Abstract
This study examined the prevalence, risk factors, and prognosis of epilepsy in patients with primary central nervous system lymphoma (PCNSL) and explored the necessity of prophylactic antiepileptic treatment in this population. In this retrospective, observational, single-center study, we analyzed clinical data from PCNSL patients who were diagnosed at our institution between January 2018 and April 2023. The cohort comprised 214 patients with PCNSL (with a median age of 62 years), of which 128 (47.6%) patients were male. Epilepsy was observed in 16.82% (36/214) of the patients, with 9.35% (20/214) presenting with seizures as the initial symptom. Cortical involvement was significantly associated with seizures (OR = 9.512, 95% CI 3.870–23.381; p = 0.036). Other potential risk factors included an edema zone > 1 cm around the lesion and PIM1 wild-type status. Antiepileptic drugs (AEDs) neither reduced seizure incidence (8.11% with AEDs vs. 8.43% with no AEDs, p = 0.970) nor improved PFS (HR = 0.613, 95% CI 0.338–1.109; p = 0.106). Therefore, AEDs should not be used as preventive measures in this population.
Similar content being viewed by others
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare but highly aggressive subtype of extranodal non-Hodgkin’s lymphoma, with an annual incidence of 0.4 per 100,000 individuals in various populations1. PCNSL accounts for approximately 4% of all primary CNS tumors and 4–6% of extranodal lymphomas, whereby it most commonly presents as diffuse large B-cell lymphomas2. Additionally, it typically manifests as a space-occupying lesion within the brain parenchyma but can also involve the eyes, leptomeninges, spinal cord, and cerebrospinal fluid. Currently, a high-dose methotrexate (HD-MTX)-based regimen is the first-line treatment for eligible patients with PCNSL3,4,5. Prompt treatment can result in a 5-year overall survival rate of approximately 30%, thus underscoring the critical need for timely diagnosis and intervention6. Despite the availability of therapeutic options, 15–25% of patients exhibit resistance to chemotherapy, whereas 25–50% experience relapse following an initial response7.
Epilepsy, a brain network disorder8, is a prevalent comorbidity among patients with brain tumors9. The diagnosis of epilepsy, which is a complex condition without a universally accessible gold standard, primarily relies on comprehensive patient histories and accurate eyewitness accounts. Treatment strategies for PCNSL, including intensified chemotherapy and autologous stem cell transplantation, can lead to neurotoxic effects, acute encephalopathy, and seizures. These complications not only adversely affect quality of life but also exacerbate the economic and psychosocial burdens on patients and their families10,11. Seizures occur in up to 33% of PCNSL patients; however, detailed knowledge regarding the specific characteristics of epilepsy, its risk factors, its impact on prognosis, and the effectiveness of antiepileptic drug (AED) use remains limited12. Neurosurgeons often prescribe long-term oral AEDs for intracranial space-occupying lesions, with levetiracetam being the most commonly used13. Nevertheless, the efficacy of this practice has not been validated in the literature14.
Lumbar puncture is routinely performed in PCNSL patients, whereby it demonstrates high concordance between mutations in cerebrospinal fluid (CSF) circulating tumor DNA (ctDNA) and those in tumor biopsy tissue15. Consequently, CSF ctDNA serves as an easily accessible biomarker for tumor mutations. In gliomas, seizure-inducing factors have been identified, including IDH mutations and their byproduct known as d-2-hydroxyglutarate16,17, as well as BRAF V600E mutations, especially in gangliogliomas18,19. The molecular subtypes of PCNSL, which are characterized by unique oncogenic pathways, transcriptomic and epigenetic signatures, tumor locations, and prognostic implications, may influence seizure occurrence20. However, whether gene mutations in PCNSL are associated with secondary epilepsy remains unclear.
