3A1 binding despite the low binding affinity of 15 nM between the 103.2 Fab and BTN3A132. Future studies can test the effect of a 103.2 bi-Fab on Vγ9Vδ2 to determine whether avidity or steric hindrance drives the 103.2 mAb antagonism of Vγ9Vδ2 activation.
We were then curious to learn more about the putative interactions between the BTN3A1 and BTN2A1 ectodomains, the established interaction between BTN2A1 and the Vγ9Vδ2 TCR and yet unknown BTN2A1 binding partners by developing mAbs against BTN2A1. Our BTN2A1 antibodies either antagonized or had no effect on Vγ9Vδ2 antagonism consistent with published reagents19,38. This suggests that the primary role BTN2A1 serves is to coordinate the formation of the pAg signaling complex as a central protein covered in critical epitopes for pAg signaling, one of which binds the Vγ9Vδ2 TCR (Fig. 4)19,20,24,38. Cellular and structural data suggest that many of our ɑ-BTN2A1 mAbs bind the Vγ9Vδ2 TCR epitope on BTN2A1 with pM-nM range affinities. However, some do not inhibit Vγ9Vδ2 T cell activation although the affinity between BTN2A1 and the HV4 loop of the Vγ9Vδ2 TCR is ~ 40 μM. Though the biophysical principles underlying TCR-signaling are still poorly understood, it is possible that antibodies with fast off-rates provide a sufficient opportunity for the Vγ9Vδ2 TCR to bind to BTN2A1 and initiate TCR-mediated signaling. None of our tested antibody reagents bound non-overlapping epitopes with the Vγ9Vδ2 TCR, therefore the roles of the BTN2A1 IgC domain as well as the IgV domain membrane distal and ABE faces remain undetermined. It is possible that additional epitopes exist to coordinate binding with BTN3A or another TCR CDR loop ligand.
We have utilized the reagents developed in this work to improve our understanding of the molecular events driving pAg signaling. While this knowledge may eventually aid in the improvement of Vγ9Vδ2 immunotherapies, our reagents themselves can be useful in a clinical setting. The 20.1 mAb is already being explored as an immunotherapy adjuvant and we report a small but significant improvement on its agonist properties with the addition of 4 amino acids to its hinge region (Fig. 1c,d). Therapeutic development surrounding BTN3A has focused on reagents with agonistic properties. An intriguing idea is the use of antagonist antibodies in clinical contexts where the presence of Vγ9Vδ2 T cells or the expression of BTNs is a poor prognostic marker. Pro-inflammatory subsets of Vγ9Vδ2 T cells have been implicated in autoimmune disorders including psoriasis, inflammatory bowel disease, celiac disease, and multiple sclerosis39. Inhibiting the activity of Vγ9Vδ2 T cells in such contexts may alleviate symptoms through the management of excessive inflammation. In the context of cancer, data surrounding the association between BTN expression in tumors and clinical outcomes are complicated and tumor dependent. High expression of BTN3A1 in ovarian and pancreatic cancers14,40,41 and BTN2A1 in metastatic renal cell carcinoma42 has been associated with reduced patient survival. Indeed, BTN3A1 has been shown to suppress αβ T cell TCR signaling in tumors by preventing the segregation of CD45 from the immune synapse41. Thus, the role of tumor-infiltrating Vγ9Vδ2 T cells, as well as how BTN3A and BTN2A1 operate outside of pAg signaling, is poorly understood. In contexts where Vγ9Vδ2 T cells or BTNs play immunosuppressive roles, targeting BTN2A1 with antagonistic antibodies may sensitize tumors to immunotherapy.
Methods
Protein expression and purification
Fabs
ɑ-BTN2A1 Fabs with a human-IgG1 Herceptin scaffold were cloned into the RH2.2 vector. Fab heavy and light chains were on the same plasmid. Fab overexpression was induced in Escherichia coli BL21 Gold (DE3) cells in 2xYT media using 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) for 4 h at 37 °C. Bacterial cells were centrifuged, resuspended and lysed by homogenization and sonication. Bacterial lysate was spun at 20,000 × g for 45’. Fabs were purified from the periplasmic fraction with Protein Ga1 or G-F resin (Kossiakoff Lab, U.Chicago), eluted with 0.1 M Glycine pH 2.3 and neutralized with a 1:5 volume ratio of 1 M Tris Buffer pH 8.0. Fabs were then dialyzed 1:1E5 into sterile phosphate buffered saline (PBS).
ɑ-BTN3A1 Fab 20.1 and 103.2 heavy and light chains with a hybrid murine-IgG1 Fab IgV and human-IgG1 Herceptin Fab IgC scaffold were cloned into the pAcGP67a Vector containing a C-terminal human rhinovirus 3C protease cleavage site, acid- and basic-zipper, respectively, and hexa-histidine tag. Baculovirus was generated by transfection of plasmid and linearized baculovirus DNA into Sf9 insect cells using Cellfectin transfection reagent. Baculovirus was then added to High-Five insect cells and proteins were expressed for 60–68 h at 27 °C. Supernatant was isolated by centrifugation at 1700 rpm for 15’ and filtered through glass-fibre. Proteins were then incubated with 3C protease overnight in HBS + 75 mM Imidazole for the removal of the acid–base zipper and hexa-histidine tag. Proteins were purified using Protein-G resin, eluted with 0.1 M Glycine pH 2.3 and neutralized with a 1:5 volume ratio of 1 M Tris Buffer pH 8.0. Fabs were then dialyzed 1:1E5 into sterile PBS.
