Abstract
Severe fever with thrombocytopenia syndrome (SFTS) is a fatal tick-borne infectious disease that lacks effective treatments. Dynamic analysis that reflects changes in the SFTS patient’s condition is needed. This study aimed to evaluate the time-dependent predictive performance of key biomarkers using a time-dependent Cox regression model. A retrospective multicenter cohort study was conducted on 440 SFTS patients hospitalized in South Korea between 2013 and 2024. Time-dependent Cox regression and time-dependent receiver operating characteristic (ROC) analyses were applied to assess the prognostic value of Blood Urea Nitrogen (BUN), Prothrombin Time (PT), and Activated Partial Thromboplastin Time (aPTT). Missing data were handled using multiple imputation. aPTT consistently demonstrated high predictive accuracy (AUC > 0.90) throughout the disease course, indicating its sustained role in coagulopathy. PT exhibited strong early-stage predictive power (AUC = 0.86 on day 2) but declined over time, reflecting its utility for early monitoring. BUN showed a progressive increase in predictive performance (AUC = 0.70 on day 2 to AUC = 0.78 on day 8), supporting its relevance in later stages of disease progression. Non-survivors exhibited significantly higher levels of BUN, PT, and aPTT compared to survivors. This study demonstrates the utility of time-dependent analysis for evaluating dynamic biomarker changes in SFTS patients. aPTT is a robust predictor throughout the disease course, while PT is valuable for early-stage assessment and BUN for later-stage management. These findings suggest the importance of integrating dynamic biomarker monitoring into clinical decision-making to improve prognosis in SFTS patients.
Similar content being viewed by others
Introduction
Severe Fever with Thrombocytopenia Syndrome (SFTS) is an acute viral disease caused by the SFTS virus, primarily transmitted through tick bites, and is a zoonotic infection. Since its first report in China in 2009, SFTS has emerged as a severe viral disease characterized by high incidence and mortality, particularly in East Asia (China, Japan, Korea, and Vietnam)1,2,3,4. There is currently no available vaccine or antiviral treatment, and the disease progresses rapidly, with a mortality rate of approximately 20%, which is higher than that observed for other infectious diseases5. Early detection and prompt treatment are crucial. Therefore, there is an urgent need to develop clinical prediction models that can accurately assess the prognosis of SFTS and optimize patient management.
Time-dependent covariates frequently arise in biomedical research evaluating patient status. For instance, a patient’s serum creatinine level, which reflects renal function, may fluctuate during hospitalization. In contrast, time-fixed covariates are variables whose values remain unchanged throughout the study period.
Previous studies have identified demographic factors, comorbidities, and initial clinical status as significant factors influencing the prognosis of SFTS6,7,8,9. These studies mainly focus on assessing the association between early biomarker values, measured on the day of hospital admission, and survival risk, using the Cox proportional hazards model (Cox model), which is based on time-fixed covariates. Although the Cox model is useful for assessing risk at a specific point in time, it has the limitation of not reflecting the patient’s dynamically changing condition10,11.
In order to make informed decisions regarding the treatment and management of patients in actual clinical settings, more advanced analyses that dynamically reflect changes in a patient’s condition are needed12. In particular, for acute infectious diseases such as SFTS, clinical outcomes in the initial state can change rapidly over time, so predicting mortality based on this is highly likely to result in unreliable results.
Recently, considerable research has been conducted on time-dependent covariate models that overcome the limitations of existing time-fixed covariate-based models and effectively reflect the dynamic characteristics of data13,14,15. Unlike models that reflect only a specific point in time, time-dependent covariate models integrate longitudinal data and include it in the analysis, allowing the dynamic characteristics of the data to be reflected in the analysis. By dynamically analyzing the impact of changes in clinical status at a specific point in time on death, more accurate predictions and interpretations can be achieved compared to single-point analysis.
Therefore, this study aimed to analyze the factors influencing in-hospital mortality in SFTS patients using a time-dependent covariate model. The objective of this study is to identify dynamically changing risk factors, accurately assess the prognosis of SFTS patients, and provide a foundation for supporting clinical decision-making.