Previous studies on epilepsy secondary to PCNSL were based on small sample sizes and lacked complete follow-up data. Most information on PCNSL-related epilepsy was obtained from brief descriptions within broader PCNSL studies, which provided minimal insight into changes in seizure incidence throughout the disease course. Detailed data on seizure frequency, the proportion of treatment-resistant patients, and the evolution of seizures during remission remain scarce. Additionally, there is no established evidence regarding the use of AEDs prophylaxis or the efficacy of specific AEDs in PCNSL management. As a result, several unknown factors remain to be investigated, including the identification of the risk factors for seizures in PCNSL patients, how epilepsy influences the prognosis of PCNSL patients, the justification of the need for the prophylactic use of antiepileptic drugs (AEDs) in PCNSL patients, and if cerebrospinal fluid (CSF) ctDNA can be utilized to elucidate the relationship between gene mutations and PCNSL-related epilepsy. This research sought to address these unknown aspects of seizures related to PCNSL.
Methods
Patients
This retrospective, observational cohort study analyzed the clinical data of 214 immunocompetent patients who were newly diagnosed with PCNSL and who were admitted to Huashan Hospital, Fudan University, between January 2018 and April 2023. The study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The inclusion criteria were as follows: (1) a PCNSL diagnosis confirmed via histopathology, vitreous biopsy, or cerebrospinal fluid cytology/flow cytometry7; (2) a diagnosis made between January 2018 and April 2023; (3) lymphoma confined to the central nervous system, as confirmed via imaging examinations; (4) no history of seizures prior to PCNSL diagnosis, except for initial epileptic events; (5) an age older than 18 years; and (6) no previous use of chemotherapy or radiotherapy. The exclusion criteria were as follows: (1) patients with primary epilepsy; (2) individuals with a history of traumatic brain injury, psychiatric disorders, systemic diseases or genetic diseases, as well as a family history of epilepsy; (3) follow-up duration of less than three months; and (4) positive HIV status. A workflow diagram detailing the patient selection process is shown in Fig. 1. This research has been performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Huashan Hospital, Fudan University (Approval number: 2022-1009).
Data collection and evaluation
Data collection, which was completed on January 23, 2024, included comprehensive assessments for PCNSL, such as ophthalmologic evaluations; brain MRI with contrast enhancement; whole-body ultrasound; chest, abdominal, and pelvic contrast-enhanced computed tomography (CT) scans; positron emission tomography-computed tomography (PET-CT) scans; bone marrow biopsies; serum and urine protein electrophoresis measurements; routine blood tests; biochemical analyses; lactate dehydrogenase (LDH) and β2 microglobulin levels; and other hematological parameters. The diagnosis of epilepsy was based on a combination of clinical symptoms and signs evaluated by neurologists, with some eligible patients also undergoing electroencephalogram (EEG) examinations. EEG abnormalities were defined as the presence of θ waves, δ waves, β waves, or spike waves in the EEG recording. The collected data included age, sex, clinical status, ECOG performance score at diagnosis, surgical approach (e.g., puncture or excision), Ki67 index, GCB classification, symptomatology, lesion localization, PET-CT findings, evidence of intraocular lymphoma, cerebrospinal fluid analysis, biochemical tests, tumor burden indicators, seizure occurrences, use of antiepileptic drugs (AEDs), EEG results, treatment plans, and treatment outcomes.
Cerebrospinal fluid ctDNA sequencing
This study involved 33 individuals with CSF samples that were donated to the biobank of the Department of Hematology, Huashan Hospital, Fudan University, and these samples were used for next-generation sequencing. Written informed consent was obtained from all patients before their donation. A 233-gene panel was employed for hybridization-capture sequencing on the Illumina next-generation sequencing (NGS) platform; this panel targeted exon regions of genes that are closely associated with PCNSL and known for high mutation frequencies. CSF samples were extracted via a cfDNA extraction kit (Nanodigbio 417622-10), whereas PBMC samples were processed via a gDNA extraction kit (TIANamp Blood DNA Kit). The sequencing data were aligned to the human reference genome GRCh37/ hg19, and cfDNA sequencing analysis was conducted according to the xGen Prism DNA Library Prep Kit analysis guide.