mAbs
ɑ-BTN2A1 mAb sequences with a hybrid human-IgG1 Herceptin Fab IgV and murine-IgG1 IgC-Hinge-Fc scaffold were cloned into the AbVec vector. Heavy and light chains were on separate plasmids. ɑ-BTN3A1 mAbs with a murine-IgG1 scaffold were cloned into the AbVec vector. MAbs were expressed in Expi293 cells at 37 °C using the ExpiFectamine™ transfection system with 0.5 μg each of heavy and light chain plasmids per 1 mL of culture. 7 days after transfection, supernatant was isolated by centrifugation at 3000 × g for 10’. mAbs were then purified using Protein G or Protein Ga1 resin, eluted with 0.1 M Glycine pH 2.3 and neutralized with a 1:5 volume ratio of 1 M Tris Buffer pH 8.0. MAbs were then dialyzed 1:1E5 into sterile PBS.
The murine IgG1 and human Herceptin Fab domains utilized in ɑ-BTN2A1 and ɑ-BTN3A1 constructs lack N-linked glycosylation sites allowing comparison of Fab and mAb protein produced with 2 different expression systems.
BTN2A1 ectodomain, BTN2A1 ectodomain C219S
BTN2A1 ectodomain (residues 1–219) and BTN2A1 ectodomain C219S (residues 1–219) were cloned into the pAcGP67a Vector containing a C-terminal human rhinovirus 3C protease cleavage site and hexa-histidine tag. Proteins were expressed in High-Five insect cells with the baculovirus expression system as previously described. Proteins were purified using Ni–NTA resin and eluted with Hanks Buffered Saline (HBS) + 200 mM and 500 mM imidazole. Proteins were de-glycosylated for 2 h at 37 °C using Endo-F3 in HBS + 15 mM imidazole at a concentration of ~ 1 mg/mL. Proteins were repurified using Ni–NTA resin and HBS + 200 mM imidazole elution to remove Endo-F3 and incubated with 3C protease overnight in HBS + 75 mM imidazole for the removal of the hexa-histidine tag. Proteins were purified further using anion-exchange chromatography over the MonoQ column in 20 mM Tris pH 8.0 with a 20 mL gradient of 30–700 mM NaCl. Proteins were subsequently utilized for structural determination.
Biotinylated BTN2A1 ectodomain + avitag and BTN3A1 ectodomain + avitag
BTN2A1 ectodomain (residues 1–217) and BTN3A1 ectodomain (residues 1–217) were cloned into the pAcGP67a Vector containing a C-terminal BirA biotinylation sequence, human rhinovirus 3C protease cleavage site and hexa-histidine tag. Proteins were expressed in High-Five insect cells with the baculovirus expression system as previously described. Proteins were purified using Ni–NTA resin as previously described. Proteins were then incubated with 3C protease overnight in HBS + 75 mM Imidazole for the removal of the hexa-histidine tag. Proteins were buffer-exchanged to HBS + 15 mM Imidazole with 0.05 M bicine buffer pH 8.3, 10 mM ATP, 10 mM MgOAc and 50 μM d-biotin and biotinylated overnight using the BirA protein. Proteins were then purified using size-exclusion chromatography (SEC) over the S200 column in HBS and verified for biotinylation by size-shift on an SDS-PAGE gel following coincubation with Traptavidin.
G115 and DP 10.7 TCRs
The γ chain of the G115 or DP 10.7 gamma constant-region TCR was cloned into the pAcGP67a Vector containing a C-terminal human rhinovirus 3C protease cleavage site, acidic-zipper and hexa-histidine tag. The δ chain of the G115 or DP 10.7 delta constant-region TCR was cloned into the pAcGP67a Vector containing a C-terminal BirA biotinylation sequence, human rhinovirus 3C protease cleavage site, basic-zipper and hexa-histidine tag. TCRs were expressed in High-Five insect cells with the baculovirus expression system as previously described. TCRs were purified using Ni–NTA resin and eluted as previously described. TCRs were then incubated with 3C protease overnight in HBS and 75 mM Imidazole for the removal of the hexa-histidine tag. For TCR being used for tetramerization, TCR was buffer exchanged to HBS + 15 mM Imidazole HBS + 15 mM Imidazole with 0.05 M bicine buffer pH 8.3, 10 mM ATP, 10 mM MgOAc and 50 μM d-biotin and biotinylated overnight using the BirA protein. Unbiotinylated and biotinylated TCR were then purified using SEC over the S200 column in HBS and verified for biotinylation by size-shift on an SDS-PAGE gel following coincubation with Traptavidin, if applicable. Biotinylated TCR was tetramerized by co-incubation with Streptavidin-PE at a ratio of 1:1.1 streptavidin monomer to biotinylated TCR monomer.
Nanobody
Histidine-tagged anti-Fab nanobody37 was expressed in E. coli BL21 (DE3) cells overnight at 20 °C post induction with 1 mM IPTG at OD600 = 0.6–0.8. The cells were harvested by sonication in 25 mM TRIS, pH 8.0, 300 mM NaCl and 10% glycerol. After centrifugation, the supernatant was passed over a 10 mL HisTrap HP column and eluted with 25 mM TRIS, pH 8.0, 300 mM NaCl, 150 mM imidazole and 10% glycerol. The protein was further purified by SEC in 20 mM HEPES, pH 7.4, and 200 mM NaCl.
