Abstract
To ascertain whether COVID-19 infection affects sperm quality and measure the scale of the effects. A cross sectional study and a longitudinal study were conducted during the SARS-CoV-2 pandemic (from September 7th 2022 to late January 2023) in China. 604 patients participated in the cross sectional study; 140 in the longitudinal study with 149 unaffected natural controls. The cross sectional study included participants who produce semen sample after COVID-19. The longitudinal study included COVID-19 positive participants who could provide semen samples before and after the infection. In addition, patients unaffected by the infection who could provide two consecutive semen samples over the same period were included as controls for the longitudinal study. Conventional sperm quality parameters including sperm count, motility, morphology and more recent parameters such as sperm DNA fragmentation index (DFI) and sperm chromatin immaturity were observed. In the cross sectional study, the exposure group demonstrated significantly lower total sperm count (159.58 × 106 vs. 185.42 × 106, P = 0.042), lower percentage of grade A sperms (5.37% vs. 8.45%, P = 0.009), lower progressive motility (24.74 ± 14.96% vs. 28.73 ± 16.65%, P = 0.023), lower total motility (32.04 ± 18.03% vs. 36.91 ± 20.86%, P = 0.022), and higher sperm DFI (17.50% vs. 11.75%, P = 0.030) than the controls. In the longitudinal study, after the infection, patients showed lower total sperm count (131.80 × 106 vs. 173.63 × 106, Δd = -20.49 × 106, P = 0.018 ), lower percentage of grade A sperms ( 2.61% vs. 8.50%, Δd = -3.18%, P < 0.001), lower progressive motility (19.82 ± 13.68% vs. 24.88 ± 14.97%, Δd = -5.07 ± 11.94%, P < 0.001) and lower total motility (26.64 ± 17.35% vs. 32.25 ± 18.69%, Δd = -5.62 ± 14.30%, P < 0.001) and higher DFI (32.10 ± 21.30% vs. 26.49 ± 18.54%, Δd = 5.61 ± 13.71%, P < 0.039) than before the infection, while the negative controls showed the opposite changes. Finally, in the longitudinal study, after the infection, 59.29% of the COVID-19 positive patients showed deteriorated sperm concentration, 57.86% deteriorated total sperm count, 71.43% Grade A sperm, 65.00% progressive motility, 69.29% total motility, and 75.00% sperm DFI, while the changes in negative controls were all less than 40% (P < 0.002). COVID-19 was associated with poor sperm quality. The findings would be useful for clinicians to manage men with fertility problems who suffered COVID-19.
Similar content being viewed by others
Introduction
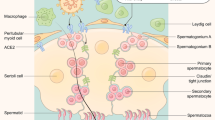
The outbreak of the coronavirus disease 2019 (COVID-19) pandemic has caused tremendous loss in life and damage to the health of mankind. We now understand that SARS-CoV-2 infection adversely affect multiple organs and potentially can affect reproductive tract as well, because studies have shown that both the receptor to SARS-CoV-2, angiotensin-converting enzyme 2 (ACE2) and the transmembrane serine protease 2 (TMPRSS2), the protease needed to cleave the viral S protein, are expressed in the male reproductive tract1. As infertility has become global health issue challenging many countries around the world, effects of SARS-CoV-2 infection or COVID-19 disease on the male reproductive system has been a focus of study for the recent years. However, no consensus has been reached concerning the effects of COVID-19 on sperm quality. Some studies have reported significant adverse impact of COVID-19 infection on semen quality2,3,4,5, and one study has linked semen quality to the severity of COVID-19 infection6. On the other hand, some studies did not find such effects7,8, and one study even reported that COVID-19 infection improved semen quality9. Nevertheless, due to the difficulty in conducting such clinical studies, the existing literature has limitations in that the sample size in the previous studies was usually small, and many study designs lacked controls which could not allow meaningful conclusions to be made. In addition, the number of reported semen parameters were relatively limited. Therefore, the question of whether COVID-19 infection affects sperm quality is still unresolved, and clinical studies with better designs are still required10.