Statistical analysis
Demographic and clinical variables were categorized according to the presence or absence of seizures. Comparative analysis of potential seizure risk factors was performed via t tests for normally distributed continuous variables, Mann–Whitney U tests for nonnormally distributed continuous variables, and chi-square tests or Fisher’s exact tests for categorical variables. Following univariate analysis, factors with p values < 0.05 were included in a binary logistic regression model to identify independent predictors. Survival outcomes were evaluated via Cox regression analysis, and hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated, with variables demonstrating p values < 0.05 being subsequently included in the multivariate Cox regression analysis. Statistical analyses were performed via SPSS version 26 (IBM, Armonk, NY, USA), with p values < 0.05 considered to be statistically significant.
Results
Patient characteristics
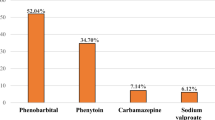
This study included 214 PCNSL patients who met the inclusion criteria. The median age at diagnosis was 62 years (range: 19–81 years). The cohort comprised 128 males (59.81%) and 86 females (40.19%). The median follow-up duration was 18.3 months (range: 0.6–71.8 months), during which 74 patients (34.58%) died. Among the patients, 124 received antiepileptic drugs, 76 were administered sodium valproate, 53 were prescribed levetiracetam, and 5 were treated with both types of drugs. The clinical characteristics are detailed in Table 1.
Epilepsy profile
Epilepsy was diagnosed in 36 of the 214 patients (16.82%). The mean follow-up duration was 1.9 years, with the incidence of first seizures in PCNSL estimated at 89.96 per 1000 person-years. At onset, 20 patients (55.56% of the epilepsy subgroup, or 9.35% of the total study population) experienced epileptic seizures. Seizures occurred during or after treatment in 16 patients (44.44% of the epilepsy subgroup). Among these patients, 4 experienced seizures during remission, whereas 12 experienced seizures when treatment was ineffective. Specifically, 3 patients experienced seizures during disease progression, 5 during stable disease (SD), and 4 at the time of relapse. For those patients who developed seizures after diagnosis, the median interval to the first seizure was 3.7 months.
Among the patients with epilepsy, 7 (19.44%) experienced persistent seizures, whereas 29 (80.56%) experienced seizures lasting from seconds to hours in duration. According to the International League Against Epilepsy (ILAE) classification21, 13 patients were classified as having generalized onset seizures (36.11%), 20 as having focal onset seizures (55.56%), and 3 as having focal-to-bilateral tonic–clonic onset seizures (8.33%). The most common seizure type was focal aware motor clonic seizures (11 out of 36 patients, 30.56%), as shown in Fig. S1.
Risk factors for epilepsy
Univariate analysis
Logistic regression was used to evaluate the relationships among demographic data, patient health status at diagnosis, pathology findings, surgical interventions, lactate dehydrogenase (LDH) levels, lesion characteristics, treatment outcomes, and epileptic seizures. Intracranial lesion resection or puncture, postoperative prophylactic drug administration, and methotrexate (MTX) response were not associated with seizure symptoms at disease onset. Consequently, these three variables were further analyzed in the No Seizures group and the Epilepsy After Diagnosis group. Comparative analysis of patients with and without epilepsy demonstrated several significant risk factors: age younger than 60 years (p = 0.007), an edema zone exceeding 1 cm around the lesion (p = 0.012), cortical lesion involvement (p < 0.001), bilateral frontal lobe involvement (p = 0.019), and the absence of PIM1 gene mutation (p = 0.020). However, factors such as EEG anomalies (p = 0.149) and antiepileptic drug use (p = 0.970) were not significantly correlated with seizure occurrence (Table 2). Additionally, DLBCL-GCB subtype, ECOG score, eye involvement, elevated CSF pressure (CSF pressure > 110mmH2O), positive CSF cytology, elevated LDH levels, multifocal lesions, deep lesions (located more than 3 cm from the brain surface), bulky disease (lesion diameter greater than 3 cm), and lesion resection were not associated with epilepsy outcomes (Supplementary Table S1 online).
