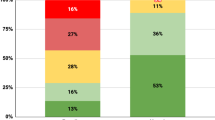
Abstract
Assessing the clinical and biochemical profiles of patients with type-2 diabetes (T2D) is vital for understanding the disease characteristics and improving disease management. This study aimed to assess the profiles of adults (> 18 years old) with T2D enrolled in a one-year diabetes management program in Pune, India. This cross-sectional study retrospectively analyzed 18,950 records from the Freedom from Diabetes Clinic, between November 2015 and June 2023. Baseline sociodemographic, biochemical, and anthropometric parameters were extracted from electronic medical records and analyzed using descriptive statistical methods. A gender-wise comparison was performed for the biochemical and anthropometric measurements. Poor glycemic control (glycated hemoglobin [HbA1c] ≥ 7%) was reported by 70.0% of the patients at baseline, despite the majority (80.2%) being on glucose lowering medications. The prevalence of overweight (body mass index [BMI] 23.0–24.9 kg/m2) and obesity (BMI ≥ 25 kg/m2) was 21.5% and 57.2%, respectively. Hypertension and hypercholesterolemia were reported in 56.8% and 25.7% of the patients, respectively. A significant association was observed between comorbidities such as hypertension, overweight/obesity, and poor glycemic control (p < 0.05). Additionally, significant gender-wise differences were observed in the clinical parameters, including homeostatic model assessment of insulin resistance, fasting insulin, and micronutrients (p < 0.05). This study highlights the high prevalence of poor glycemic control, overweight/obesity, and comorbidities among T2D patients enrolled in a one-year diabetes management program in India. Gender-specific differences emphasize the need for tailored management approaches. These findings underscore the importance of comprehensive lifestyle interventions to improve glycemic control, address comorbidities, and reduce complication risks in the Indian population with T2D.
Similar content being viewed by others
Introduction
Type 2 diabetes (T2D) is a global health concern that is primarily attributed to lifestyle and associated comorbidities. The International Diabetes Federation (IDF) reports that approximately 537 million adults currently have diabetes, with the number expected to rise to 783 million by 20451. India ranks second in its contribution to the global diabetes epidemic, with nearly 7.2 million people affected in 20211,2. The Asian Indian population is at a higher risk due to specific risk factors such as central obesity, a characteristic "thin-fat" phenotype, and a lower median age at diagnosis3. Furthermore, India has a considerable burden of T2D-related comorbidities, such as hypertension, dyslipidemia, and obesity, which present additional challenges in the effective management of T2D4,5. Glycemic control, a critical aspect of T2D management, is often intertwined with these comorbidities, which complicate patient management6. Considering the complexities of T2D management in this population, it is essential to comprehensively assess clinical and biochemical characteristics to improve overall disease outcomes.
Previous large-scale studies in India, including population-based surveys and limited real-world evidence studies, have primarily focused on reporting the glycemic status, associated risk factors, and complications in specific cohorts of patients with T2D. For instance, the ICMR-INDIAB survey, conducted on a representative population across three Indian states on patients with self-reported diabetes, revealed that 70% had poor glycemic control (HbA1c ≥ 7%)7, highlighting a significant gap in diabetes management in the region. Other studies providing real-world evidence have either focused specifically on glycemic control in those receiving antidiabetic therapy in routine healthcare settings8 or on younger adults (18–45 years) with diabetes5.
The present study, in the context of limited real-world evidence, aimed to undertake an extensive evaluation of the fundamental characteristics of individuals diagnosed with T2D, aged between 18 and 70 years who participated in a one-year intensive lifestyle intervention (ILI)-based diabetes management program from across 560 cities in India. By focusing on this specific cohort, which exhibits significant phenotypic and genotypic differences from Western populations, this study sought to provide a nuanced perspective on the factors influencing glycemic control in the context of structured diabetes management initiatives.
Methods
Study design and setting
This was a cross-sectional study of adult (> 18 years old) T2D patients who were part of a one-year in-person (November 2015–January 2020) or a one-year online (February 2020–June 2023) integrated ILI (program for diabetes management in Pune, India. Retrospective data on the baseline anthropometric and biochemical parameters of patients who met the eligibility criteria were extracted from an electronic database maintained by the Freedom from Diabetes Clinic in India.
Eligibility criteria
The inclusion criteria were age between 18 and 70 years and a confirmed diagnosis of T2D according to the American Diabetes Association (ADA) guidelines9. Exclusion criteria were pancreatitis or drug-induced diabetes (steroids), other types of diabetes (type 1 diabetes mellitus, diabetes insipidus, maturity-onset diabetes of the young, latent autoimmune diabetes in adults, or Gestational Diabetes), and myocardial infarction within the last 6 months.