SARS-CoV-2 is known highly contagious. The Chinese government previously adopted strict control measures and effectively prevented it from causing widespread infection in the general population of mainland China. However, due to the fact that large proportion of the population in China had been vaccinated and the virulence of the virus had been decreasing, the Chinese government has adjusted its strategy on the control and prevention of COVID-19 since December 7, 2022. As a result, a large proportion of the population (over 80%) was infected and the number of COVID-19 patients has increased dramatically in the short period of time soon after the strategy adjustment. During that period of time, some patients came to our fertility center to have their semen examined for infertility reasons soon after they recovered from their overt clinical manifestations. This gave us a unique opportunity to intensively observe the effects of COVID-19 on the male sperm quality in a relatively short period of time. We therefore conducted a cross sectional study for patients coming to our center. We recruited the COVID-19 affected (exposure group) or unaffected (control group) participants who had their semen examined after December 7th at our center, and compared semen parameters between the two groups. In order to confirm the cross sectional study results and to measure the scale of the effect, we further conducted a longitudinal study to compare the semen parameters in the same individuals (COVID-19 positive patients) before and after the virus infection. In addition, besides the conventional semen parameters such as sperm count, sperm motility and sperm morphology, more recent sperm quality indicators including sperm DNA fragmentation index (FDI) and chromatin immaturity were also studied. Therefore, the purpose of this study was to report our findings in order to ascertain the effects of COVID-19 infection on the sperm quality. We hope that results from this study may help clinicians in their consultation or management of patients with fertility problems while encountering COVID-19.
Materials and methods
Study design and participants
The current study was designed to be both a cross sectional study and a longitudinal study. A convenient sampling method was adopted. Participants were men who came to our fertility center for receiving semen analysis due to infertility during the period of early December of 2022 to late January 2023. Participants consisted of patients who had or had not suffered mild COVID-19 and they were asked to provide information in relation to the COVID-19 infection status. The structured questions related to the SARS-Cov2 infection became a routine part of disease history taking process during that special period of time due to the fact that a large proportion of the population were infected, though all of them had been previously vaccinated. However, informed consent was obtained from all the participants, and this study was performed in accordance with the ethical standards of Helsinki Declaration.
The cross sectional study included participants who could produce at least one semen sample after 7th of December. We compared the semen parameters between the patients who had contracted COVID-19 (exposure group) and those who had not (control group). The longitudinal study included COVID-19 positive participants who not only could provide one semen sample after he recovered from the infection, but also had at least one semen sample examined before the infection, thus allowing a comparison of semen parameters before and after the onset of COVID-19 in these individuals. In the longitudinal study, in order to provide control groups for the longitudinal (before and after, respectively) comparison of the COVID-19 positive group, we additionally recruited patients unaffected by the infection (COVID-19 negative group) who could provide two consecutive semen samples (former and latter, respectively) right over the same period of time. Patients with pre-COVID-19 baseline azoospermia were excluded. In addition, when motility was calculated, patients with severe pre-COVID-19 baseline oligozoospermia (< 5 × 106/mL) were excluded. The detailed process of collecting data is described in Fig. 1.
Semen analysis
Semen parameters analyzed in this study included conventional parameters such as semen volume, sperm concentration, total sperm count, percentage of grade A sperms, progressive sperm motility, total sperm motility and normal sperm morphology as well as more recent parameters of the sperm quality such as the percentage of immature sperm nucleoprotein and sperm DFI. Semen analysis was performed according to the standard procedure provided in the WHO Laboratory Manual for the Examination and Processing of Human Semen11. Briefly, semen samples were obtained by ejaculation via masturbation into a container in a dedicated semen collection room next to the semen analysis laboratory after a 3–7 day abstinence period. The samples were allowed to liquefy for less than 60 min before the analysis. Ejaculate volume was directly measured. Concentration and motility of sperms were measured with WLJY9000, a computer-aided semen analysis (CASA) instrument. The WHO criteria (2021) were applied to define the parameters such as count, motility, and morphology of the sperms. For sperm morphology analysis, semen smear was stained with Papanicolaou method and sperm morphology was observed with optical microscopy. Sperm motility was graded into 4 categories according to the sperm movement pattern: rapid progressive motility (grade A), slow progressive motility (grade B), non-progressive motility (grade C) and immotility (grade D). The former two grades were collectively referred to as progressive motility, while the former three grades collectively defines the total motility11. Immature sperm nucleoprotein (histone) was stained with aniline blue using a commercially available kit and the percentage of immature sperm nucleoprotein was calculated by dividing the number of sperms with immature nucleoprotein by the total number of sperms. Finally, sperm DNA fragmentation was stained with acridine orange and the sperm DFI was obtained with BriCyte E6 flow cytometry.