Methods
Study design and patient selection
This retrospective, multicenter cohort study was conducted nationwide in South Korea, utilizing data from ten tertiary referral hospitals with infectious diseases specialists. Patients with a confirmed diagnosis of SFTS who were hospitalized and treated between May 2013 and October 2024 were included. SFTS virus infection was confirmed by detecting viral RNA in patient serum during the acute phase of illness using real-time polymerase chain reaction (RT-PCR) or conventional reverse transcription PCR. Patients who were not admitted to the ward-namely, those evaluated only in the outpatient clinics or who left the hospital against medical advice-were excluded. Additionally, patients with no laboratory tests performed within one day of hospital admission were excluded, as baseline laboratory results were unavailable for the admission day or the following day.
The study protocol was approved by the Institutional Review Boards (IRBs) of each participating hospital, and the list of IRB approvals and numbers is provided in Supplementary Table 1. Due to the retrospective nature of the study and the use of anonymized patient data, the requirement for written informed consent was waived.
Data collection
Demographic, clinical, and laboratory data were collected from the electronic medical records (EMRs) of each hospital. Laboratory data comprised test results obtained throughout the hospital stay. Key hematological and biochemical markers such as white blood cell (WBC) count, platelet count, aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine, lactate dehydrogenase (LDH), albumin, C-reactive protein (CRP), prothrombin time (PT), and activated partial thromboplastin time (aPTT) were included. The primary outcome variable for this study was in-hospital mortality, defined as death occurring during the hospital stay.
Statistical analysis
All statistical analyses were performed using R software version 4.4.2 (R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were expressed as mean ± standard deviation or median (interquartile range) based on the data distribution, and categorical variables were presented as frequencies and percentages.
To assess the dynamic effect of biomarkers on mortality, a time-dependent Cox regression model was applied. The dataset for time-dependent Cox regression analysis was created using the tmerge function from the survival package. Each laboratory test result was categorized into time intervals based on the test date and converted into time-dependent covariates.
To handle missing laboratory test data, multiple imputation was performed using the mice (Multivariate Imputation by Chained Equations) package in R. Twenty imputed datasets were generated using predictive mean matching with 20 iterations. Time-dependent Cox regression was conducted on each of the 20 imputed datasets using the coxph function from the survivalpackage11, and the results were pooled using Rubin’s rules to obtain final estimates, standard errors, and confidence intervals (CIs).
Univariate Cox regression was used to evaluate the association between each laboratory test, defined as a time-dependent covariate, and mortality. Variables with p ≤ 0.05 were included in the multivariate Cox regression model.
Multivariate Cox regression with stepwise selection was then applied, and hazard ratios (HRs) with 95% CIs were calculated. During the analysis, violations of the proportional hazards assumption were evaluated using Schoenfeld residuals, and key covariates were selected through multivariate analysis. The final selected covariates were included in a Cox regression model to evaluate their independent impact on mortality. Model fit and prediction performance were further verified through time-dependent receiver operating characteristic (ROC) analysis. The cumulative/dynamic time-dependent ROC analysis was performed using the timeROC package, and the area under the curve (AUC) was calculated to evaluate the prediction accuracy of each variable. The optimal cutoff value was derived based on the Youden index, and the results were organized in graphs and tables.
Results
Patients characteristics
A total of 440 patients diagnosed with SFTS were included in this study. The demographic and clinical characteristics of the patients are summarized in Table 1. The mean age of the patients was 66.94 ± 13.20 years, and 48.2% were male. Seventy-six patients (17.3%) died from SFTS, as recorded in the EMRs. The mean age of deceased patients was 73.28 ± 8.89 years, which was significantly higher than that of survivors (65.62 ± 13.57 years, p < 0.001). There was no significant difference in gender distribution between survivors and non-survivors (p = 0.778).