Data collection
Baseline data of eligible 18,950 patients were retrospectively extracted on the following parameters—
-
a.
Sociodemographic data included age, gender, education, and occupation.
-
b.
Medical history included the duration of diabetes, comorbid conditions (e.g., cardiovascular disease, hypertension, dyslipidemia, obesity, and kidney function), and medication history. Clinical parameters included hemoglobin, liver, renal, and thyroid function tests, micronutrient status (vitamins D and B12), and serum iron levels.
-
c.
Glycemic control included fasting and postprandial blood glucose, HbA1c, and fasting insulin levels. Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) and Homeostatic Model Assessment of Beta-cell function (HOMA-B) were calculated using standard formulas10. Patients on external insulin were excluded from the HOMA-IR and HOMA-B analyses.
Ethical approval
This study adhered to ethical guidelines and all procedures involving human participants were conducted in accordance with the Declaration of Helsinki. Ethical approval for this study was received from the Institutional Ethics Committee of Freedom from Diabetes Research Foundation (Ref. No. FFDRF /IEC/2024/7). This study is registered in the Clinical Trials Registry of India (CTRI) (CTRI/2024/03/064596). The retrospectively extracted data were anonymized and handled with strict confidentiality to ensure the privacy of individuals involved in the study. Personal identifiers were removed to prevent identification of individual participants.
Patient consent
Due to the retrospective nature of the study, the requirement for informed consent was waived by the Ethics Committee.
Statistical analyses
Data were analyzed using descriptive statistics including frequency and percentage. Mean ± standard deviation and median (interquartile range) were used to describe normally distributed and skewed data, respectively. The association between categorical variables was assessed using the chi-square test. All analyses were performed using SPSS version 21. Statistical significance was set at p < 0.05.
Results
The mean age of the study population (n = 18,950) was 52.7 ± 10.6 years, and the majority were male (64.6%). The sociodemographic characteristics of the study population are presented in Table 1. The study included patients from 560 cities in India.
Glycemic control and medication status
Glycemic parameters showed that the mean HbA1c level was 8.1 ± 1.8% among the study population, which suggests poor glycemic control despite the majority (80.2%) being on glucose-lowering medication (Oral Hypoglycemic Agents (OHAs) and/or insulin) (Table 2). The median fasting and postprandial blood glucose levels were 131 (110–162) mg/dl and 160 (127–214) mg/dl, respectively. The median fasting insulin levels, HOMA-IR, and HOMA-B were 7.9 (5.0–12.4) µU/mL, 2.7 (1.6–4.4), and 42.6 (23.6–75.5), respectively. Insulin resistance, as measured by HOMA-IR (≥ 2.5)11was observed in more than half (54.2%) of the population. A significant difference was observed in the gender-wise comparison of fasting insulin and HOMA-IR with higher levels observed in females than males (p < 0.001).
Comorbidities
The prevalence of comorbidities showed that more than half of the study population (57.2%) was obese (BMI ≥ 25 kg/m2) with significantly higher percentage of obesity seen in females than males (p < 0.001) (Table 3). The prevalence of hypertension (blood pressure ≥ 140/90 mmHg without medication or on anti-hypertensive medication) in the population was 56.8% (42% were on medication). Dyslipidemia, defined as high cholesterol (> 200 mg/dl), elevated triglyceride levels (> 150 mg/dl) and/or high low-density lipoprotein cholesterol (LDL-C) levels (> 100 mg/dl), was also notable. Irrespective of statin therapy status, hypercholesterolemia, elevated triglycerides, and elevated LDL-C was observed in 25.7%, 38.5%, and 53.4% of the population, respectively; 45.3% of participants with dyslipidemia were on statin therapy. The mean LDL-C, total cholesterol, triglyceride, and high-density lipoprotein cholesterol (HDL-C) levels were 104.2 ± 38.3 mg/dl, 172.1 ± 45.6 mg/dl, 153.7 ± 97.9 mg/dl, and 42.1 ± 9.9 mg/dl, respectively. Low HDL-C levels were observed in 57.9% of the population. The mean HDL-C levels were 40.2 ± 9.0 mg/dl (males) and 45.6 ± 10.5 mg/dl (females). Mean triglyceride levels between males and females were comparable; (156.1 ± 105.2 mg/dl vs. 149.4 ± 83.1 mg/dl). Females were significantly more obese than males [BMI 29 (26.8–31.6) kg/m2 vs. 27.5 (26.1–29.3) kg/m2; (p < 0.001)]. Medication status for comorbidities is shown in Table 3.