Statistical analysis
Data analysis was executed by employing the Statistical Package for Social Sciences (SPSS 20.0 for Windows). Statistical significance level was set at < 0.05 for all statistical tests (2-tailed). Continuous variables were presented as mean and standard deviations or median and interquartile range (IQR), based on data distribution evaluated by Kolmogorov–Smirnov test. Differences between groups are evaluated by independent samples t-test, paired samples t-test, Mann Whitney U test or Paired Wilcoxon test as appropriate. Categorical variables are presented as counts and percentages and were compared by χ2 test.
Ethical approval
The Medical Ethics Committee of Shengjing Hospital approved the study (2023PS004F).
Results
The cross-sectional study included 604 participants who could provide the semen sample for analysis after December 7th when the Chinese government adopted new strategy for controlling COVID-19. Out of the 604 patients, 515 (85.26%) had developed COVID-19 (exposure group) before their semen examination while the rest 89 (14.74%) had not developed the disease (control group). The average age of the COVID-19 exposure and control groups were 32.56 ± 4.58 and 33.9 ± 6.20 respectively, and there was no statistically significant difference between them. In addition, among the 515 cases of COVID-19, 140 patients (referred to as COVID-19 positive group in the longitudinal study) could provide two consecutive semen samples, one before the onset of COVID-19 and the other after the infection. Finally, in order to provide a natural (negative) control to the 140 COVID-19 positive patients in the longitudinal study, 149 patients who had not contracted COVID-19 and had two consecutive semen samples during the same period of time was randomly selected. The average age of COVID-19 positive patients and COVID-19 negative patients in the longitudinal study were 33.68 ± 4.33 and 33.71 ± 3.52 respectively, also showing no statistically significant difference.
Comparison of semen parameters between the COVID-19 exposure and control groups
In the cross sectional study, comparison of semen parameters between the COVID-19 exposure and control groups was carried out, and the results were shown in Table 1. As shown in Table 1, the semen parameters such as semen volume, sperm concentration, percentage of normal sperm morphology and percentage of immature sperm nucleoprotein were not statistically significant between the two groups. However, COVID-19 exposure group demonstrated significantly lower total sperm count, lower percentage of grade A sperms, lower progressive motility, lower total motility, and higher sperm DFI than the control group.
Comparison of semen parameters of COVID-19 positive patients before and after the onset of the infection in the longitudinal study
In order to illustrate the scale of COVID-19 impact on sperm quality, comparison of the semen parameters before and after the onset of the infection was performed in the 140 COVID-19 positive patients who could provide two consecutive semen samples across the onset point of COVID-19, and the results were shown in Table 2. We found that, after the onset of COVID-19, patients’ total sperm count, percentage of grade A sperms, progressive sperm motility and total sperm motility were all significantly lower and sperm DFI was higher than those before the onset, while the other parameters did not show statistically significant difference.
Comparison of semen parameters between the two consecutive semen samples of the COVID-19 negative natural control group during the same period of time in the longitudinal study
In order to describe the natural change (i.e. free from the COVID-19 influence) in semen parameters of the patients who had not contracted the infection during the same period of time and to rule out the possibility of confounding influence by factors such as weather, climate or environmental changes other than the factor of COVID-19, a comparison between the two consecutive semen samples, i.e. the former samples and the latter samples of the COVID-19 negative patients in the longitudinal study was performed. Results of the comparison were shown in Table 2. We found that, in the natural situation of our patients, semen parameters of the latter samples including sperm concentration, total sperm count, progressive motility, total sperm motility were significantly higher and the sperm DFI lower than those of the former samples, respectively, whereas the difference in other parameters were not statistically significant.