Laboratory results at admission showed significant differences between survivors and non-survivors. The platelet count in non-survivors was 60.83 × 10³/µL, significantly lower than that in survivors (75.82 × 10³/µL, p = 0.003). Non-survivors also exhibited significantly higher creatinine levels (1.29 vs. 1.01, p = 0.001) and lower albumin levels (3.27 g/dL vs. 3.63 g/dL, p < 0.001) compared to survivors. Furthermore, BUN levels were elevated in non-survivors (28.49 vs. 19.80, p < 0.001). Inflammatory and organ dysfunction markers, such as AST (560.94 IU/L vs. 236.84 IU/L, p < 0.001), ALT (155.85 IU/L vs. 102.75 IU/L, p = 0.003), and LDH (1873.88 IU/L vs. 884.82 IU/L, p < 0.001), were significantly higher in non-survivors. Coagulation-related markers, including PT (13.43 vs. 12.08, p < 0.001) and aPTT (57.77 s vs. 42.13 s, p < 0.001), were also significantly prolonged in non-survivors.
Independent risk factors for mortality in SFTS patients
In the univariate Cox regression analysis with time-dependent covariates, several variables were significantly associated with increased in-hospital mortality (Table 2). Key variables included elevated WBC count (HR 1.181, 95% CI 1.115–1.251, p < 0.001), elevated neutrophil count (HR 1.066, 95% CI 1.036–1.098, p < 0.001), and reduced platelet count (HR 0.987, 95% CI 0.978–0.996, p = 0.006). Among biochemical markers, elevated potassium levels (HR 2.454, 95% CI 1.867–3.325, p < 0.001), elevated BUN levels (HR 1.042, 95% CI 1.033–1.050, p < 0.001), prolonged PT (HR 1.160, 95% CI 1.095–1.230, p < 0.001), and prolonged aPTT (HR 1.036, 95% CI 1.029–1.042, p < 0.001) emerged as predictive variables for mortality.
The results of the multivariate Cox regression analysis with time-dependent covariates, based on variables selected in the univariate analysis, are summarized in Table 2. Independent predictors for mortality were identified as neutrophil count (HR 1.063, 95% CI 1.017–1.112, p = 0.007), LDH (HR 1.000, 95% CI 1.000–1.000, p < 0.001), total bilirubin (HR 1.311, 95% CI 1.111–1.546, p = 0.002), albumin (HR 0.469, 95% CI 0.263–0.835, p = 0.011), BUN (HR 1.031, 95% CI 1.023–1.040, p < 0.001), PT (HR 1.146, 95% CI 1.080–1.215, p < 0.001), and aPTT (HR 1.014, 95% CI 1.004–1.025, p = 0.008).
Time-Dependent ROC analysis and predictive performance
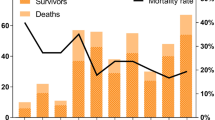
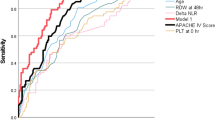
Time-dependent ROC analysis was conducted to evaluate the prognostic accuracy of mortality predictors. A time-dependent ROC curve was generated to assess prediction performance over time, with AUC values calculated at each time point from day 2 to day 8 (Fig. 1 (a, c, e)). The analysis identified BUN, PT, and aPTT as key variables predicting in-hospital mortality among SFTS patients. In particular, aPTT consistently demonstrated the highest prediction accuracy, indicating its reliability in evaluating SFTS prognosis. Although PT exhibited high predictive accuracy in the early stages, its performance decreased over time, suggesting its utility is limited to early-stage monitoring. Conversely, BUN demonstrated relatively stable performance with a gradual increase in predictive power over time, indicating its reliability as a predictive variable throughout the disease course.
Moreover, the dynamic profiles of the three key mortality predictors (BUN, PT, and aPTT) identified through multivariate Cox regression and time-dependent ROC analysis were illustrated in Fig. 1 (b, d, f). The graphs depict the fluctuations in predictor levels over the course of SFTS progression. In survivors, BUN remained within the normal range, but in non-survivors, it gradually increased over time. Both PT and aPTT exhibited consistently higher levels in non-survivors. Notably, aPTT was prolonged beyond the normal range from the early stage, consistently maintaining a distinct separation between survivors and non-survivors throughout the disease course, supporting its robust predictive performance at all time points.
