Abstract
This study aims to investigate the incidence of new-onset atrial fibrillation (AF) in individuals with type 2 diabetes mellitus (T2DM) across different categories of steatotic liver disease (SLD). Using a health examination database between 2009 and 2012, this study included 2,480,880 patients. Participants were categorized into five groups based on hepatic steatosis (fatty liver index ≥ 60), cardiometabolic risk factors, and alcohol consumption. Cox regression analyses were performed. The metabolic dysfunction-associated steatotic liver disease (MASLD) group showed an increased risk of new-onset AF (adjusted hazard ratio (aHR), 1.10; 95% confidence interval (CI), 1.08–1.11). The MASLD with other combined group demonstrated increased AF development (aHR, 1.22; 95% CI, 1.18–1.26). In metabolic dysfunction and alcohol-related steatotic liver disease (MetALD) and alcohol-related liver disease (ALD) with metabolic groups, heavy to excessive alcohol consumption increased the risk of AF incidence, with the highest aHR associated with greater alcohol intake (aHR, 1.26; 95% CI, 1.22–1.29, 1.48; 95% CI, 1.41–1.55). MASLD increased the risk of AF in patients with T2DM, with a higher risk observed when accompanied by other liver diseases. Alcohol consumption was associated with proportional increase in the risk of AF, with excessive alcohol consumption associated with the highest risk of AF.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF), one of the most common cardiac arrhythmias, is a prominent cardiovascular health concern because of its association with thromboembolic stroke, heart failure, and increased risk of mortality1,2. It is anticipated that reducing the burden of AF will improve patients’ quality of life. Projection studies indicate that by 2050, the prevalence of AF in the United States will exceed 10 million3. Although the exact mechanisms remain unclear, many studies have suggested that diabetes mellitus (DM) and AF share common risk factors4,5,6, with recent research further exploring the metabolic risk factors associated with AF7,8,9. Metabolic dysfunction-associated steatotic liver disease (MASLD) is the most recent term for steatotic liver disease (SLD) associated with metabolic syndrome. Unlike the mechanisms underlying non-alcoholic fatty liver disease (NAFLD), which mainly focuses on liver fat accumulation, MASLD encompasses broader metabolic dysfunctions, including obesity, insulin resistance, and dyslipidemia10,11. While studies show a 99% overlap between NAFLD and MASLD, younger patients with early-stage, mild NAFLD may not always meet MASLD diagnostic criteria12. MASLD is strongly associated with an increased risk of developing type 2 DM (T2DM) and shares common pathophysiological mechanisms13,14. A recent meta-analysis demonstrated that MASLD is significantly associated with an increased risk of developing incident AF, with a random-effects hazard ratio (HR) of 1.20 (95% confidence interval (CI), 1.10–1.32) and this association remained significant even after adjusting for T2DM15. In the context of the increasing emphasis on early prevention due to the additive deleterious effects of T2DM on MASLD, the specific impact of SLD categories on AF development in T2DM patients remains underexplored16. Therefore, we investigated the incidence of new-onset AF in a nationwide cohort of individuals with T2DM, stratified by SLD categories.
Results
Baseline characteristics
A total of 2,480,880 individuals were divided into the following five groups: 1,778,355 (71.68%), no-steatosis group; 510,905 (20.59%), MASLD group; 100,568 (4.05%), metabolic dysfunction and alcohol-related steatotic liver disease (MetALD) group; 34,597 (1.39%), alcohol-related liver disease (ALD) with metabolic group; and 56,455 (2.28%), MASLD with other combined groups. Table 1 presents the baseline characteristics of the participants. The median age of the total population was 57 years, and 60.1% of the patients were male.
Participants in the MetALD and ALD with metabolic groups were more likely to be current smokers and predominantly men, with a higher prevalence of new-onset DM. They exhibited higher values of systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting glucose, estimated glomerular filtration rate (eGFR), triglycerides (TG), gamma-glutamyl transferase (GGT), and lower values of low-density lipoprotein (LDL) cholesterol than the other groups. The MASLD with other combined group showed a higher prevalence of comorbidities, including hypertension (HTN), dyslipidemia, myocardial infarction (MI), congestive heart failure (CHF), transient ischemic attack (TIA), thromboembolism, and peripheral arterial disease (PAD).