Multivariate analysis
Variables with a p value less than 0.05 in the univariate analysis were included in the multivariate analysis. Cortical involvement was observed to significantly increase the risk of seizures in PCNSL patients (OR = 9.512, 95% CI = 3.870–23.381; p = 0.036). Additionally, there was a trend toward epilepsy observed in patients with edema zones surrounding the lesion (OR = 0.038, 95% CI = 0.001–1.345; p = 0.072) and in those lacking PIM1 mutations (OR = 0.092, 95% CI = 0.006–1.440; p = 0.089), as shown in Supplementary Table S2 online. Prophylactic use of AEDs did not decrease the incidence of epilepsy in PCNSL patients with high-risk factors, such as cortical lesions (OR = 0.711, 95% CI = 0.248–2.039; p = 0.526).
Analysis of prognostic factors for PCNSL
The progression-free survival (PFS) and overall survival (OS) of PCNSL patients were assessed by using the log-rank test via univariate analysis. As detailed in Table 3, Supplementary Table S3 online, and Fig. 2A–H, several factors, including seizures (p = 0.009), atypical EEG patterns (p = 0.016), high ECOG score (p < 0.001), multiple lesions (p = 0.021), poor MTX response (p < 0.001), prophylactic AED use (p = 0.039), deep lesion localization (p = 0.007), and larger tumor size (p = 0.012), were significantly associated with reduced PFS. For overall survival (OS), factors such as high ECOG score (p < 0.001), elevated LDH level (p = 0.012), and poor MTX response (p < 0.001) were significantly associated with adverse outcomes (Fig. 2I–K). In contrast, sex, age, GCB subtype, and an edema zone > 1 cm were not related to PFS or OS.
Kaplan–Meier survival curve and prognosis analysis. (A) Univariate analysis of seizures for PFS. (B) Univariate analysis of electroencephalography for PFS. (C) Univariate analysis of ECOG score for PFS. (D) Univariate analysis of PFS in patients with multiple lesions. (E) Univariate analysis of response to HD-MTX for PFS. (F) Univariate analysis of AED use for PFS. (G) Univariate analysis of deep lesions for PFS. (H) Univariate analysis of bulky lesions for PFS. Bulky lesions were defined as lesions with diameter ≥ 3 cm. (I) Univariate analysis of ECOG score for OS. (J) Univariate analysis of LDH levels for OS. Elevated: > upper limit of normal [ULN]; normal: ≤ ULN. (K) Univariate analysis of response to HD-MTX for OS.
The multivariate Cox regression analysis for PFS included variables with p values less than 0.05 from the univariate analysis. The results identified a normal EEG (HR = 1.960, 95% CI = 1.017–3.775; p = 0.044) and a favorable MTX response (HR = 0.037, 95% CI = 0.015–0.089; p < 0.001) as independent predictors of improved PFS (Supplementary Table S4 online). In contrast, the multivariate analysis for OS identified a high ECOG score (HR = 2.356, 95% CI = 1.352–4.106; p = 0.002) and ineffective MTX treatment (HR = 0.343, 95% CI = 0.199–0.589; p < 0.001) as independent predictors of poor OS (Supplementary Table S5 online). Neither epilepsy nor AED use had a significant effect on PFS or OS.
Discussion
Our study cohort, which consisted exclusively of HIV-negative individuals, had a seizure prevalence of 16.82% (36/214), with 9.36% (20/214) experiencing seizures at onset, thus resulting in an incidence rate of 89.96 per 1,000 person-years. Most of these seizures were transient and focal. The literature reports seizure onset rates ranging from 7 to 33% among PCNSL patients22,23,24. Fox et al. reported a higher seizure incidence rate of 224.4 per 1,000 person-years in a retrospective cohort study of 36 PCNSL patients. In their study, two-thirds of the patients presented with seizures at diagnosis, whereby they primarily experienced generalized seizures (58.33%), followed by focal seizures with retained consciousness (16.66%)25. Decave et al. reported that 11% (10/101) of immunocompetent PCNSL patients were hospitalized due to seizures26. In a 2016 retrospective study, Langner et al. reported a seizure occurrence rate of 4.5% (8/177) in PCNSL patients at relapse or progression27. Notably, Bayraktar et al. reported that HIV-positive PCNSL patients had a significantly greater risk of seizures compared with their HIV-negative counterparts (38% vs. 11%, respectively; p = 0.004)28, thus underscoring the potential influence of HIV status on seizure risk.