Discussion
SFTS is a viral disease characterized by high mortality and rapid progression, particularly among vulnerable populations such as the elderly and immunocompromised individuals2,3,4,9,16. Most existing studies on mortality prediction in SFTS have focused on evaluating prognosis and analyzing relevant factors based on clinical outcomes measured at the time of hospital admission. Although this approach is useful for assessing a patient’s condition at a specific point in time, it fails to reflect the dynamic nature of acute infectious diseases such as SFTS, where clinical outcomes change rapidly over time.
To address these limitations, this study applied a time-dependent Cox regression model to dynamically analyze the impact of biomarker changes on mortality risk throughout hospitalization. The conventional Cox model assumes that covariates remain constant over time, limiting its ability to measure biomarker fluctuations during disease progression. In contrast, the time-dependent Cox model integrates time-varying covariates, making it more suitable for analyzing the evolving nature of acute conditions such as SFTS. Given the rapid progression of SFTS, the rate and pattern of biomarker changes are critical determinants of prognosis, necessitating an analytical method that reflects these temporal variations. By using time-dependent covariates, this study effectively measured biomarker dynamics and provided more accurate risk predictions. Thus, the time-dependent Cox model serves as an appropriate statistical method for prognostic analysis in SFTS and offers a robust basis for investigating the time-dependent characteristics of acute infectious diseases. This study utilized a time-dependent Cox regression approach to analyze all laboratory data collected during hospitalization from a cohort of 440 SFTS patients across 10 hospitals in South Korea. The analysis revealed that coagulation markers (PT and aPTT) and renal function markers (BUN) played a significant role in predicting in-hospital mortality risk.
In this study, aPTT, a key marker of the intrinsic coagulation pathway, maintained consistently high AUC values throughout the study period., indicating that coagulation dysfunction related to the intrinsic pathway plays a sustained role in mortality risk. The intrinsic pathway is critical in regulating long-term coagulation and thrombus formation, particularly following the activation of the extrinsic pathway during the early phase of infection17. The persistent predictive value of aPTT in our study emphasizes its potential as an important biomarker for assessing overall coagulopathy in SFTS patients. Similarly, Ye Wang et al. reported that aPTT and other coagulation-related markers were significantly prolonged in SFTS patients, identifying aPTT as an independent risk factor for SFTS-related mortality18. Several other studies have also shown that hemorrhagic manifestations were more frequent in deceased SFTS patients and that aPTT was significantly prolonged in the early stages of the disease19,20,21. Moreover, aPTT levels have been positively correlated with SFTSV viral load, suggesting a mechanistic link between viral pathogenesis and coagulation dysfunction22. Mechanistically, SFTSV has been found to cause vascular endothelial damage and activate the intrinsic coagulation system, ultimately leading to prolonged aPTT23. These findings are consistent with the results of the present study, indicating that prolonged aPTT is a critical risk factor for poor prognosis in SFTS patients.
PT, another marker reflecting the extrinsic coagulation pathway, was also identified as a risk factor for poor prognosis in SFTS patients. These findings are consistent with previous studies that have identified PT as an important predictor of mortality in SFTS patients24,25, further supporting the role of coagulation abnormalities in SFTS mortality. However, most previous studies have focused on PT values measured at the time of hospital admission, providing only a static assessment of coagulation function. The present study analyzed the temporal changes in PT throughout hospitalization, providing a more comprehensive perspective on its prognostic value. Our results showed that the AUC value of PT decreased over time, suggesting that PT is particularly useful as a prognostic marker during the initial phase of SFTS, reflecting the significant role of extrinsic coagulation dysfunction in the early pathophysiology of the disease.
BUN is a widely used serum biomarker for assessing kidney function due to its ease of measurement and clinical applicability. Renal impairment is commonly observed in the early stages of various infectious diseases, and previous studies have reported that BUN levels are significantly elevated in severe cases of SFTS and COVID-19, indicating its potential as a prognostic marker6,26,27,28. In this study, BUN exhibited a progressive increase in AUC values over time, suggesting that kidney dysfunction becomes increasingly relevant as the disease progresses. This finding indicates the increasing impact of renal impairment on SFTS prognosis in later stages and proposes the need for continuous monitoring of kidney function in critically ill patients29,30,31.