Comparison of semen parameters between the COVID-19 positive patients and COVID-19 negative patients in the longitudinal study
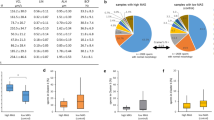
In order to further illuminate the scale of the effect of COVID-19 infection on the semen parameters, samples of COVID-19 positive patients in the longitudinal study before and after the infection were compared with the former and latter samples of the natural control group (COVID-19 negative), respectively. Results of the comparison were shown in Table 2. We found that, in the comparison between the former sample of the natural control group (n = 149) and the before-infection sample of the positive group (n = 140), the positive group showed higher concentration, higher total motility and lower sperm DFI than the negative control group, while other parameters did not show any significant difference. However, comparison between the after-infection sample of the positive group and the latter sample of the negative natural control group showed that semen parameters of the positive group such as sperm concentration, total sperm count, total sperm motility, percentage of grade A sperms, and progressive sperm motility were all significantly lower than those of the natural control group, while the difference in sperm DFI was no longer significant (Table 2; Fig. 2).
Changes in semen parameters of COVID-19 positive patients (n = 140) and negative patients (n = 149) across the onset of the disease in the longitudinal study. (a) Semen volume (ml); (b) Sperm concentration (×106/ml); (c) Total sperm count (×106); (d) Grade A sperm (%); (e) Progressive motility (%); (f) Total motility (%); (g) Normal sperm morphology (%); (h) Immature sperm nucleoprotein (%); (i) Sperm DFI (%).
Distribution of semen parameter changes among COVID-19 positive patients and negative patients in the longitudinal study
In order to further illuminate extent of the influence of COVID-19 on semen parameters, distribution of semen parameter changes were listed and compared between the COVID-19 positive patients and negative patients in the longitudinal study (Table 3). As shown in Table 3, the proportion of patients who had deteriorated semen parameters including sperm concentration, total sperm count, Grade A sperm, progressive motility, and total motility of the positive group were significantly higher than those of COVID-19 negative patients. With respect to DFI, most (75.0%) of the COVID-19 positive patients showed deterioration, whereas only a small proportion (38.10%) of the COVID-19 negative patients showed deterioration.
Discussion
This study was designed to determine whether COVID-19 affects sperm quality in men and to identify which sperm parameters were affected. Impact of COVID-19 on the semen parameters were demonstrated from different perspectives (with different study designs), including a cross sectional study of 515 patients who contracted COVID-19 (exposure group) and 89 controls who were free from the disease, and a longitudinal study (n = 140) with comparison of parameters before and after the onset of the infection in the same individuals. In addition, we included a COVID-19 negative natural control group (n = 149) with two consecutive samples over the same period of time to rule out the possibility of natural parameter change (worsening) over time due to other potential confounding factors such as adverse climate or environmental changes rather than the COVID-19 infection. As a result, we were able to generate several interesting findings and tentative conclusions: (1) The total sperm count and the motility including progressive motility and total motility were all significantly reduced while the sperm DFI was increased after the onset of COVID-19 in both the cross sectional and longitudinal observations, which strongly indicated that COVID-19 was associated with impaired sperm quality; (2) The finding that sperm quality of the latter samples were better than the former samples in the COVID-19 negative natural control group indicated that patients attending the fertility center generally benefited from the consultation or treatment by the physicians; (3) Comparison of samples between the COVID-19 positive and negative groups in the longitudinal study revealed that after the onset of COVID-19, sperm quality was worsened, further implicating COVID-19 as the causative factor; (4) The finding that in comparison with the COVID-19 negative group, a larger proportion of COVID-19 positive patients showed decreased sperm count and motility and increased DFI further indicated the adverse impact of COVID-19 on sperm quality from another perspective.
Total sperm count reflects the capacity of the testis to produce sperms, and is considered one of the most important conventional sperm quality parameters. COVID-19 was generally believed to be able to impair sperm production. However, there are still controversies as some studies did not reach the same conclusions12. Our findings in this study supported the conclusion that sperm count was adversely affected by COVID-19, because the COVID-19 exposure group showed much fewer total sperm count than the controls, and in the longitudinal study, over half (57.86%, 81/140) of the cases showed reduction in the total sperm count, with scale of the reduction being 11.8% (a actual reduction of 20.49 × 106 sperms in number) after COVID-19. Our study results contrasted with some previous studies, but were in line with a recent meta-analysis which showed that the infection was associated with a reduction of SMD = − 0.30 in total sperm count13. We speculate that the causes of the discrepancies might be due to differences in the study design or smaller sample size in the other studies9,14.