Phage display selection
To obtain high-affinity binders, five rounds of selection were performed using the phage display selection protocol previously described35,43. Briefly, Biotinylated BTN2A1 was immobilized onto streptavidin-coated paramagnetic beads for five rounds of phage selection. In the first round, 1 μM of BTN2A1 was immobilized on 200 μl SA magnetic beads and was incubated with 1 mL phage library (1010 CFU) for 1 h at room temperature with gentle shaking. The beads were washed three times to remove nonspecific phage, added to log phase E. coli XL-1 blue cells and incubated for 20 min at room temperature. Then, media containing 100 μg/mL ampicillin and 10 9 p.f.u./mL of M13K07 helper phage was added for overnight phage amplification at 37 °C. The amplified phage was precipitated in 20% PEG/2.5 M NaCl for 20 min on ice for subsequent rounds. Before each round, the phage pool was negatively selected against empty paramagnetic beads for 30‘ with shaking to eliminate nonspecific binders. The final antigen concentration was dropped systematically from 1 μM to 10 nM from the first to the fifth round (2nd round: 200 nM, third round: 50 nM, fourth round 20 nM, and fifth-round 10 nM). After phage binding, the beads were subjected to five washing rounds with 0.5% BSA/PBST. The bound phages were eluted using 0.1 M Glycine, pH 2.6, and neutralized with TRIS–HCl, pH 8. Then, the phage eluate was used for E. coli infection and phage amplification, as described above. Additional selection pressure using 1 μM of not biotinylated BTN3A1 in all washes was applied to ensure specificity to BTN2A1. After the fourth and fifth rounds the infected cells were plated on ampicillin agar and 192 colonies were picked to produce phage clones for single-point phage ELISA assay. The promising clones demonstrating high specificity were sequenced and reformatted into a RH2.2 expressing vector.
Affinity analyses
Cellular affinity
For determination of ɑ-BTN2A1 mAb cellular affinity, Daudi cells were resuspended in PBS + 2% FBS and incubated with increasing concentrations of ɑ-BTN2A1 mAb. ɑ-BTN2A1 mAbs were stained with 1:200 fluorescently-tagged secondary mAb and staining was assessed by flow-cytometry.
Enzyme-linked immunosorbent assays (ELISA)
Single-point phage ELISA was used to test specificity of ɑ-BTN2A1 Fabs for BTN2A1 binding. 50 nM of BTN2A1 or BTN3A1 protein was directly immobilized on high-binding experimental wells for 30’, followed by extensive blocking with 2% BSA for 1 h. After 15’ of incubation with phage, the wells were extensively washed three times with 0.5% BSA/PBST and incubated with Protein L-HRP (1:5000 dilution in HBST) for 20’. The plates were again washed and developed with TMB substrate and quenched with 10% H3PO4, followed by the absorbance at A450 determination.
BLI
For 20.1 and 103.2 engineered variants relative affinity determination, biotinylated BTN3A1 ectodomain in HBS was immobilized on Streptavidin biosensor tips until Binding reached between 3 and 4 nm. Tips were blocked with 1 μM biotin for 30″. After baseline was established in PBS for 30″, the biosensor tip was exposed to 1.3 μM mAb for 90″. Dissociation in PBS was then assessed for 120”.
For ɑ-BTN2A1 Fab competition with the Vγ9Vδ2 TCR experiments, biotinylated BTN2A1 ectodomain in HBS was immobilized for 150″ on Streptavidin biosensor tips. Tips were blocked with 1 μM biotin in HBS for 30 s followed by 2 mg/mL BSA for 180″. The biosensor tip was exposed to 66 μM ɑ-BTN2A1 Fab concentration for 120″. Tips were immediately placed into 66 μM ɑ-BTN2A1 Fab + 80 μM Vγ9Vδ2 TCR for 120″, in HBS. Dissociation in HBS was then assessed for 120”.
Surface plasmon resonance analysis
All Surface Plasmon Resonance (SPR) analyses were performed on a MASS-1. BTN2A1 was immobilized via a 6 × His-tag to a Ni–NTA sensor chip. Fabs in two-fold dilutions were run as analytes at 30 μl/min flow rate at 20 °C. Sensograms were corrected through double referencing, and a 1:1 binding model was fit using Sierra Analyzer.
Cell-lines
The γ and δ chain of the G115 Vγ9Vδ2 TCR clone were cloned into the pMSC-V vector with puromycin and zeocin selection genes, respectively. Jurkat JRT3.5 cells were transfected with these plasmids and selected first for γ chain expression for 7–14 days using 1 mg/mL puromycin. Cells were then selected for ~ 4 weeks with 200 mg/mL zeocin. Cells were stained with ɑ-γ9 and ɑ-δ2 antibodies and sorted on TCR expression at both 1 and 4 weeks. Daudi-Cas9 ΔAAVS1, ΔBTN2A1, ΔBTN3A1 cell lines were generated as described33. Daudi-Cas9 ΔAAVS1 were generated with a CRISPR guide RNA targeting AAVS1, a safe harbor site that acted as a CRISPR cutting control. JRT3.5 Jurkat cells expressing the G115 TCR, DaudiΔAAVS1, DaudiΔ2A1, and DaudiΔ3A1 were cultured in RPMI-1640 supplemented with 10% Fetal Bovine Serum (FBS), 2 mM L-glutamine and 10 U/mL Penicillin/Streptomycin (R10). Cells were split to 0.3E6 cells/mL daily and split 24 h prior to the start of an activation assay.