These results suggest that the management strategy for SFTS patients should be adjusted according to the disease progression. In the early stages, it is critical to rapidly assess and correct coagulation dysfunction by monitoring aPTT and PT, whereas in the later stages, continuous monitoring and management of kidney function markers, such as BUN, are essential to improving prognosis.
Although this stepwise approach is an important sign that the patient’s condition is progressing to severity, it cannot be confirmed through fixed covariate analysis. However, the time-dependent covariate model effectively evaluates the prognosis of SFTS patients by reflecting changes in clinical outcomes over time (and mortality). It is anticipated that such an approach can contribute to reducing in-hospital mortality risk. In particular, it emphasizes the need to flexibly adjust management and treatment strategies as the patient’s condition evolves. Continuous monitoring of the patient’s condition and timely implementation of appropriate interventions through dynamic clinical outcome analysis are essential for improving the survival rate of SFTS patients.
The clinical decision support system (CDSS), which is integrated with EMR systems, is essential for the practical implementation of time-dependent analyses in clinical settings. Currently, most healthcare institutions rely on clinicians to manually review patient data and make decisions accordingly; however, real-time risk prediction could be achieved through automated data collection and analysis using CDSS. CDSS embedded with the time-dependent algorithm could enhance clinical utility by providing automated alerts when specific biomarker fluctuations reach critical thresholds.
Limitations
This study has several limitations. First, missing laboratory data were inevitable due to the retrospective design and variability in clinical practice across institutions. To address this limitation, multiple imputations were employed; however, imputation may introduce bias depending on the underlying data distribution. Second, as a retrospective multicenter observational analysis, it inherently limits the ability to measure the impact of interventions on biomarker dynamics and patient prognosis. In particular, patients with greater disease severity were more likely to receive intensive treatment, introducing potential selection bias that may have influenced the results. Furthermore, treatment strategies varied across institutions, and clinical decisions were made at the discretion of individual physicians, further complicating the interpretation of findings. These factors present challenges in accurately determining causal relationships between treatment interventions and biomarker changes. Third, the generalizability of our findings requires careful consideration. This study was conducted using multicenter data from South Korea, and our findings are consistent with previous studies conducted in China, which identified prognostic biomarkers for SFTS-related mortality. However, due to differences in environmental, genetic, and healthcare system factors, direct generalization of our results to other endemic regions, such as China and Japan, remains challenging. To improve generalizability, external validation using independent datasets is necessary, and further multinational studies are required to confirm these findings across diverse populations.
Conclusions
In summary, our study demonstrates that using a time-dependent Cox model may significantly improve the prognostic evaluation of SFTS by measuring dynamic changes in key biomarkers. Notably, coagulation markers such as PT and aPTT, along with renal function indicator BUN, provide robust predictive power throughout the disease course. These findings support the integration of dynamic biomarker monitoring into clinical decision-making to facilitate timely interventions. Future prospective studies are needed to further validate these results and enhance risk stratification in the treatment of SFTS.
Data availability
The data sets generated and analyzed in the study are available from the corresponding author upon reasonable request.
Abbreviations
- SFTS:
-
Severe fever with thrombocytopenia syndrome
- AST:
-
Aspartate aminotransferase
- ALT:
-
alanine aminotransferase
- LDH:
-
lactate dehydrogenase
- BUN:
-
blood urea nitrogen
- CRP:
-
C-reactive protein
- CK-MB:
-
creatine phosphokinase MB fraction
- PT:
-
prothrombin time
- aPTT:
-
activated partial thromboplastin time
- eGFR:
-
estimated glomerular filtration rate
References
Yu, X. J. et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl. J. Med. 364, 1523–1532. https://doi.org/10.1056/NEJMoa1010095 (2011).
Kim, K. H. et al. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg. Infect. Dis. 19, 1892–1894. https://doi.org/10.3201/eid1911.130792 (2013).