Risk of AF incidence according to alcohol consumption, metabolic risk, and MASLD
Compared to the no steatosis group (reference), the MASLD group showed an increased risk of AF development (aHR, 1.10; 95% CI, 1.08–1.11) after adjustment for age, sex, income, smoking, regular exercise, body mass index (BMI), Charlson Comorbidity Index (CCI) score, fasting glucose, DM duration, insulin use, oral hypoglycemic agent (OHA), chronic kidney disease (CKD), MI, CHF, dyslipidemia, stroke, TIA, thromboembolism, PAD, and CHA2DS2-VASc score. (Model 5, Table 2). In the MetALD and ALD with metabolic groups, heavy to excessive alcohol consumption further increased the risk of AF incidence, with the highest aHR observed with greater alcohol intake (aHR, 1.26; 95% CI, 1.22–1.29 for MetALD; aHR, 1.48; 95% CI, 1.41–1.55 for ALD with metabolic group). The MASLD with other combined group, when having concomitant liver disease or ALD, showed increased AF incidence (aHR, 1.22; 95% CI, 1.18–1.26).
Subgroup analysis
Subgroup analyses of new-onset AF incidence stratified by age, sex, BMI, DM duration, fasting glucose, insulin use or OHA, HTN, dyslipidemia, regular exercise, and CHA2DS2-VASc score were performed after adjusting for Model 5 (Supplementary Table 1). These trends are consistent with the primary outcome. A significant trend in HRs was observed, demonstrating a stepwise increase from the MASLD group to the MetALD group, and further to the ALD group. Additionally, we explored the interaction between the main exposure and outcome across age, sex, BMI, DM duration, insulin or OHA use, HTN, dyslipidemia, regular exercise, and CHA2DS2-VASc score (P for interaction < 0.05). AF incidence was generally higher among individuals aged ≥ 60 years; however, the relative risk increase was more evident in younger patients within the MetALD and ALD with metabolic groups, compared to those older than 60 years. Women showed a higher AF risk in the ALD with metabolic group compared to men. A longer diabetes duration of ≥ 5 years was associated to an increased AF risk across all categories, suggesting prolonged diabetes may contribute to AF development. Notably, individuals with CHA2DS2-VASc < 3, and those without dyslipidemia or HTN exhibited a higher AF risk, indicating that MASLD and ALD may independently contribute to AF development regardless of traditional cardiovascular risk factors. In addition, across all groups, individuals with a lower BMI (< 25) exhibited a greater AF risk, particularly in the ALD with metabolic and MASLD with other combined groups, suggesting a possible association between lean MASLD and AF. Furthermore, an additional subgroup analysis was conducted to compare the risk of AF between lean and non-lean MASLD and MetALD patients, stratified by BMI (< 23 vs. ≥ 23), showing that the risk of AF was higher in the lean group (BMI < 23) (Supplementary Table 2).
Risk of AF incidence according to the degree of hepatic steatosis in the alcohol consumption group
We further categorized the participants based on hepatic steatosis using the fatty liver index (FLI) categories and alcohol intake (none, mild, heavy, and excessive drinking). As shown in Table 3, compared with the group with FLI < 30 or 30 ≤ FLI < 60, the group with FLI ≥ 60 exhibited increased aHRs for AF development. Moreover, as alcohol consumption increased from mild to heavy and then to excessive levels, the risk of AF increased.
Sensitivity analysis, excluding individuals diagnosed with ALD or concomitant liver disease, demonstrated a similar trend in the aHR, suggesting a consistent association with AF development (Supplementary Table 3). Additionally, a sensitivity analysis using a different model for SLD (FLI ≥ 30) was performed, which also demonstrated a consistent association with AF development (Supplementary Table 4). Furthermore, Supplementary Table 5 shows that advanced fibrosis (BARD score ≥ 2) was associated with a higher incidence of AF, particularly in the ALD with metabolic group.