Isolation and expansion of Vγ9Vδ2 T cells from peripheral blood
Peripheral blood was isolated from healthy donors through the IRB13-0666 protocol, in accordance with all guidelines and regulations, and after informed consent was obtained from all participants. Blood was subjected to density gradient centrifugation using Ficoll. Lymphocytes were isolated and brought to 1E6 cells/mL in RPMI-1640 supplemented with 10% FBS, 2 mM L-glutamine, 0.1% β-me, 0.5X non-essential amino acids (Corning), 1 mM sodium pyruvate and 10 U/mL Penicillin/Streptomycin (R10 +). Cells were pulsed with 5 μM Zoledronate and 100 U/mL IL-2 and incubated at 37 °C. 100 U/mL IL-2 was supplemented into the media every 2–3 days and the culture volume was doubled on day 6 with fresh R10 + media supplemented with 100 U/mL IL-2. One day prior to use in activation assays, 100 U/mL IL-2 was pulsed into the media. Vγ9Vδ2 T cells were utilized on Day 9–10 for activation assays and assayed via flow-cytometry for expansion at such time.
Agonism/antagonism of Vγ9Vδ2 T cells
In 96-well round-bottom plates with edge-wells containing 200 μL PBS, 0.05E6 Daudi target cells were incubated with PBS, mAb, or Fab + /− HMBPP, Pamidronate or MOPC isotype mAb in R10 for 2 h in 100 μL. Target cells were washed with 200 μL warm PBS and centrifuged at 300 × g for 5′ 3 X and co-incubated with 0.1E6 Jurkat or Primary Vγ9Vδ2 T cells per condition overnight in 200 μL R10 or R10 + , respectively. 1.6 μg/mL PHA was added to wells with T cells alone prior to overnight incubation as a positive control for activation. T Cell activation was assessed via flow-cytometry.
Cellular mAb:TCR competition for BTN2A1 binding assay
Full-length BTN2A1 was cloned into the pAcGP67a Vector containing a C-terminal human rhinovirus 3C protease cleavage site and hexa-histidine tag. BTN2A1 was expressed in High-Five insect cells with the baculovirus expression system as previously described. BTN2A1-expressing Insect cells were seeded at 1.5E5 cells/well in 96-well round-bottom plates with edge-wells containing 200 μL PBS and incubated with 150 nM ɑ-BTN2A1 mAb for 30’. Insect cells were washed with 200 μL PBS and centrifuged at 300 × g for 5’. Insect cells were then sequentially stained with DAPI followed by additional washes and stained with 120 nM G115 tetramer labeled with the PE-fluorophore. MAb:tetramer competition was assessed via flow-cytometry.
Flow cytometry
For Jurkat T cell activation assays, target and T cells from each condition were resuspended in PBS + 2% FBS. Cells were stained with Live-Dead fluorescent dye, ɑ-CD19 to gate target-cells, ɑ-Vγ9 to gate T cells and ɑ-CD69 fluorophore-conjugated mAbs as a marker for T cell activation. For Primary T cell activation assays, target and T cells from each condition were stained with 1:1500 dilution Live/dead Fixable Near-IR Dead Cell Stain, 1:50 dilution of ɑ-Vγ9, ɑ-Vδ2 to gate T cells, and 1:50 dilution of ɑ-CD25, ɑ-CD107a and ɑ-CD69 fluorophore-conjugated mAbs as markers for activation.
For mAb and TCR-tetramer competition, cells were resuspended in PBS + 2% FBS and stained with DAPI and fluorescently-labeled G115 TCR tetramers. Data were collected on either the Aurora (Cytek) or AttuneNxt (ThermoFisher Scientific).
X-ray crystallography
Sample preparation
De-glycosylated and purified BTN2A1 ectodomain C219S was mixed with purified Fab at a 1:1 ratio. Complexes were concentrated and purified over SEC using the S200 column in HBS. Complexes were then concentrated to 6.9 mg/mL and tested for crystallization using the Morpheus I protein crystallization screen (Molecular Dimensions) in 3-well sitting drop plates. Crystallization conditions from well G9 were optimized to 0.1 M Buffer System 3, pH 8.3 with 0.1 M Carboxylic Acids and 20% Precipitant mix 1. Optimized crystals were cryoprotected with 20% Ethylene Glycol in 0.1 M Buffer System 3, pH 8.3 with 0.1 M Carboxylic Acids and 25% Precipitant mix 1 and flash-frozen in Liquid Nitrogen.
Data collection and processing
X-ray datasets were collected at the Stanford SSRL beamline 14–1 on a Pilatus 6 M (Dectris) at 100 K. A complete dataset was collected at a wavelength of 1.1950E-10 m. XDS, truncate, pointless, freeR, and aimless were used for data reduction and scaling. An initial molecular replacement solution was obtained using PHASER through Phenix44 with PDB ID code 8DFW30: Chain B and 4RRP45: Chains D,J with CDR loop atoms deleted (D:25–33, 49–61, 90–96. J:28–34, 50–58, 95–100). The initial model was improved using iterative rounds of manual building with coot46 followed by refinement with Phenix44. A second round of phasing was performed using Chains C,D and J from the refined BTN2A1-Fab complex structure and followed by subsequent rounds of refinement.