In the context of PCNSL, factors predisposing patients to seizures, especially those factors related to clinical, radiological, and therapeutic aspects, are seldom detailed in the literature. Reports suggest that epileptogenic activities are primarily cortical in nature and occur near the tumor mass29. The literature has identified young age, cortical tumor location, and immune deficiency as potential seizure risk factors in PCNSL12, which is consistent with the findings of glioma research that demonstrated associations between increased seizure risk and cerebral cortex involvement30. Our study confirmed this association, thus demonstrating a significant link between cortical involvement and an increased incidence of seizures. Patients with cortical tumor involvement exhibited a higher seizure rate than did those without such involvement. Additionally, our analysis did not reveal a significant correlation between the histological subtype, Ki-67 proliferation index, or triple-hit status and seizure frequency in PCNSL patients.
Intrinsic epileptogenic potential and genetic changes may influence seizure development in specific tumor subtypes31,32. For example, the interaction between IDH mutations and NMDA receptor glutamate activity is speculated to increase seizure susceptibility in diffuse glioma patients17. Certain genetic alterations in PCNSL may also increase seizure risk. This study is the first to examine the correlation between genetic mutations and seizure episodes in a PCNSL cohort, whereby it identified a potential link between PIM1 mutation status and epilepsy. Previous research has shown that kainic acid-induced seizures upregulate PIM-1 mRNA in the dentate gyrus of the rat hippocampus, thereby suggesting an association between PIM-1 expression and seizure activity33. Further explorations are needed to understand the roles of molecular mutations in PCNSL-related seizures.
In this study, 92 individuals underwent electroencephalogram (EEG) testing during the interictal phase. Although EEG abnormalities do not directly correlate with seizure episodes, they are significantly associated with reduced progression-free survival (PFS). Research has indicated that approximately 44.4% of PCNSL patients with epilepsy exhibit epileptic discharges on EEG, thus highlighting its diagnostic value in epilepsy31. Other studies have shown that a longer duration of epilepsy from the initial seizure to surgery and abnormal interictal EEG results are predictors of unfavorable outcomes in patients with low-grade neuroepithelial tumors (LEATs), with odds ratios (ORs) of 1.01 (p < 0.001) and 5.91 (p = 0.005), respectively34.
The primary goal of AED therapy is to reduce seizure frequency while minimizing side effects. Preventive treatment with AEDs does not significantly reduce seizure frequency within the first six months following a brain tumor diagnosis35. Guidelines from the American Society of Neuro-Oncology (SNO) and the European Association of Neuro-Oncology (EANO) advise against the prophylactic use of AEDs in newly diagnosed brain tumor patients due to insufficient evidence of seizure risk reduction (class 1A evidence)36. In a study by Happold et al., an analysis of four randomized controlled trials involving 1,869 glioblastoma patients revealed that neither valproic acid nor levetiracetam improved progression-free survival (PFS) or overall survival (OS)37. Similarly, a Norwegian national registry study of 1,263 glioblastoma patients indicated no survival benefit from AED use38. The current standard protocol involves the administration of levetiracetam for seven days following craniotomy and supratentorial tumor resection. Compared with valproic acid, levetiracetam has proven to be effective in managing brain tumor-related epilepsy (BTRE) without causing drug interactions or hematologic or neurocognitive side effects, although it does demonstrate a greater incidence of adverse psychiatric effects39. A significant majority of patients with WHO grade 2 glioma-related epilepsy remain seizure-free for two years after starting levetiracetam treatment40. Our findings highlight the fact that AEDs (including levetiracetam and sodium valproate) do not reduce the incidence of epilepsy or improve PFS or OS in PCNSL patients. Therefore, we do not recommend the prophylactic use of AEDs in newly diagnosed PCNSL patients who have not experienced seizures after surgery, even for those patients with high-risk factors for epilepsy, such as cortical tumor involvement. However, for patients who experience seizures, levetiracetam remains the first-line treatment choice, and the decision to withdraw AEDs should be made collaboratively between the physician and the patient after a thorough evaluation41.