Takahashi, T. et al. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J. Infect. Dis. 209, 816–827. https://doi.org/10.1093/infdis/jit603 (2014).
Tran, X. C. et al. Endemic severe fever with thrombocytopenia syndrome, Vietnam. Emerg. Infect. Dis. 25, 1029–1031. https://doi.org/10.3201/eid2505.181463 (2019).
Brault, A. C., Savage, H. M., Duggal, N. K., Eisen, R. J. & Staples, J. E. Heartland Virus Epidemiology, Vector Association, and Disease Potential. Viruses 10 (2018). https://doi.org/10.3390/v10090498
Xu, X. et al. Analysis of clinical features and early warning indicators of death from severe fever with thrombocytopenia syndrome. Int. J. Infect. Dis. 73, 43–48. https://doi.org/10.1016/j.ijid.2018.05.013 (2018).
Wang, X. et al. The predictive effect of the platelet-to-lymphocyte ratio (PLR) and the neutrophil-to-lymphocyte ratio (NLR) on the risk of death in patients with severe fever with thrombocytopenia syndrome (SFTS): a multi-center study in China. Ann. Transl Med. 9, 208. https://doi.org/10.21037/atm-20-4736 (2021).
Liu, Z. et al. High levels of C-reactive protein-to-albumin ratio (CAR) are associated with a poor prognosis in patients with severe fever with thrombocytopenia syndrome in early stage. J. Med. Virol. 94, 5375–5384. https://doi.org/10.1002/jmv.27972 (2022).
Liu, Q., He, B., Huang, S. Y., Wei, F. & Zhu, X. Q. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect. Dis. 14, 763–772. https://doi.org/10.1016/S1473-3099(14)70718-2 (2014).
Fisher, L. D. & Lin, D. Y. Time-dependent covariates in the Cox proportional-hazards regression model. Annu. Rev. Public. Health. 20, 145–157. https://doi.org/10.1146/annurev.publhealth.20.1.145 (1999).
Therneau, T., Crowson, C. & Atkinson, E. Using time dependent covariates and time dependent coefficients in the Cox model. Survival Vignettes, 1–25 (2017).
Blanche, P., Aurélien, Latouche & Viallon, V. Time-dependent AUC with right-censored data: a survey. Risk Assess. Evaluation Predictions, 239–251 (2013).
Wang, G. et al. Structured learning in time-dependent Cox models. Stat. Med. 43, 3164–3183. https://doi.org/10.1002/sim.10116 (2024).
Zeng, L., Zhang, J., Chen, W., Ding, Y. & tdCoxSNN Time-dependent Cox survival neural network for continuous-time dynamic prediction. J. R Stat. Soc. Ser. C Appl. Stat. 74, 187–203. https://doi.org/10.1093/jrsssc/qlae051 (2025).
Sun, Z. & Cao, H. Regression analysis of multiplicative hazards model with time-dependent coefficient for sparse longitudinal covariates. ArXiv Preprint arXiv. 2310, 15877 (2023).
Lin, T. L. et al. The first discovery of severe fever with thrombocytopenia syndrome virus in Taiwan. Emerg. Microbes Infect. 9, 148–151. https://doi.org/10.1080/22221751.2019.1710436 (2020).
Lyu, C. J. Introduction to coagulation system. J. Korean Soc. Neonatology, 1–5 (2011).
Wang, Y. et al. Blood Urea nitrogen to albumin ratio is a novel predictor of fatal outcome for patients with severe fever with thrombocytopenia syndrome. J. Med. Virol. 96, e29731. https://doi.org/10.1002/jmv.29731 (2024).
Song, L., Zhao, Y., Wang, G., Huang, D. & Sai, L. Analysis of risk factors associated with fatal outcome among severe fever with thrombocytopenia syndrome patients from 2015 to 2019 in Shandong, China. Eur. J. Clin. Microbiol. Infect. Dis. 41, 1415–1420. https://doi.org/10.1007/s10096-022-04506-4 (2022).
Kim, M. et al. The evaluation of surrogate laboratory parameters for predicting the trend of viral loads in patients with severe fever with thrombocytopenia syndrome: Cross-Correlation analysis of time series. Infect. Chemother. 54, 470–482. https://doi.org/10.3947/ic.2022.0073 (2022).