Cryo-electron microscopy
Sample preparation
Deglycosylated and purified BTN2A1 ectodomain was mixed with purified Fab and Nanobody37 at a 1:1.2:1.44 ratio. Complexes were concentrated and purified over SEC using the S200 column in HBS. Purified complexes were concentrated to ~ 2.3 mg/mL and diluted to 0.85 mg/mL in HBS containing CHAPSO detergent at 0.25 × CMC. Complexes were frozen on grids using the FEI Vitrobot (ThermoFisher Scientific).
Data collection and processing
Cryo-EM data were acquired using a Titan Krios G3i (ThermoFisher Scientific) equipped with a Gatan BioQuantum K3 energy filter and camera at the University of Chicago Advanced Electron Microscopy Core (RRID: SCR_019198). Exposure acquisition settings are given in (Supplementary Table 4).
Data processing steps were carried out using RELION version 5.0-beta247. Details for the processing workflow are given in (Supplementary Figs. 10 and 11). Briefly, exposure movies were motion corrected using the patch-based implementation in RELION, and the contrast transfer function (CTF) for each resulting micrograph estimated using CTFFIND4 version 4.1.1448 based on movie amplitude spectra sums covering a dose of ~ 4.0 e-/Å2. Micrographs with outlier results from these preprocessing steps or with significant non-vitreous ice contamination were removed, resulting in 4073 micrographs used for downstream processing. A random 500 micrograph subset was processed first, from which potential particle positions were picked by Topaz49 using its included general (pre-trained) model. These particles were subject to iterative 2D classification, 3D initial model generation, and 3D classification. The results were used to train a Topaz model that was then used to pick particle locations from the full micrograph set. These particles were similarly subject to iterative 2D and 3D classification, including a final fixed-pose 3D classification step based on results of multi-body refinement of each complex half. This was followed by iterating CTF refinement (per-particle defocus, per-micrograph astigmatism, and per-image shift position beamtilt) and 3D auto-refinement until resolution failed to improve, and two rounds of particle polishing. Finally, symmetry expansion was performed and the results used for multi-body refinement. One of the resulting body maps was used for model building and refinement. Throughout this workflow, 3D auto-refinement steps alternated between using SIDESPLITTER50 and Blush51 for regularization; multi-body refinement steps used Blush regularization. Cryo-EM model building.
A previous crystal structure of the BTN2A1 ectodomain (PDB ID: 8DFW30) and AlphaFold252 predicted models for the Fab heaby and light chains and nanobody were used as starting models. These were refined by iterating between manual changes in Coot46, molecular dynamics manipulation in ISOLDE53, and automated real-space refinement in Phenix44. A version of the map that was sharpened and de-noised by LAFTER54 was used for work done in Coot and ISOLDE; the map output directly from multi-body refinement was used in Phenix44, including for refinement of the final model and its atomic displacement parameters (ADPs)/B-factors.
Data analysis
General
Flow-Jo (v10.9.0) was utilized to analyze flow-cytometry data. All results from flow-cytometry, and affinity-determination experiments were plotted and statistically analyzed in GraphPad Prism. Adobe Illustrator was used to design figures.
Analysis of protein complex structures
CCP4-Contact was used to determine van der Waals interactions with a distance cut-off of 4.001. PDB-ePISA55 was used to find salt-bridge and hydrogen-bond interactions between Fab and BTN2A1.
Statistics
All statistical analyses were performed in Graphpad Prism. All data sets were representative of at least 3 replicates with means displayed as points and standard deviations (SD) displayed as error bars. Data from activation assays involving titration of a reagent were displayed on an XY graph and, in specific cases, analyzed using non-linear regression (variable-slope, 4 parameters). Data from specific concentrations within a titration were assessed for statistical significance using multiple t-tests with the Dunnett’s method. Activation assays involving use of a reagent at a single concentration and the TCR competition insect-cell assay were analyzed using multiple t-tests with the Dunnett’s method. Crystallography statistical analyses were carried out in Phenix.
Antibodies | ||
Anti-huBTN3 (CD277) clone 20.1 | ||
Anti-huBTN3 (CD277) clone 20.15 | This manuscript | |
Anti-huBTN3 (CD277) clone 20.110 | This manuscript | |
Anti-huBTN3 (CD277) clone 20.115 | This manuscript | |
Anti-huBTN3 (CD277) clone 20.130 | This manuscript | |
Anti-huBTN3 (CD277) clone 103.2 | ||