Our multifactorial analysis revealed that a favorable response to high-dose methotrexate (HD-MTX) was positively associated with prolonged overall survival (OS) and progression-free survival (PFS) in PCNSL patients. However, the occurrence of seizures did not significantly affect survival outcomes. This finding aligns with a comprehensive review of 21 studies, which reported an unclear association between epilepsy and mortality rates in PCNSL patients12. In contrast, a recent study revealed that patients with epilepsy had a shorter PFS of 9.6 months than non-epileptic patients with a median PFS of 14.1 months (hazard ratio [HR] 1.4, 95% CI 1.0–1.9, p = 0.03). While there was no significant shorter OS rates in patients with PCNSL and epilepsy, with a median OS of 17 months, compared with 44.1 months in the group without epilepsy in univariable analysis ( log-rank test, p = 0.09)24.
The primary limitations of this study include its retrospective, single-center design and the relatively small sample size for cerebrospinal fluid next-generation sequencing. Additionally, we only enrolled immunocompetent patients, and the study lacked video electroencephalogram data, which is valuable for determining the type and prognosis of epilepsy42. Moreover, the relatively short median follow-up duration of 1.5 years may also lead to an underestimation of seizure occurrence, which can manifest in the later stages of the disease.
Despite these limitations, this study provides a valuable, comprehensive evaluation of seizure prevalence, classification, clinical manifestations, genetic variations, risk factors, and their prognostic implications in the context of PCNSL. We confirmed that epilepsy affects a subset of PCNSL patients, with cortical involvement being identified as a significant risk factor for seizures, as well as a broad edema zone surrounding the lesion being identified as a potential determinant of seizure occurrence. Additionally, we explored the role of molecular mutations in PCNSL-related seizures and identified a potential link between PIM-1 expression and seizure activity. Furthermore, seizures did not significantly impact survival outcomes, and the use of AEDs neither reduced the occurrence of epilepsy nor improved survival outcomes.
PCNSL is a rare malignant neoplasm in the brain that is often associated with the development of seizures and can significantly affect patient quality of life. A multidisciplinary team, including hematologists, neurologists, radiologists, and nursing professionals, is crucial for proactively identifying risk factors and collaboratively developing effective prevention and management strategies to improve outcomes for patients with PCNSL-induced epilepsy. Future research could involve prospective, multicenter studies that include both immunocompetent and immunocompromised patients, with more detailed clinical data collection and genetic mutation information. Prophylactic strategies for epilepsy based on preliminary findings could be explored and validated. Additionally, extending the follow-up period and conducting multidimensional and subgroup analyses would deepen the understanding of epilepsy in the context of PCNSL and refine treatment and care approaches, ultimately reducing the incidence of epilepsy in PCNSL patients and improving the quality of life for those with PCNSL-related epilepsy.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Shiels, M. S. et al. Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br. J. Haematol. 174(3), 417–424 (2016).
Dandachi, D. et al. Primary central nervous system lymphoma in patients with and without HIV infection: a multicenter study and comparison with U.S national data. Cancer Causes Control. 30(5), 477–488 (2019).
Ferreri, A. J. et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 3(5), e217–e227 (2016).
Houillier, C. et al. Radiotherapy or autologous stem-cell transplantation for primary CNS lymphoma in patients age 60 years and younger: long-term results of the randomized phase II PRECIS study. J. Clin. Oncol. 40(32), 3692–3698 (2022).
Ferreri, A. J. M. et al. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol. 4(11), e510–e523 (2017).