Sperm motility is another important conventional semen parameters found affected by COVID-19 in this study. In this study, we considered total motility as well as progressive sperm motility and grade A motility, because progressive sperm motility was known related to pregnancy rates15,16, and grade A motility was useful for predicting outcomes of the assisted reproductive technology17,18,19. Our study showed that in comparison with the controls, the exposure group had much lower sperm motility, and the longitudinal study more specifically revealed that after COVID-19, most (69.29%, 97/140) of the cases showed reduction in the total motility, and the scale of reduction is 17.4% (an actual reduction of -5.62%). It is interesting as well as important to note that among all the categories of the motility, the most affected is grade A sperm motility. As shown in the longitudinal study, after COVID-19, as high as 71.43% (100/140) of the cases showed reduction in grade A sperm motility, and scale of the reduction was as high as 37.41% (an actual reduction of -3.18%). Similarly, although not as pronounced as with grade A motility, the exposure group also showed much lower progressive motility, and the longitudinal study showed that after COVID-19, 65.0% (91/140) of the cases showed reduction in the progressive motility and the scale of reduction was 20.38% (an actual reduction of -5.07%). Similar findings have been reported in previous studies by other researchers, though their sample size was smaller20,21 or the study design was different22. Again, there are some discrepancies. For example, one study found there were a decrease only in total motility, not in the progressive motility21. We speculate that the slight differences in finding might be due to the smaller sample size or the long interval between onset of the disease and the semen collection (e.g. median = 111.5 days in one study ) in the previous studies.
Another important finding of this study was about the effect of COVID-19 on sperm DNA integrity: Both the cross sectional and the longitudinal study demonstrated that COVID-19 was associated with increased sperm DFI. Specifically, in the cross sectional study, the exposure group had much higher sperm DFI than the controls; in the longitudinal study, 75% (21/28) of the COVID-19 positive patients showed increased sperm DFI after onset of the disease, and the scale of increase was 21.18% (an actual increase of + 5.61%); whereas in the COVID-19 negative patients, sperm DFI was actually decreased by 12.68% (an actual decrease of -4.80%) over the same period of time. This finding is important in that it would be useful for clinicians in the field of reproduction to consult or manage patients, because it had been known that elevated sperm DNA fragmentation (SDF) affected outcomes of assisted reproductive techniques (ART) such as pregnancy rates and live birth rates, and high DFI was also associated with miscarriage23. This finding is important also because research on the effect of COVID-19 on sperm DNA integrity has been sparse, and the limited literature had inconsistent opinions. For example, one previous cohort study (n = 120) by Donders GGG and colleagues24 showed that DNA damage was most pronounced in the early stage following the onset of COVID-19, and they found abnormal DFI (defined as DFI > 25%) in 29% of the early samples as compared with 11-15% of the more later (1–2 months) samples after the onset. Similarly, Dipankar and colleagues25 reported impact of COVID-19 on sperm DNA integrity in 30 patients suffering from mild COVID-19; they found that DFI was high (74.25%) at the first semen sampling after patients’ recovery from COVID-19 and became lower (66.94%) at the second semen sampling 74 days after the first sampling. Finally, Falahieh FM and colleagues26 reported their study on 20 patients who had previously suffered moderate COVID-19, and found that DFI of patients at Day 120 after the diagnosis was lower than that at Day 14 after the diagnosis which fell within the normal range (defined as DFI < 30%). One of the issues with the above three studies was that they lacked baseline value of DFI before COVID-19. In this regard, it is interesting to note that Moryousef J27 recently published a case report in which pre-COVID-19 values of DFI were included, and the patient showed substantially elevated sperm DFI one month following the onset of COVID-19 as compared with the pre-COVID-19 baseline values, and the DFI returned to normal level 4 months later.These aforementioned study results indicated that the COVID-19 could affect sperm DNA integrity. In contrast, however, a more recent study done by Paoli and colleagues8 reported that sperm DFI of the recovered COVID-19 patients was within the normal range, and DFI was not related to severity or presence/absence of COVID-19, concluding that COVID-19 had no effects on DFI. Our findings supported the conclusion that sperm DNA integrity was adversely affected by COVID-19. We think that discrepancy or uncertainty over the issue probably occurred due to the fact that many previous studies lacked controls with COVID-19 negative patients and lacked the controls with baseline values of semen parameters before the onset of COVID-19, and their sample sizes were usually small. In addition, with respect to Paoli’s study8, the fact that their COVID-19 patients were those already 3 months recovered from COVID-19 could be the reason for the null finding. All in all, we guess that the study designs in previous studies could make it difficult to reach a firm conclusion on the impact of COVID-19. In this sense, our finding with DFI might be important in that it helped in clarifying the issue.