Discussion
This population-based retrospective study sheds light on the relationship between the SLD categories and the development of new-onset AF in patients with T2DM. Additionally, we explored the dose-dependent association between alcohol consumption and the risk of AF, with excessive alcohol consumption presenting the highest risk. We also found that MASLD significantly increased the risk of AF, particularly when accompanied by other liver diseases. Furthermore, the AF risk was further elevated in individuals with MASLD who had advanced fibrosis (BARD score ≥ 2).
Recent epidemiological evidence suggests a strong concordance between the NAFLD and MASLD definitions, with approximately 99% of individuals with NAFLD meeting the MASLD criteria17. The newly proposed term, MASLD, highlights its close association with metabolic syndrome, emphasizing the interplay between hepatic steatosis and cardiometabolic risk factors. The prevalence of metabolic syndrome varies globally between 12.5% and 31.4% according to meta-analyses, MASLD has emerged as a major public health issue, notably because of its association with increased cardiovascular disease risk18,19,20. A recent systematic review of NAFLD cohorts reported a pooled cardiac-specific mortality rate of 4.20 (1.34–7.05) per 1000 person-years21. Consistent with our findings, a previous meta-analysis found that among patients with NAFLD, those with T2DM had the highest risk of developing AF22. In China, patients with metabolic dysfunction-associated fatty liver disease (MAFLD) were associated with a significantly higher prevalence of AF risk in cross-sectional and longitudinal studies23. In a retrospective study, MAFLD with T2DM patients had a significantly higher prevalence of paroxysmal AF (6.3% vs. 1.3%) than those the non-MAFLD group24. During the 9-year follow-up period, liver cirrhosis was identified as an independent risk factor for the development of AF (aHR, 1.46; 95% CI, 1.18–1.80) in the Korean population25. A previous study confirmed the positive correlation between NAFLD and AF risk, indicating a higher risk of incident AF in individuals with higher FLI scores26. Similarly, a Swedish cohort study found that the histologically confirmed MASLD group exhibited higher rates of incident AF (aHR, 1.26; 95% CI, 1.18–1.35), particularly among patients with liver cirrhosis27. Consistent with this, our study found increased aHRs for AF development in MASLD, with higher rates observed for FLI ≥ 60 (Table 3) and advanced fibrosis (BARD score ≥ 2) (Supplementary Table 5). In a recent study, a 1-year follow-up after catheter ablation revealed that the weight loss group exhibited a significant reduction in recurrent AF compared to the control group28. A previous study postulated an association between the triglyceride-glucose index (TyG), a novel indicator of insulin resistance, and a higher risk of AF in patients with NAFLD, suggesting a relationship between insulin resistance and AF29,30. One study found that lean body mass may be an important factor affecting AF risk31. Our subgroup analysis showed that both lean MASLD and lean MetALD patients had a higher risk of AF compared to their non-lean counterparts. The increased AF risk in individuals with lower BMI but with MASLD, abdominal adiposity suggested a strong link between body composition and AF development. However, it remains unclear whether improvements in cardiometabolic risk factors, including body composition changes, lead to long-term cardiovascular benefits, including reduced AF risk, and further studies are needed to investigate these complex interactions.
Our findings revealed that AF risk increased with increased alcohol consumption across the MASLD, MetALD, and ALD with metabolic groups, with aHRs increasing as follows: MASLD (aHR = 1.10), MetALD (aHR = 1.26), and ALD (aHR = 1.48). In a randomized controlled trial involving 140 patients with AF, the AF burden over 6 months was significantly lower in the alcohol abstinence group than that in the control group (> 10 drinks/week) (0.5% vs. 1.2%; P = 0.01)32. In a previous study involving patients newly diagnosed with T2DM, alcohol abstinence was associated with a low risk of AF incidence (aHR, 0.81; 95% CI, 0.68–0.97) compared with consistent alcohol consumption (≥ 20 g/d)33. A recent updated meta-analysis found that the association between AF risk and alcohol consumption was dose-dependent34. Additionally, a linear relationship was observed in men, whereas a potentially nonlinear J-shaped relationship was observed in women. In one study involving an asymptomatic healthy population, frequent binge drinkers had a 3.2 times higher risk of AF than infrequent light drinkers35. Explanations for the effects of alcohol include its direct toxicity and indirect contributions to obesity and HTN, as well as impact on the autonomic nervous system, which has been widely investigated36,37.