Jarius, S. et al. Update on the diagnosis and treatment of neuromyelits optica spectrum disorders (NMOSD) - revised recommendations of the Neuromyelitis Optica Study Group (NEMOS). Part I: Diagnosis and differential diagnosis. J. Neurol. 270(7), 3341–3368 (2023).
Ferreri, A. J. M. et al. Primary central nervous system lymphoma. Nat. Rev. Dis. Primers. 9(1), 29 (2023) (Published 2023 Jun 15).
Royer, J. et al. Epilepsy and brain network hubs. Epilepsia. 63(3), 537–550 (2022).
Chen, D. Y., Chen, C. C., Crawford, J. R. & Wang, S. G. Tumor-related epilepsy: epidemiology, pathogenesis and management. J. Neurooncol. 139(1), 13–21 (2018).
Psimaras, D. et al. Complications neurologiques centrales des chimiothérapies cytotoxiques et des thérapies ciblées [Central nervous system complications in patients undergoing cytotoxic chemotherapy and targeted therapies]. Bull Cancer. 99(9), 851–863 (2012).
Schenone, L. et al. Intensive chemotherapy followed by autologous stem cell transplantation in primary central nervous system lymphomas (PCNSLs). Therapeutic outcomes in real life-experience of the French Network. Bone Marrow Transplant. 57(6), 966–974 (2022).
Aboubakr O, et al. Epileptic seizures in patients with primary central nervous system lymphoma: A systematic review. Rev. Neurol. (2023).
van der Meer, P. B. et al. Prescription preferences of antiepileptic drugs in brain tumor patients: An international survey among EANO members [published correction appears in Neurooncol Pract. 2022 Oct 19;10(1):106. 10.1093/nop/npac082]. Neurooncol. Pract. 9(2), 105–113 (2021) (Published 2021 Oct 2).
Youngerman, B. E. et al. Patterns of seizure prophylaxis after oncologic neurosurgery. J. Neurooncol. 146(1), 171–180 (2020).
Mutter, J. A. et al. Circulating tumor DNA profiling for detection, risk stratification, and classification of brain lymphomas. J. Clin. Oncol. 41(9), 1684–1694 (2023).
Easwaran, T. P. et al. Molecular classification of gliomas is associated with seizure control: a retrospective analysis. Neuromolecular Med. 23(2), 315–326 (2021).
Chen, H. et al. Mutant IDH1 and seizures in patients with glioma. Neurology. 88(19), 1805–1813 (2017).
Xing, H., Song, Y., Zhang, Z. & Koch, P. D. Clinical characteristics of BRAF V600E gene mutation in patients of epilepsy-associated brain tumor: A meta-analysis. J. Mol. Neurosci. 71(9), 1815–1824 (2021).
Mortazavi, A. et al. IDH-mutated gliomas promote epileptogenesis through d-2-hydroxyglutarate-dependent mTOR hyperactivation. Neuro Oncol. 24(9), 1423–1435 (2022).
Hernández-Verdin, I. et al. Molecular and clinical diversity in primary central nervous system lymphoma. Ann. Oncol. 34(2), 186–199 (2023).
Fisher, R. S. et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia. 58(4), 531–542 (2017).
Houillier, C. et al. Management and outcome of primary CNS lymphoma in the modern era: An LOC network study. Neurology. 94(10), e1027–e1039 (2020).
Puligundla, C. K. et al. Clinicopathological features and outcomes in primary central nervous system lymphoma: a 10-year experience. Indian J. Med. Paediatr. Oncol. 38(4), 478–482 (2017).
Aboubakr, O. et al. Epilepsy in patients with primary CNS lymphoma: Prevalence, risk factors, and prognostic significance. Neurology. 103(5), e209748 (2024).
Fox, J. et al. Seizures in patents with primary central nervous system lymphoma: Prevalence and associated features. J. Neurol. Sci. 400, 34–38 (2019).