On the other hand, our study did not find any effect of COVID-19 on sperm morphology, and this finding was in line with the study result by Can Balcı MB who reported a pronounced reduction of sperm concentration in COVID-19 positive patients as compared with COVID-19 negative patients but no difference in sperm morphology was found between the two groups28. We also explored sperm chromatin maturity (immature sperm nucleoprotein) in this study because previous study had indicated that chromatin maturity or condensation was a valuable parameter in assessing male infertility29 and sperm chromatin maturity was related to zygote development in ART programs30. However, we did not identify any change in sperm nucleoprotein in either the cross sectional study or the longitudinal study.
Our study has some strengths. First, to our knowledge, our study had the largest sample size among all the observational studies up to date concerning the impact of COVID-19 on sperm quality. Second, design of the study with both cross sectional and longitudinal observations with COVID-19 negative controls allowed us reaching relatively confident conclusions concerning the effect of COVID-19 on semen parameters. In addition, with this type of design we could measure the scale of the effect, which should be useful for the clinicians to evaluate condition of the patients. Third, this study was done at the time of change in the control strategy for COVID-19 in China when over 80% of the population were infected with the virus in a concentrated period of time (less than two months). By seizing the window period for the virus infection, we were able to recruit large number of participants for the study. More important, because of the concentration of time and number of COVID-19 patients, many of the potential confounding factors for the study such as uncertainties over the diagnosis of COVID-19 infection or possible influences from climate or environment change could be eliminated so that results of the study might be more reliable. Finally, our study provided a relatively more complete description of the effect of COVID-19 on sperm qualities because more sperm parameters including the conventional semen parameters and the more recent parameters such as sperm chromatin maturity and sperm DNA fragmentation were explored.
Nevertheless, some limitations in this study should be noted. First, all the semen samples came from one reproductive center, and a multi-center study involving more cases is still needed. Second, due to the concentration of the outbreak, some baseline information on the sample was not fully collected. Third, the study only reported the semen characteristics shortly after COVID-19 recovery. We can conduct follow-up on these cases and perform semen analysis in later periods to understand if the changes acquire completely reversion. Furthermore, studies on chromosomic aberrations and imprinting changes, capable of being transmitted, could also be a subject for the future research.
Although WHO has declared that “COVID-19 is now an established and ongoing health issue which no longer constitutes a public health emergency of international concern”, the long-term careful management of COVID-19 pandemic is still advocated by WHO31. More important, SARS-CoV-2 virus is still currently rampant in many parts of the world, and fertility centers are bound to encounter many patients recovering from or currently suffering COVID-19. Therefore, proper handling of COVID-19 is definitely required in the diagnosis and management of male infertility. Based on our experience with the SARS-CoV-2 infection and the knowledge on its effects on sperm quality, we strongly suggest that screening or diagnosis of COVID-19 should be integrated into the workup of the patients with male infertility. In addition, keeping in mind the fact that SARS-CoV-2 continues to evolve, clinicians should remain vigilant against any potential adverse effects of different SARS-CoV-2 variants. In this sense, our study with SARS-CoV-2 infection might serve as a good example to observe effects of a specific SARS-CoV-2 variant on sperm quality. COVID-19 in our study has been known caused by a newly identified variant of the novel coronavirus, Omicron. Omicron is more infectious than previous variants, but less pathogenic in terms of causing pneumonia32,33. Present study is very important, because up to date, the effect of Omicron on sperm quality has not been reported and our study provided novel information in this regard. In addition, because Omicron is more contagious than the previous variants, large population will be infected with the variant and the characteristics of the infection should be addressed specifically. In this sense, our study findings may be very meaningful in clinical terms.
In conclusion, our study showed that COVID-19 was associated with poor sperm quality manifested by reduced sperm count and sperm motility, and increased sperm DNA fragmentation. Further research is needed to observe the long-term effect of COVID-19 on sperm quality or reproductive outcomes. In addition, there may be a need to constantly monitor the SARS-CoV-2 infection and integrate screening and diagnosis of the disease in the management of male infertility.
Data availability
The data that support the finding of this study are available from the corresponding author upon reasonable request.