Other clinical parameters
Nearly 30% of females in the study population had anemia (hemoglobin < 12 g/dl) (Table 4). Additionally, nearly half (46.2%) of the population was Vitamin D deficient, and males were significantly more deficient in micronutrients than females (p < 0.001). Elevated high-sensitivity C-reactive protein (hs-CRP) levels were observed in 72.8% of the population, indicating a moderate to high cardiovascular risk. Data on liver, renal, and thyroid functions, along with serum iron and C-reactive protein (CRP) levels, have also been reported; microalbuminuria was observed in more than one-fifth of the population (21.6%). Significant gender-wise differences were observed for total bilirubin, serum albumin, serum creatinine, uric acid, urine microalbumin, thyroid function, and inflammatory markers, namely CRP and hs-CRP (p < 0.001).
Association between comorbidities and glycemic control
We observed a significant association between comorbid conditions, such as hypertension, overweight/obesity, and poor glycemic control (HbA1c ≥ 7%) (Table 5). Comorbidities such as overweight/obesity (BMI ≥ 23.0 kg/m2) and hypertension (blood pressure ≥ 140/90 mmHg) were significantly associated with poor glycemic control (HbA1c ≥ 7%). Additionally, more than six years of diabetes duration demonstrated a significant positive association with poor glycemic control (p < 0.05).
Discussion
This cross-sectional study provides a comprehensive analysis of the baseline characteristics and glycemic challenges of 18,950 patients diagnosed with T2D who participated in an intensive lifestyle intervention program for diabetes management in India. The prevalence of poor glycemic control among most of the study population, coupled with significant associations between suboptimal glycemic control and comorbidities, such as hypertension, dyslipidemia, and obesity, underscores the need for effective and targeted interventions in the management of T2D.
We report the prevalence of poor glycemic control, as assessed by HbA1c levels (≥ 7%), in a substantial proportion of patients (70%) despite the use of glucose-lowering medications. This finding is consistent with the results of the multicenter ICMR-INDIAB study, which reported poor glycemic control in 69% of the population7. Similarly, another large-scale cross-sectional study of 55,639 individuals showed that 76.6% of the population had uncontrolled T2D8.The reason for this suboptimal glycemic control could be attributed to previous reports of increased non-adherence to pharmacotherapy management in the Asian population30, or it could highlight the inadequacy of pharmacotherapy as a standalone management tool for T2D, especially in the Asian/Asian-Indian population.
Our study highlights the significant burden of T2D and comorbidities, with nearly half of participants being overweight or obese, 56.8% showing hypertension, 26–53% showing dyslipidemia, and 70% having T2D for over 6 years, emphasizing the need for tailored interventions. Comorbidities such as hypertension, dyslipidemia, and cardiovascular disease increase the risk of diabetes-related complications, posing challenges in management and secondary prevention while also impacting the quality of care for patients with T2D31,32. Further, we found that poor glycemic control was significantly associated with elevated blood pressure (which is a leading cause of cardiovascular-related and premature deaths worldwide, and an independent risk factor for macrovascular and microvascular complications)33, higher BMI (> 23.0 kg/m2), and longer disease duration (> 6 years), consistent with previous studies34,35. These findings underscore the importance of integrated management strategies that address lifestyle factors and early intervention to improve glycemic control and reduce complications.
Gender-wise comparison of comorbidities in our study found higher levels of cardiometabolic risk factors, including dyslipidemia (high LDL-C, high total cholesterol with low HDL-C), obesity and hypothyroidism among women, whereas men showed higher cardiac medication use, reflecting distinct disease patterns36. Our findings on gender-specific lipid profile align with previous studies that report high LDL-C and triglyceride levels among women, contributing to a substantial cardiovascular risk among them37,38. Nevertheless, few research on lipid profile differences in T2D populations have reported that men with T2D tend to have high triglyceride levels, predisposing them to cardiovascular risk especially at younger ages37,39. Further, studies also indicate that while men with diabetes have a 3.8-fold increased risk of coronary heart disease (CHD), women face a significantly higher 14.7-fold risk, even after adjusting for traditional factors40. Women may be more vulnerable to cardiovascular disease (CVD) due to multiple factors, including the loss of estrogen’s protective effects post-menopause, which contributes to a more atherogenic lipid profile41. Additionally, women often exhibit worse cardiometabolic profiles than men, with higher visceral adiposity, hypertension, and dyslipidemia, further increasing CVD risk42. These results highlight the need to consider gender-specific risk factors when addressing CVD risk in patients with diabetes.