Decavèle, M. et al. Clinical features and outcome of patients with primary central nervous system lymphoma admitted to the intensive care unit: a French national expert center experience. J. Neurol. 268(6), 2141–2150 (2021).
Langner-Lemercier, S. et al. Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. Neuro Oncol. 18(9), 1297–1303 (2016).
Bayraktar, S., Bayraktar, U. D., Ramos, J. C., Stefanovic, A. & Lossos, I. S. Primary CNS lymphoma in HIV positive and negative patients: comparison of clinical characteristics, outcome and prognostic factors. J. Neurooncol. 101(2), 257–265 (2011).
Bourdillon, P. et al. Differential cortical layer engagement during seizure initiation and spread in humans. Nat. Commun. 15(1), 5153 (2024).
Chassoux, F. et al. Dysembryoplastic neuroepithelial tumors: epileptogenicity related to histologic subtypes. Clin. Neurophysiol. 124(6), 1068–1078 (2013).
Barba, C. et al. Intrinsic epileptogenicity of gangliogliomas may be independent from co-occurring focal cortical dysplasia. Epilepsy Res. 97(1–2), 208–213 (2011).
Feldman, J. D. et al. Seizure activity induces PIM-1 expression in brain. J. Neurosci. Res. 53(4), 502–509 (1998).
Xie, M. G. et al. The cognitive functions and seizure outcomes of patients with low-grade epilepsy-associated neuroepithelial tumors. J Neurooncol. 160(1), 1–12 (2022).
Hauff, N. S. & Storstein, A. Seizure management and prophylaxis considerations in patients with brain tumors. Curr. Oncol. Rep. 25(7), 787–792 (2023).
Walbert, T. et al. SNO and EANO practice guideline update: Anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Neuro Oncol. 23(11), 1835–1844 (2021).
Happold, C. et al. Does valproic acid or levetiracetam improve survival in glioblastoma? A pooled analysis of prospective clinical trials in newly diagnosed glioblastoma. J. Clin Oncol. 34(7), 731–739 (2016).
Knudsen-Baas, K. M., Engeland, A., Gilhus, N. E., Storstein, A. M. & Owe, J. F. Does the choice of antiepileptic drug affect survival in glioblastoma patients?. J. Neurooncol. 129(3), 461–469 (2016).
van der Meer, P. B. et al. Management of epilepsy in brain tumor patients. Curr. Opin. Oncol. 34(6), 685–690 (2022).
Fairclough, S., Goodden, J., Chumas, P., Mathew, R. & Maguire, M. Levetiracetam as a first-line antiseizure medication in WHO grade 2 glioma: Time to seizure freedom and rates of treatment failure. Epilepsia. 64(4), 857–865 (2023).
Avila, E. K. et al. Brain tumor-related epilepsy management: A Society for Neuro-oncology (SNO) consensus review on current management. Neuro Oncol. 26(1), 7–24 (2024).
Ge, H. et al. Does epilepsy always indicate worse outcomes? A longitudinal follow-up analysis of 485 glioma patients. World J. Surg. Oncol. 20(1), 297 (2022) (Published 2022 Sep 19).
Asadi-Pooya, A. A., Brigo, F., Lattanzi, S. & Blumcke, I. Adult epilepsy. Lancet. 402(10399), 412–424 (2023).
Funding
This study was part of a research project (SHDC12020112) that was funded by Shanghai Shenkang Clinical Innovation Project, and it was also supported by a research program (HSBY2021007) from Huashan Hospital, Fudan University, Shanghai, China.
Author information
Authors and Affiliations
Contributions
MZ and BC conceived the study. JM and ZL participated in the study design. YL conducted the data collection and analysis and YZ analyzed the magnetic resonance results. QL and HK performed the statistical analyses. MZ draffed the manuscript. YM edited and checked the manuscript. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, M., Ling, Y., Zeng, Y. et al. Antiepileptic drugs failed to prevent initial seizures or improve survival outcomes in patients with primary CNS lymphoma. Sci Rep 15, 10017 (2025). https://doi.org/10.1038/s41598-025-94477-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-94477-1