DM-related AF is associated with myocardial fibrosis primarily due to atrial structural remodeling38. Uncontrolled glycemia induces oxidative stress, leading to inflammation and subsequent fibrosis, which are associated with myocardial tissue damage and an increased incidence of AF in the diabetic population39. Metabolic defects, such as insulin resistance and impaired glucose tolerance contribute to endothelial dysfunction and accelerate atherogenesis, potentially explaining the occurrence of AF40,41. Previous research found that patients with DM and AF faced higher risks of diabetic-related complications, including nephropathy (HR, 1.23; 95% CI, 1.16–1.30), macrovascular complications (HR, 1.12; 95% CI, 1.09–1.16), and diabetic foot (HR, 1.13; 95% CI, 1.09–1.17)42. Moreover, in both the ADVANCE and ORBIT-AF studies, the presence of AF was associated with increased mortality and adverse cardiovascular outcomes in patients with DM43,44. Our study particularly emphasizes the modification of cardiometabolic risk factors and lifestyle, including reducing alcohol consumption and weight, smoking cessation, and promoting regular exercise for individuals with risk factors to prevent AF.
The strength of this study lies in its nationwide population-based analysis with a large sample size. To our knowledge, this is the first study to analyze the association between MASLD, AF incidence, and alcohol consumption in individuals with T2DM, particularly in an Asian population. Using multivariate Cox regression analysis adjusted for multiple potential covariates, subgroup analyses according to several variables, further stratified analyses based on alcohol consumption and FLI categories, and sensitivity analysis, we provide robust evidence for our findings.
However, several limitations should be acknowledged. First, our definition of AF did not consider the potential influence of valvular heart disease, which may have affected our results. Additionally, because of the impracticality of using invasive techniques in asymptomatic individuals undergoing routine health check-ups, hepatic steatosis was characterized using FLI, although liver biopsy remains the gold standard for diagnosis. Zhang et al. demonstrated that the FLI score could be used as a diagnostic tool for patients with NAFLD, with a sensitivity of 87% and specificity of 58.5%, compared with the magnetic resonance imaging-derived proton density fat fraction (MRI-PDFF)45. This methodological approach may have introduced some degree of variability to the analysis. Moreover, the potential for undetected asymptomatic or paroxysmal AF may have led to underdiagnosis in our study. Furthermore, the lack of clinical data in our database, including glycated hemoglobin (HbA1c) and C-reactive protein (CRP) levels and echocardiographic findings, limited our ability to fully assess the burden of AF in our cohort. Finally, given the retrospective observational design of this nationwide cohort study, caution should be exercised when inferring causality from the results. Despite these limitations, our study contributes valuable evidence to the understanding of the relationship between MASLD and AF risk in patients with T2DM. Individuals in the MASLD, MetALD, and ALD with metabolic groups had a significantly increased risk of developing AF. Our findings also highlighted the cumulative effect of alcohol consumption on AF risk in these individuals.
Methods
Study population and design
We used the comprehensive Korean National Health Insurance Service (NHIS) database, which covers the entire Korean population from January 2009 to December 2022. The NHIS, which encompasses two key healthcare programs, the National Health Insurance (NHI) and Medical Aid (MA), covers approximately 97% of the population under NHI, with the remaining 3% covered by MA, targeting individuals with the lowest income.