Anti-huBTN3 (CD277) clone 103.25 | This manuscript | |
Anti-huBTN3 (CD277) clone 103.210 | This manuscript | |
Anti-huBTN3 (CD277) clone 103.215 | This manuscript | |
Anti-huBTN3 (CD277) clone 103.230 | This manuscript | |
Anti-huBTN2 clone 2A1.1 | This manuscript | |
Anti-huBTN2 clone 2A1.2 | This manuscript | |
Anti-huBTN2 clone 2A1.3 | This manuscript | |
Anti-huBTN2 clone 2A1.4 | This manuscript | |
Anti-huBTN2 clone 2A1.5 | This manuscript | |
Anti-huBTN2 clone 2A1.6 | This manuscript | |
Anti-huBTN2 clone 2A1.7 | This manuscript | |
Anti-huBTN2 clone 2A1.8 | This manuscript | |
Anti-huBTN2 clone 2A1.9 | This manuscript | |
Anti-huBTN2 clone 2A1.10 | This manuscript | |
Anti-huBTN2 clone 2A1.11 | This manuscript | |
Anti-huBTN2 clone 2A1.12 | This manuscript | |
Anti-huBTN2 clone 2A1.13 | This manuscript | |
Anti-huBTN2 clone 2A1.14 | This manuscript | |
Anti-huBTN2 clone 2A1.15 | This manuscript | |
Anti-IgG1 clone MOPC-21 | Biolegend | Cat#400102 |
Anti-huTCRVγ9 clone B3-APC | Biolegend | Cat#331310 |
Anti-huTCRVδ2 clone-FITC | Biolegend | Cat#331405 |
Anti-huCD19 clone HIB19-BV421 | Biolegend | Cat#302233 |
Anti-huCD69 clone FN50-BV421 | Biolegend | Cat#310930 |
Anti-huCD69 clone FN50-PE | Biolegend | Cat#310906 |
Anti-huCD107a (LAMP-1) clone H4A3-APC | Biolegend | Cat#328620 |
Anti-huCD25 clone BC96-BV711 | Biolegend | Cat#302636 |
Anti-muIgG1k clone RMG1-1-APC | Biolegend | Cat#406609 |
Anti-muIgG1k clone RMG1-1-PE | Biolegend | Cat#406607 |
muIgG1 kappa isotype clone MOPC-21-PE | Biolegend | Cat#400112 |
muIgG1 kappa isotype clone MOPC-21-APC | Biolegend | Cat#400120 |
muIgG1 kappa isotype clone MOPC-21-BV421 | Biolegend | Cat#400157 |
muIgG1 kappa isotype clone MOPC-21-BV711 | Biolegend | Cat#400168 |
human TruStain FcX | Biolegend | Cat#422302 |
Insect, bacterial and virus strains | ||
Sf9 cells in Sf-900 II SFM | Thermo scientific | Cat#11496015 |
High-five cells in express five medium | Thermo scientific | Cat#B85502 |
DH5α | Thermo scientific | Cat#18265017 |
BL21 (DE3) | New England biolabs | Cat#C2527H |
XL-1 E. coli | Agilent/stratagene | Cat#200249 |
M13K07 helper phage | New England biolabs | N0315S |
Chemicals, peptides and recombinant proteins | ||
Live/dead fixable near-IR dead cell stain kit | Thermo scientific | Cat#L34975 |
DAPI | Fisher scientific | Cat#EN62248 |
HMBPP | Sigma aldrich | Cat#95098 |
Critical commercial assays | ||
Expifectamine transfection kit | Thermo scientific | Cat#A14524 |
Experimental models: cell lines | ||
Daudi-Cas9ΔAAVS1 (DaudiΔAAVS1) | Mamedov et al.33 | Guide 12 |
Daudi-Cas9Δ2A1 (DaudiΔ2A1) | Mamedov et al.33 | N/A |
Daudi-Cas9Δ3A1 (DaudiΔ3A1) | Mamedov et al.33 | N/A |
Jurkat JRT3.5—G115 TCR | Gu et al.22 | N/A |
Primary PBMCs | This manuscript | N/A |
Software, algorithms, databases | ||
Flow-Jo | FlowJo LLC | v10.9.0 |
GraphPad Prism | GraphPad Inc | v10.0.3 |
Coot | v0.9.8.92 | |
Phenix | ||
Adobe Illustrator | Adobe | v28.0 |
Snapgene | Dotmatics | v7.1.b |
Microsoft Suite | Microsoft Corp | v16.8 |
Endnote | Clarivate | v21.2 |
UCSF ChimeraX | Goddard et al.56 | v1.7 |
Relion | Zivanov et al.47 | v4.0.1 |
XQuartz | Apple Inc | v2.8.0 |
PDB | RCSB57 | N/A |
SBGrid | Morin et al.58 | v2.9.7 |
Phenix | Liebschner et al.44 | v1.21rc1-5015 |
CCP4 | Agirre et al.59 | |
PDB-ePISA | Krissinel et al.55 | v1.52 |
Data availability
The datasets generated and/or analyzed during the current study are available in the RCSB Protein Data Bank (RCSB PDB) repository, under entry numbers 8VC7 and 9DPE and are available from the corresponding author on request.
References
Pauza, C. D. et al. Gamma delta T cell therapy for cancer: It is good to be local. Front. Immunol. https://doi.org/10.3389/fimmu.2018.01305 (2018).
Hoeres, T. et al. Improving the efficiency of Vγ9Vδ2 T-cell immunotherapy in cancer. Front. Immunol. https://doi.org/10.3389/fimmu.2018.00800 (2018).
Constant, P. et al. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science 264 (5156), 267–270 (1994).
Ravens, S. et al. Microbial exposure drives polyclonal expansion of innate γδ T cells immediately after birth. Proc. Natl. Acad. Sci. USA 117 (31), 18649–18660 (2020).
Fichtner, A. S. et al. TCR repertoire analysis reveals phosphoantigen-induced polyclonal proliferation of Vγ9Vδ2 T cells in neonates and adults. J. Leukoc. Biol. 107 (6), 1023–1032 (2020).
Papadopoulou, M. et al. TCR sequencing reveals the distinct development of fetal and adult human Vγ9Vδ2 T cells. J. Immunol. 203 (6), 1468–1479 (2019).
Perera, M. K. et al. Transient increase in circulating gamma/delta T cells during Plasmodium vivax malarial paroxysms. J. Exp. Med. 179 (1), 311–315 (1994).
Jouen-Beades, F. et al. In vivo and in vitro activation and expansion of gammadelta T cells during Listeria monocytogenes infection in humans. Infect. Immun. 65 (10), 4267–4272 (1997).