Our study further highlights gender-specific differences in other clinical parameters. Consistent with previous research, females had higher fasting insulin levels and HOMA-IR, likely due to greater visceral fat, contributing to metabolic dysfunction and cardiovascular risk43,44,45. Additionally, men showed more vitamin B12 and D deficiencies, while women had higher thyroid dysfunction (elevated T3, T4, and TSH levels) and lower iron levels, likely due to hormonal, dietary, and physiological factors46,47.
Despite a higher prevalence of obesity, women in our study maintained higher vitamin D levels than men, which can be attributed to multiple physiological and behavioral factors. Increased use of vitamin D supplements and awareness of its sources may contribute to better vitamin D status despite increased adiposity48. Estrogen enhances vitamin D metabolism by increasing serum levels of its active form, potentially mitigating the effects of obesity on vitamin D levels49. Additionally, women have a higher proportion of subcutaneous fat, which may store and release vitamin D more effectively than visceral fat, which is more common in men and associated with lower vitamin D levels50. Lifestyle factors such as sun exposure and higher consumption of dairy or fortified foods among women may also contribute to these findings51.
Overall, such gender-specific associations with various clinical parameters highlight the complexity of gender differences and emphasize the need for tailored approaches for effective T2D management. Considering the multifactorial aspects of diabetes management, healthcare professionals must consider lifestyle, culture, socioeconomic status, and medication regimens when providing personalized care8. By using targeted interventions such as patient education, lifestyle counselling, and tailored medication management, healthcare providers can help improve glycemic control and reduce the clinical burden of comorbidities in patients with T2D.
Limitations
This cross-sectional study provides valuable insights into the baseline characteristics of a large cohort of patients with T2D in India. However, it has certain limitations. As a retrospective analysis of data from a clinical database, this study was susceptible to biases related to data completeness. The cross-sectional design enabled the identification of associations between variables, but did not establish causation or track disease progression and treatment response over time. Additionally, the single-center design may have introduced a selection bias. Although this could have limited the generalizability of the study to the broader Indian population, the hybrid nature of the program helped mitigate this to some extent by enrolling patients from 560 cities across India. The subscription-based model may also have introduced socioeconomic selection bias, restricting participation to those who could afford it and potentially underrepresenting lower-income groups, which may have impacted the findings. Furthermore, this study did not assess the history of self-reported complications, which could have influenced the overall assessment.
Conclusion
Despite these limitations, our study on Indian population from across 560 Indian cities provides a comprehensive assessment of T2D patients enrolled in a diabetes management program. With 70% of patients experiencing poor glycemic control despite treatment, targeted interventions are urgently needed. The strong association between population-specific comorbidities such as hypertension, dyslipidemia, obesity, and suboptimal glycemic control underscores the complexity of T2D management. A holistic approach, considering sociodemographic factors and comorbidities, is essential for effective diabetes care. Future longitudinal studies are needed to explore causative relationships and trends in glycemic control.
Data availability
All data are available from the corresponding author and can be shared following a reasonable request. In such cases, patient information will be de-identified before sharing.
References
IDF Diabetes Atlas 2021 | IDF Diabetes Atlas. https://diabetesatlas.org/atlas/tenth-edition/.
Sathyanath, S. et al. An economic evaluation of diabetes mellitus in India: A systematic review. Diabetes Metab. Syndr. 16, 102641 (2022).
Vijayakumar, V., Mavathur, R. & Sharma, M. N. K. Ethnic disparity and increased prevalence of type 2 diabetes among South Asians: Aetiology and future implications for diabetes prevention and management. Curr. Diabetes Rev. 14, 518–522 (2018).
Bansode, B. & Prasad, J. B. Burden of comorbidities among diabetic patients in Latur, India. India. Clin. Epidemiol. Glob Health 13, 100957 (2022).
Saboo, B. et al. REAL-world evidence of risk factors and comorbidities in YOUNG Indian adults with type 2 diabetes mellitus: A REAL YOUNG (diabetes) study. J. Family Med. Prim. Care 10, 3444 (2021).
Urina-Jassir, M. et al. The effect of comorbidities on glycemic control among Colombian adults with diabetes mellitus: A longitudinal approach with real-world data. BMC Endocr. Disord. https://doi.org/10.1186/s12902-021-00791-w (2021).