This government-operated database includes a claims dataset containing patient demographic information, health check-up records, diagnoses coded according to the International Classification of Disease, 10th revision (ICD-10) codes, and prescription details. The health examination dataset included responses from health-related behavior questionnaires, comprehensive medical histories, anthropometric data, and laboratory results. We further accessed mortality records from the Korean National Statistical Office, which included death dates and causes coded according to the revised 10th International Statistical Classification of Diseases. This study was approved by the Institutional Review Board (IRB) of Samsung Medical Center (approval no. SMC 2024-03-104), Seoul, Republic of Korea, which waived the requirement for participant informed consent because all data provided to the researchers were de-identified.
Patients with T2DM aged 20 years and above who underwent general health examinations between 2009 and 2012 (N = 2,741,135) were included in the study. The following exclusion criteria applied: age under 20 years (N = 390), missing data (N = 136,836), a diagnosis of liver cancer before the index date (N = 6,408), past history of liver transplantation (N = 566), a previous diagnosis of AF (N = 81,970), on oral anticoagulant therapy at baseline (N = 4,787), and death or AF diagnosis within 1 year of screening (N = 29,298). The final cohort included 2,480,880 individuals.
Measurements and definitions
Height, body weight, and waist circumference (WC) were measured during health examinations. BMI was calculated as body weight (kg) divided by height (m) squared in meters. CKD was defined as an eGFR ≤ 60 mL/min/1.73 m2. T2DM was defined as a fasting blood glucose ≥ 126 mg/dL, the use of specific drugs (OHAs, glucagon-like peptide (GLP) 1 receptor agonist, and insulin) or at least one claim using codes E11-14. OHAs include metformin, sulfonylureas, dipeptidyl peptidase (DPP) 4 inhibitors, thiazolidinediones, alpha-glucosidase inhibitors, and meglitinides. Insulin use was defined as one or more prescriptions of insulin per year or three or more prescriptions per year in an outpatient setting. New-onset DM was defined as a fasting blood glucose level of 126 mg/dL or higher at the time of health screening, with no prior prescription of DM medication before the health check-up. HTN was identified as a SBP ≥ 140 mmHg, DBP ≥ 90 mmHg or taking antihypertensive medication or having at least one claim using codes I10-13 and I15. Dyslipidemia was defined as having at least one claim per year using code E78 and at least one claim per year for the prescription of a lipid-lowering agent or a total cholesterol level of ≥ 240 mg/dL. Alcohol abuse/misuse, or ALD were defined by the following ICD-10 codes: E24.4, F10, G31.2, G62.1, G72.1, I42.6, K29.2, K70, K86.0, Q35.4, R78.0, T51.0, T51.8, T51.9, X65, Y15, Y57.3, Y90, Y91, Z50.2, Z71.4. Concomitant liver diseases were also defined by ICD-10 codes and included viral hepatitis (B16–B19, B00.8), drug-induced (toxic) liver disease (K71), hepatic veno-occlusive disease (I82), liver abscess (K75.0, A06.4), hemochromatosis (E83.1), Wilson’s disease (E83.0), alpha-1 antitrypsin deficiency (E88.0), autoimmune hepatitis (K75.4), primary biliary cholangitis (K74.3, K74.4), other cholangitis (K83), and glycogen storage disease (E74). Stroke was defined by ICD-10 codes I63 and I64; TIA by G458 and G459; thromboembolism by I74, I26, and I802; PAD by I70 and I73; MI by I21 and I22; and CHF by I50. The ALD and concomitant liver disease ICD-10 codes used for classification of MASLD and MASLD with other combined group. The CCI is a comorbidity score comprised of 12 conditions. Income was classified based on the quartile (Q) of the study population (Q4: highest; Q1: lowest). Regular exercise was also evaluated through the questionnaire and was defined as meeting any one of the following two criteria: (i) ≥ 3 d/ week of vigorous activity (causing extreme shortness of breath) for at least 20 min/d, or (ii) ≥ 5 d/ week of moderate-intensity activity (causing significant shortness of breath) for at least 30 min/d. The CHA2DS2-VASc score includes CHF, HTN, age (≥ 65 years = 1 point; ≥75 years = 2 points), DM, previous stroke/TIA (2 points), vascular disease (PAD, previous MI, and aortic atheroma), and sex (female sex). Alcohol consumption was assessed using questionnaires on participants’ drinking behaviors. The participants were asked about their average frequency (days per week) and amount (standard glass). A standard glass sample was calculated as 8 g of pure alcohol. The average amount of alcoholic beverages consumed was converted into pure alcohol (g) consumed per day.