Raziuddin, S. et al. Gamma delta T cells and the immune response in visceral leishmaniasis. Eur. J. Immunol. 22 (5), 1143–1148 (1992).
Sumida, T. et al. Predominant expansion of V gamma 9/V delta 2 T cells in a tularemia patient. Infect. Immun. 60 (6), 2554–2558 (1992).
Gober, H. J. et al. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 197 (2), 163–168 (2003).
Liu, Y. & Zhang, C. The role of human gammadelta T cells in anti-tumor immunity and their potential for cancer immunotherapy. Cells 9 (5), 1206 (2020).
Künkele, K. P. et al. Vγ9Vδ2 T cells: Can we re-purpose a potent anti-infection mechanism for cancer therapy?. Cells 9, 829 (2020).
Chen, S. et al. Prognostic and therapeutic Significance of BTN3A proteins in tumors. J. Cancer 12 (15), 4505–4512 (2021).
Tanaka, Y. et al. Natural and synthetic non-peptide antigens recognized by human γδ T cells, in nature. Nature 375, 155–158 (1995).
Correa, I. et al. Most γδ T cells develop normally in β2-microglobulin-deficient mice. Proc. Natl. Acad. Sci. USA 89 (2), 653–657 (1992).
Morita, C. T. et al. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human γδ T cells. Immunity 3 (4), 495–507 (1995).
Harly, C. et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood 120 (11), 2269–2279 (2012).
Rigau, M. et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science 367, 6478 (2020).
Karunakaran, M. M. et al. Butyrophilin-2A1 directly binds germline-encoded regions of the Vg9Vd2 TCR and is essential for phosphoantigen sensing. Immunity 52, 487-498.e6 (2020).
Sandstrom, A. et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2T Cells. Immunity 40 (4), 490–500 (2014).
Gu, S. et al. Phosphoantigen-induced conformational change of butyrophilin 3A1 (BTN3A1) and its implication on Vγ9Vδ2 T cell activation. Proc. Natl. Acad. Sci. USA 114 (35), E7311–E7320 (2017).
Karunakaran, M. M. et al. A distinct topology of BTN3A IgV and B302 domains controlled by juxtamembrane regions favors optimal human gammadelta T cell phosphoantigen sensing. Nat. Commun. 14 (1), 7617 (2023).
Zhang, M. et al. Cryo-EM structural insights into Vγ9Vδ2 TCRactivationvia2 multiple butyrophilins. Biorxiv.org https://doi.org/10.1101/2024.10.02.616253 (2024).
Hsiao, C. H. C. et al. Ligand-induced interactions between butyrophilin 2A1 and 3A1 internal domains in the HMBPP receptor complex. Cell Chem. Biol. 29 (6), 985-995.e5 (2022).
Yuan, L. et al. Phosphoantigens glue butyrophilin 3A1 and 2A1 to activate Vγ9Vδ2 T cells. Nature 621 (7980), 840–848 (2023).
Wang, H. et al. Critical roles for coiled-coil dimers of butyrophilin 3A1 in the sensing of prenyl pyrophosphates by human Vgamma2Vdelta2 T cells. J. Immunol. 203 (3), 607–626 (2019).
Peigné, C.-M. et al. The juxtamembrane domain of butyrophilin BTN3A1 controls phosphoantigen-mediated activation of human Vγ9Vδ2 T cells. J. Immunol. 198 (11), 4228–4234 (2017).
Willcox, C. R. et al. Phosphoantigen sensing combines TCR-dependent recognition of the BTN3A IgV domain and germline interaction with BTN2A1. Cell Rep. 42 (4), 112321 (2023).
Fulford, T. S. et al. Vγ9Vδ2 T cells recognize butyrophilin 2A1 and 3A1 heteromers. Nat. Immunol. 25, 1355–1366 (2024).
Lai, A. Y. et al. Cutting edge: Bispecific gammadelta T cell engager containing heterodimeric BTN2A1 and BTN3A1 promotes targeted activation of Vgamma9Vdelta2(+) T cells in the presence of costimulation by CD28 or NKG2D. J. Immunol. 209 (8), 1475–1480 (2022).
Palakodeti, A. et al. The molecular basis for modulation of human Vγ9Vδ2 T cell responses by CD277/butyrophilin-3 (BTN3A)-specific antibodies. J. Biol. Chem. 287 (39), 32780–32790 (2012).
Mamedov, M. R. et al. CRISPR screens decode cancer cell pathways that trigger γδ T cell detection. Nature 621 (7977), 188–195 (2023).
Davodeau, F. et al. Close correlation between Daudi and mycobacterial antigen recognition by human gamma delta T cells and expression of V9JPC1 gamma/V2DJC delta-encoded T cell receptors. J. Immunol. 151 (3), 1214–1223 (1993).
Paduch, M. et al. Generating conformation-specific synthetic antibodies to trap proteins in selected functional states. Methods 60 (1), 3–14 (2013).
Fellouse, F. A. et al. High-throughput generation of synthetic antibodies from highly functional minimalist phage-displayed libraries. J. Mol. Biol. 373 (4), 924–940 (2007).
Ereno-Orbea, J. et al. Structural basis of enhanced crystallizability induced by a molecular chaperone for antibody antigen-binding fragments. J. Mol. Biol. 430 (3), 322–336 (2018).
Cano, C. E. et al. BTN2A1, an immune checkpoint targeting Vγ9Vδ2 T cell cytotoxicity against malignant cells. Cell Rep. 36 (2), 109359 (2021).