Unnikrishnan, R. et al. Glycemic control among individuals with self-reported diabetes in India–the ICMR-INDIAB Study. Diabetes Technol. Ther. 16, 596–603 (2014).
Borgharkar, S. S., Das, S. S., Surendra, D. & Borgharkar, S. Real-world evidence of glycemic control among patients with type 2 diabetes mellitus in India: the TIGHT study. BMJ Open Diabetes Res. Care 7, e000654 (2019).
Committee, A. D. A. P. P. et al. 2. Diagnosis and classification of diabetes: Standards of care in diabetes—2024. Diabetes Care 47, S20–S42 (2024).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and fl-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Das, R. R. et al. Prevalence of insulin resistance in urban Indian school children who are overweight/obese: A cross-sectional study. Front Med 8, 613594 (2021).
Saxena, P., Prakash, A. & Nigam, A. Efficacy of 2-hour post glucose insulin levels in predicting insulin resistance in polycystic ovarian syndrome with infertility. J. Hum. Reprod. Sci. 4, 20 (2011).
Solano, M. P. & Goldberg, R. B. Lipid management in type 2 diabetes. Clin. Diabetes 24, 27–32 (2006).
Misra, A. Ethnic-specific criteria for classification of body mass index: A perspective for Asian Indians and American diabetes association position statement. Diabetes Technol. Ther. 17, 667–671 (2015).
Kim, H. J. & Kim, K. Il. Blood pressure target in type 2 diabetes mellitus. Diabetes Metab. J. 46, 667–674 (2022).
Kumar, P., Mukherji, A. & Roy, A. Prevalence of hypothyroidism in the population of west Bokaro coal mine area, Jharkhand: A hospital-based observational study. Cureus https://doi.org/10.7759/cureus.28733 (2022).
Billett, H. H. Hemoglobin and Hematocrit. Anesthesiology 28, 763–763 (1990).
Singla, R. et al. Vitamin B12 deficiency is endemic in Indian population: A perspective from north India. Indian J. Endocrinol. Metab. 23, 211 (2019).
Amrein, K. et al. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 74, 1498–1513 (2020).
Rai, S., Shetty, S., Bhandary, R., Rao, A. V. & Student, P. G. Reference interval of serum bilirubin panel in healthy individuals of attending tertiary care hospital-a cross sectional study. Int. J. Clini. Biochem. Res. 4, 73 (2017).
Busher, J. T. Serum Albumin and Globulin. Clinical Methods: The History, Physical, and Laboratory Examinations (1990).
Anand, S. et al. Prevalence of chronic kidney disease in two major Indian cities and projections for associated cardiovascular disease. Kidney Int. 88, 178–185 (2015).
Rajagopalan, P. et al. Population-based estimation of renal function in healthy young Indian adults based on body mass index and sex correlating renal volume, serum creatinine, and cystatin C. Int. J. Nephrol. Renovasc. Dis. 9, 243 (2016).
Is it time to revise the normal range of serum uric acid levels? https://www.europeanreview.org/article/7352.
Hosten, A. O. BUN and Creatinine. Clinical Methods: The History, Physical, and Laboratory Examinations (1990).
Veeramuthumari, P., Isabel, W. & Kannan, K. A study on the level of T3, T4, TSH and the association of A/G polymorphism with CTLA-4 gene in graves’ hyperthyroidism among south Indian population. Indian J. Clin. Biochem. 26, 66 (2011).
Mahajan, A. et al. High-sensitivity C-reactive protein levels and type 2 diabetes in urban north Indians. J. Clin. Endocrinol. Metab. 94, 2123–2127 (2009).
Nehring, S. M., Goyal, A., Bansal, P. & Patel, BCc. Reactive protein. StatPearls 65, 237–244 (2023).
Ridker, P. M. & Cook, N. Clinical usefulness of very high and very low levels of C-reactive protein across the full range of Framingham risk scores. Circulation 109, 1955–1959 (2004).
Asiri, R., Todd, A., Robinson-Barella, A. & Husband, A. Ethnic disparities in medication adherence? A systematic review examining the association between ethnicity and antidiabetic medication adherence. PLoS One 18, e0271650 (2023).
Eilat-Tsanani, S., Margalit, A. & Golan, L. N. Occurrence of comorbidities in newly diagnosed type 2 diabetes patients and their impact after 11 years’ follow-up. Sci. Rep. 11, 1–10 (2021).