He, F., Zheng, X. & Zhang, Z. Clinical features of severe fever with thrombocytopenia syndrome and analysis of risk factors for mortality. BMC Infect. Dis. 21, 1253. https://doi.org/10.1186/s12879-021-06946-3 (2021).
Zhang, Y. Z. et al. Hemorrhagic fever caused by a novel bunyavirus in China: pathogenesis and correlates of fatal outcome. Clin. Infect. Dis. 54, 527–533. https://doi.org/10.1093/cid/cir804 (2012).
Wang, Y. et al. Clinical laboratory parameters and fatality of severe fever with thrombocytopenia syndrome patients: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 16, e0010489. https://doi.org/10.1371/journal.pntd.0010489 (2022).
Zhong, F. et al. Establishment and validation of a clinical risk scoring model to predict fatal risk in SFTS hospitalized patients. BMC Infect. Dis. 24, 975. https://doi.org/10.1186/s12879-024-09898-6 (2024).
Xiao, W. et al. Development and validation of a clinical and laboratory-based nomogram to predict mortality in patients with severe fever with thrombocytopenia syndrome. BMC Infect. Dis. 24, 1206. https://doi.org/10.1186/s12879-024-10106-8 (2024).
Gai, Z. T. et al. Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patients. J. Infect. Dis. 206, 1095–1102. https://doi.org/10.1093/infdis/jis472 (2012).
Liu, Y. M. et al. Kidney Function Indicators Predict Adverse Outcomes of COVID-19. Med 2, 38–48 e32 (2021). https://doi.org/10.1016/j.medj.2020.09.001
Li, H. et al. Epidemiological and clinical features of laboratory-diagnosed severe fever with thrombocytopenia syndrome in China, 2011-17: a prospective observational study. Lancet Infect. Dis. 18, 1127–1137. https://doi.org/10.1016/S1473-3099(18)30293-7 (2018).
Chen, N. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395, 507–513. https://doi.org/10.1016/S0140-6736(20)30211-7 (2020).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3 (2020).
Yang, X. et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 8, 475–481. https://doi.org/10.1016/S2213-2600(20)30079-5 (2020).
Acknowledgements
This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant number: HI22C0797).
Author information
Authors and Affiliations
Contributions
HJW, TKK, JHH, and MGK conceived and designed the study. STH, JRY, MK, JO, IGB, SB, YRY, JHH, MH, HAK, SIJ, KTK, SH, UJK, GK, YJK, JHY, TEK, HJW and MGK collected and organized the data. HJW and MGK analyzed the data, prepared the figures and tables, and wrote the manuscript.All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the institutional review board of Chonnam National University Hospital (IRB# CNUH-2023-213), Chonnam National University Hwasun Hospital (IRB# CNUH-2024-150), Gyeongsang National University Hospital (IRB# GNUH 2024-08-020), Jeju National University Hospital (IRB# JEJUNUH 2024-08-002), Jeonbuk National University Hospital (IRB# CUH 2022-01-067), Keimyung University Dongsan Hospital (IRB# DSMC 2024-08-008), Kyungpook National University Chilgok Hospital (IRB# DGIRB 2022-05-004), Kyungpook National University Hospital (IRB# DGIRB 2023-07-001), Wonkwang University Hospital (IRB# WKUH 2024-08-005) and Konkuk University Medical Center (IRB# KUMC 2024-09-001). The data was kept confidential; no one except the research team had access to the files.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Woo, H.J., Heo, S.T., Yoo, J.R. et al. Dynamic biomarkers and Cox regression with time-dependent covariate for mortality prediction in severe fever with thrombocytopenia syndrome. Sci Rep 15, 9293 (2025). https://doi.org/10.1038/s41598-025-94416-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94416-0
Keywords
This article is cited by
-
Procalcitonin/albumin to urea nitrogen ratio: a novel prognostic indicator for severe fever with thrombocytopenia syndrome
BMC Infectious Diseases (2026)