References
Maleki, B. H. & Tartibian, B. COVID-19 and male reproductive function: A prospective, longitudinal cohort study. Reproduction (Cambridge, England) 161(3), 319–331. https://doi.org/10.1530/REP-20-0382 (2021).
Gacci, M. et al. Semen impairment and occurrence of SARS-CoV-2 virus in semen after recovery from COVID-19. Human Reprod. (Oxford, England) 36, 1520–1529. https://doi.org/10.1093/humrep/deab026 (2021).
Tiwari, S. et al. Semen parameters in men recovered from COVID-19: A systematic review and meta-analysis. Middle East Fertil. Soc. J. 26, 44. https://doi.org/10.1186/s43043-021-00089-w (2021).
Wang, S. et al. Association between COVID-19 and male fertility: Systematic review and meta-analysis of observational studies. World J. Men’s Health https://doi.org/10.5534/wjmh.220091 (2022).
Che, B. W. et al. Effects of mild/asymptomatic COVID-19 on semen parameters and sex-related hormone levels in men: A systematic review and meta-analysis. Asian J. Androl. https://doi.org/10.4103/aja202250 (2022).
Adamyan, L. et al. A review of recent studies on the effects of SARS-CoV-2 infection and SARS-CoV-2 vaccines on male reproductive health. Med. Sci. Monitor Int. Med. J. Exp. Clin. Res. 28, e935879. https://doi.org/10.12659/MSM.935879 (2022).
Al-Alami, Z. M. et al. COVID-19 and semen fluid parameters, a retrospective study from infertility clinics. Life 12, 2076. https://doi.org/10.3390/life12122076 (2022).
Paoli, D. et al. Male reproductive health after 3 months from SARS-CoV-2 infection: A multicentric study. J. Endocrinol. Invest. 46, 89–101. https://doi.org/10.1007/s40618-022-01887-3 (2023).
Kumar, T. et al. Effects of the COVID-19 pandemic on semen quality in male partners of infertile couples: A hospital-based observational study. Asian J. Androl. https://doi.org/10.4103/aja202278 (2022).
Tian, Y. & Zhou, L. Q. Evaluating the impact of COVID-19 on male reproduction. Reproduction (Cambridge, England) 161, R37-r44. https://doi.org/10.1530/rep-20-0523 (2021).
WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen (6th). (World Health Organization, 2021).
Ata, B. et al. SARS-CoV-2, fertility and assisted reproduction. Hum. Reprod. Update 29, 177–196. https://doi.org/10.1093/humupd/dmac037 (2023).
Xie, Y. et al. SARS-CoV-2 effects on sperm parameters: A meta-analysis study. J. Assisted Reprod. Genet. 39, 1555–1563. https://doi.org/10.1007/s10815-022-02540-x (2022).
Gul, A., Zengin, S., Dundar, G. & Ozturk, M. Do SARS-CoV-2 infection (COVID-19) and the medications administered for its treatment impair testicular functions?. Urol. Int. 105, 944–948. https://doi.org/10.1159/000517925 (2021).
Zinaman, M. J., Brown, C. C., Selevan, S. G. & Clegg, E. D. Semen quality and human fertility: A prospective study with healthy couples. J. Androl. 21, 145–153 (2000).
Larsen, L. et al. Computer-assisted semen analysis parameters as predictors for fertility of men from the general population. The Danish First Pregnancy Planner Study Team. Human Reprod. (Oxford, England) 15, 1562–1567. https://doi.org/10.1093/humrep/15.7.1562 (2000).
Barratt, C. L., Björndahl, L., Menkveld, R. & Mortimer, D. ESHRE special interest group for andrology basic semen analysis course: A continued focus on accuracy, quality, efficiency and clinical relevance. Human Reprod. (Oxford, England) 26, 3207–3212. https://doi.org/10.1093/humrep/der312 (2011).
Sifer, C. et al. World Health Organization grade ‘A’ motility and zona-binding test accurately predict IVF outcome for mild male factor and unexplained infertilities. Human Reprod. (Oxford, England) 20, 2769–2775. https://doi.org/10.1093/humrep/dei118 (2005).
Björndahl, L. The usefulness and significance of assessing rapidly progressive spermatozoa. Asian J. Androl. 12, 33–35. https://doi.org/10.1038/aja.2008.50 (2010).