Pati, S. & Schellevis, F. G. Prevalence and pattern of co morbidity among type2 diabetics attending urban primary healthcare centers at Bhubaneswar (India). PLoS One 12, e0181661 (2017).
Mills, K. T., Stefanescu, A. & He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 16, 223–237 (2020).
Xing, X. Y. et al. Glycemic control and its influencing factors in type 2 diabetes patients in Anhui, China. Front. Public Health 10, 980966 (2022).
Alzaheb, R. A. & Altemani, A. H. The prevalence and determinants of poor glycemic control among adults with type 2 diabetes mellitus in Saudi Arabia. Diabetes, Metab. Syndr. Obes. 11, 15–21 (2018).
Madariaga, A. G., Santos Palacios, S., Guillén-Grima, F. & Galofré, J. C. The incidence and prevalence of thyroid dysfunction in Europe: A meta-analysis. J. Clin. Endocrinol. Metab. 99, 923–931 (2014).
Zhang, X. et al. Gender disparities in lipid goal attainment among type 2 diabetes outpatients with coronary heart disease: results from the CCMR-3B study. Sci. Rep. 7, 1–7 (2017).
Russo, G. et al. Age- and gender-related differences in LDL-cholesterol management in outpatients with type 2 diabetes mellitus. Int. J. Endocrinol. 2015, 957105 (2015).
Ambrož, M. et al. Sex differences in lipid profile across the life span in patients with type 2 diabetes: A primary care-based study. J. Clin. Med. 10, 1775 (2021).
Juutilainen, A. et al. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care 27, 2898–2904 (2004).
Wakabayashi, I. Gender differences in cardiovascular risk factors in patients with coronary artery disease and those with type 2 diabetes. J. Thorac. Dis. 9, E503–E506 (2017).
Li, T. et al. Type 2 diabetes is more predictable in women than men by multiple anthropometric and biochemical measures. Sci. Rep. 11, 1–10 (2021).
Kautzky-Willer, A., Leutner, M. & Harreiter, J. Sex differences in type 2 diabetes. Diabetologia 66, 986–1002 (2023).
Mauvais-Jarvis, F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol. Sex Differ. 6, 14 (2015).
Straub, R. H. The complex role of estrogens in inflammation. Endocr. Rev. 28, 521–574 (2007).
Yang, X. et al. Meta-analysis of serum vitamin B12 levels and diabetic retinopathy in type 2 diabetes. Arch. Med. Res. 54, 64–73 (2023).
Alharbi, A. A. et al. Gender-specific differences in the awareness and intake of Vitamin D among adult population in Qassim Region. J. Family Commun. Med. 25, 148–154 (2018).
Gallagher, J. C., Riggs, B. L. & Deluca, H. F. Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 51, 6–1364 (1980).
Wierzbicka, A. & Oczkowicz, M. Sex differences in vitamin D metabolism, serum levels and action. Br. J. Nutr. 128, 2115–2130 (2022).
Alyahya, K. O. Poor dietary consumption and limited sun exposure are risk factors for vitamin D deficiency in premenopausal Kuwaiti women: A cross-sectional study. Qatar Med. J. https://doi.org/10.5339/qmj.2020.15 (2020).
Akın, S. & Bölük, C. Prevalence of comorbidities in patients with type–2 diabetes mellitus. Prim. Care Diabetes 14, 431–434 (2020).
Acknowledgements
The authors would like to acknowledge the Freedom from Diabetes Research Foundation for providing logistic assistance during this study. The authors are grateful to all study participants.
Author information
Authors and Affiliations
Contributions
The research question and idea were conceived and conceptualized by PT and NK. The manuscript was further revised and structured with contributions from MG and BSa. TK, DT, AV, and BSh accessed and extracted relevant data. AV, BSh, and TK cleaned the data and assessed the accuracy of the analyses. DT and TK collaborated on data analyses. NK provided input for statistical analysis and revision of the manuscript. PT, NK, and TK led the writing with input from DT, AV, and BSh. The final version of the manuscript was critically analyzed by PT, NK, and BSa. All authors reviewed the manuscript, contributed to the content, and approved the final version of the manuscript. NK was responsible for the submission of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tripathi, P., Kadam, N., Kathrikolly, T. et al. Baseline clinical and biochemical profiles of type 2 diabetes patients enrolled in a lifestyle management program in India, a cross-sectional study. Sci Rep 15, 10275 (2025). https://doi.org/10.1038/s41598-025-94694-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94694-8