Tyler, C. J. et al. Human Vgamma9/Vdelta2 T cells: Innate adaptors of the immune system. Cell Immunol. 296 (1), 10–21 (2015).
Cubillos-Ruiz, J. R. et al. CD277 is a negative co-stimulatory molecule universally expressed by ovarian cancer microenvironmental cells. Oncotarget 1 (5), 329–338 (2010).
Payne, K. K. et al. BTN3A1 governs antitumor responses by coordinating ab and gd T cells. Science 369 (6506), 942–949 (2020).
Billon, E. et al. Soluble BTN2A1 is a potential prognosis biomarker in pre-treated advanced renal cell carcinoma. Front. Immunol. 12, 670827 (2021).
Slezak, T. & Kossiakoff, A. A. Engineered ultra-high affinity synthetic antibodies for SARS-CoV-2 neutralization and detection. J. Mol. Biol. 433 (10), 166956 (2021).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. Sect. D, Struct. Biol. 75, 861–877 (2019).
Kuzin, A., et al. Crystal Structure of the Fab complexed with antigen Asf1p, Northeast Structural Genomics Consortium (NESG) Target PdR16. (2014).
Emsley, P. et al. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66 (Pt 4), 486–501 (2010).
Zivanov, J. et al. A Bayesian approach to single-particle electron cryo-tomography in RELION-40. Elife 11, e83724 (2022).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192 (2), 216–221 (2015).
Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16 (11), 1153–1160 (2019).
Ramlaul, K. et al. Mitigating local over-fitting during single particle reconstruction with SIDESPLITTER. J. Struct. Biol. 211 (2), 107545 (2020).
Kimanius, D. et al. Data-driven regularization lowers the size barrier of cryo-EM structure determination. Nat. Methods 21 (7), 1216–1221 (2024).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596 (7873), 583–589 (2021).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D Struct. Biol. 74 (Pt 6), 519–530 (2018).
Ramlaul, K., Palmer, C. M. & Aylett, C. H. S. A local agreement filtering algorithm for transmission EM reconstructions. J. Struct. Biol. 205 (1), 30–40 (2019).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Goddard, T. D. et al. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Berman, H. M. et al. The protein data bank. Nucleic Acids Res. 28 (1), 235–242 (2000).
Morin, A. et al. Cutting edge: Collaboration gets the most out of software. Elife https://doi.org/10.7554/eLife.01456 (2013).
Agirre, J. A. O. et al. The CCP4 suite: integrative software for macromolecular crystallography. Biol. Crystallogr. 79, 449–461 (2023).
Acknowledgements
We would like to thank the University of Chicago Cytometry and Antibody Technology Core Facility (RRID: SCR_017760) and Dr. James Fuller of the Advanced Electron Microscopy Core Facility (RRID:SCR_019198) for maintaining critical instruments and providing technical expertise during experiment planning, data collection and analysis. We would like to thank Drs. Sumit Bandekar and Melvin Yamsek for input during Cryo-Electron Microscopy Data analysis and Dr. Caitlin Castro for substantial help during manuscript submission and revision. This work was supported by grants to the University of Chicago M.S.T.P by the United States National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) (T32GM007281) and to E.J.A. by the NIH (R01-AI155984). M.R.M. was supported by the Cancer Research Institute (CRI) Irvington Fellowship and the Human Vaccines Project Michelson Prize for Human Immunology.
Author information
Authors and Affiliations
Contributions
Conception of the study: E.J.A., A.R., Design of experiments: A.R., S.R. Acquisition of data: A.R., T.S., J.F., H.G., M.R.M. Analysis of data: A.R., S.R., J.F. Interpretation of data: A.R., S.R., E.J.A., A.K., A.M. First draft of the manuscript: A.R. Substantial revisions: A.R., S.R., C.C., E.J.A.
Corresponding author
Ethics declarations
Competing interests
A.M. is a co-founder of Arsenal Biosciences, Function Bio, Spotlight Therapeutics, and Survey Genomics, serves on the boards of directors at Function Bio, Spotlight Therapeutics and Survey Genomics, is a member of the scientific advisory boards of Arsenal Biosciences, Function Bio, Spotlight Therapeutics, Survey Genomics, NewLimit, Amgen, Tenaya, and Lightcast owns stock in Arsenal Biosciences, Function Bio, Spotlight Therapeutics, NewLimit, Survey Genomics, Tenaya, and Lightcast and has received fees from Arsenal Biosciences, Spotlight Therapeutics, NewLimit, Amgen, 23andMe, PACT Pharma, Juno Therapeutics, Tenaya, Survey Genomics, Lightcast, Gilead, Trizell, Vertex, Merck, Genentech, AlphaSights, Rupert Case Management, Bernstein, GLG, ClearView Healthcare Partners, and ALDA. A.M. is an investor in and informal advisor to Offline Ventures and a client of EPIQ. The Marson laboratory has received research support from Juno Therapeutics, Epinomics, Sanofi, GlaxoSmithKline, Gilead, and Anthem and reagents from Illumina and Genscript. M.M. is an advisor to Laguna Bio. All of the remaining authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ramesh, A., Roy, S., Slezak, T. et al. Mapping the extracellular molecular architecture of the pAg-signaling complex with α-Butyrophilin antibodies. Sci Rep 15, 12162 (2025). https://doi.org/10.1038/s41598-025-94347-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94347-w