Holtmann, N. et al. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil. Steril. 114, 233–238. https://doi.org/10.1016/j.fertnstert.2020.05.028 (2020).
Pazir, Y. et al. Impaired semen parameters in patients with confirmed SARS-CoV-2 infection: A prospective cohort study. Andrologia 53, e14157. https://doi.org/10.1111/and.14157 (2021).
Ertaş, K. et al. Examining changes on testicular structure and sperm analysis of COVID-19 patients. Andrologia 54, e14609. https://doi.org/10.1111/and.14609 (2022).
Marinaro, J. A. & Schlegel, P. N. Sperm DNA damage and its relevance in fertility treatment: A review of recent literature and current practice guidelines. Int. J. Mol. Sci. 24(2), 1446. https://doi.org/10.3390/ijms24021446 (2023).
Donders, G. G. G. et al. Sperm quality and absence of SARS-CoV-2 RNA in semen after COVID-19 infection: A prospective, observational study and validation of the SpermCOVID test. Fertil. Steril. 117, 287–296. https://doi.org/10.1016/j.fertnstert.2021.10.022 (2022).
Dipankar, S. P. et al. Semen quality in males suffering from COVID-19: A pilot study. Cureus 14, e31776. https://doi.org/10.7759/cureus.31776 (2022).
Falahieh, F. M. et al. Effects of moderate COVID-19 infection on semen oxidative status and parameters 14 and 120 days after diagnosis. Reprod. Fertil. Dev. 33, 683–690. https://doi.org/10.1071/rd21153 (2021).
Moryousef, J., Alkandari, M. H. & Zini, A. Case - Sperm DNA fragmentation associated with COVID-19 infection. Can. Urol. Assoc. J.= J. l’Association des Urologues du Canada 16, E301-e303. https://doi.org/10.5489/cuaj.7721 (2022).
Can Balcı, M. B. & Can Çilesiz, N. Investigation of the relationship between COVID-19 disease and semen parameters in idiopathic male infertility patients. Eur. Rev. Med. Pharmacol. Sci. 27, 378–383. https://doi.org/10.26355/eurrev_202301_30891 (2023).
Hammadeh, M. E. et al. Predictive value of sperm chromatin condensation (aniline blue staining) in the assessment of male fertility. Arch. Androl. 46, 99–104 (2001).
Asmarinah, et al. Sperm chromatin maturity and integrity correlated to zygote development in ICSI program. Syst. Biol. Reprod. Med. 62, 309–316. https://doi.org/10.1080/19396368.2016.1210695 (2016).
WHO. Statement on the Fifteenth Meeting of the IHR (2025). (Emergency Committee on the COVID-19 Pandemic, 2023).
Wang, J. & Dong, W. COVID-19: The possibility, ways, mechanisms, and interruptions of mother-to-child transmission. Arch. Gynecol. Obstetr. 307(6), 1687–1696. https://doi.org/10.1007/s00404-022-06639-5 (2022).
Ren, S. Y., Wang, W. B., Gao, R. D. & Zhou, A. M. Omicron variant (B11529) of SARS-CoV-2: Mutation, infectivity, transmission, and vaccine resistance. World J. Clin. Cases 10, 1–11. https://doi.org/10.12998/wjcc.v10.i1.1 (2022).
Acknowledgements
We thank all the participants for their cooperation. Bochen Pan has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This study is supported by Liaoning Provincial Government Education Department Project (JYTMS20230102), Shengjing Hospital Clinical Research Project (M0071), 345 Talent Project (M1463), and “Tree Planting” (M1590) Project to Bochen Pan. The funders played no role in the study. Researchers are independent from funders and that all authors, external and internal, had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Contributions
Study proposal: Bochen Pan, Lulu Yuan. Data collection: Bochen Pan, Zihan Dong, Xiaobin Wang, Qiang Du, Renhao Guo, Ping Li, Xu Leng, Haizhen Liang. Data analysis and drafting of the manuscript: Lulu Yuan, Wei Sun, Lu Lu, Bochen Pan. All authors read and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yuan, L., Sun, W., Dong, Z. et al. COVID-19 infection was associated with poor sperm quality: a cross-sectional and longitudinal clinical observation study. Sci Rep 15, 11380 (2025). https://doi.org/10.1038/s41598-025-94570-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-94570-5