Definition of hepatic steatosis and MASLD
We evaluated hepatic steatosis using the FLI, determined through the following equation 46: (e 0.953×loge (TG) +0.139×BMI+0.718×loge (GGT) +0.053×WC−15.745)/(1 + e 0.953×loge (TG) +0.139×BMI+0.718×loge (GGT) +0.053×WC−15.745).
MASLD diagnosis was established when participants with hepatic steatosis exhibited at least one of the following cardiometabolic risk factors, with hepatic steatosis defined as FLI ≥ 60: (1) BMI ≥ 23 kg/m2 or WC ≥ 90 cm in men and ≥ 80 cm in women; (2) fasting blood glucose levels ≥ 100 mg/dL or T2DM or specific drug treatment; (3) blood pressure (BP) ≥ 130/85 mmHg or specific drug treatment; (4) TG ≥ 150 mg/dL or specific drug treatment; (5) high-density lipoprotein (HDL) cholesterol < 40 mg/dL for men and < 50 mg/dL for women or specific drug treatment.
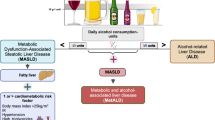
We classified patients into five groups as follows: (1) no steatosis group (FLI < 60); (2) MASLD group, where mild alcohol consumption was defined as < 30 g/d for men and < 20 g/d for women, excluding individuals with ICD codes for ALD and concomitant liver disease; (3) MetALD group, defined as heavy alcohol consumption (≥ 30 g/d but < 60 g/d for men and ≥ 20 g/d but < 50 g/d for women) with FLI ≥ 60 and ≥ 1 cardiometabolic risk factor; (4) ALD with metabolic group, defined as excessive alcohol consumption (≥ 60 g/d for men and ≥ 50 g/d for women) with FLI ≥ 60 and ≥ 1 cardiometabolic risk factor; (5) MASLD with other combined group, defined as MASLD and a diagnosis of concomitant liver disease or ALD within 1 year before screening, as identified by relevant ICD codes.
Among patients with SLD (FLI ≥ 60), advanced liver fibrosis was determined using the BARD score. This score is calculated by assigning two points for an aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio of ≥ 0.8, one point for a BMI of ≥ 28 kg/m², and one point for the presence of DM. A total score ranging from two to four indicates advanced hepatic fibrosis47.
Outcomes
The primary outcome was new-onset AF, identified by ICD-10 codes I48.0–I48.4 and I48.9, and documented in either one hospitalization record or at least two outpatient clinic records. Participants were monitored from the initial health examination until the incidence of the primary outcome event. The period between 2009 and 2012 was defined as the baseline period, with the date of the last examination as the reference point. The study cohort was observed from baseline until the earliest event of death, onset of AF, or December 31, 2022.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD), while categorical variables are presented as numbers with frequencies (%). One-way ANOVA was used to compare continuous baseline characteristics, and the chi-square test was used to compare categorical baseline variables. The incidence of AF was calculated by dividing the number of incident cases by the total follow-up duration (person-years).
We used Cox proportional hazards models to assess HRs and 95% CIs for the incidence of AF. Model 1 is a crude model. Model 2 is adjusted for age and sex. Model 3 was further adjusted for income, smoking status, regular exercise, BMI, and CCI scores. Model 4 also included adjustments for fasting glucose level, DM duration, insulin use, OHA use, and CKD. Model 5 included adjustments for MI, CHF, dyslipidemia, stroke, TIA, thromboembolism, PAD, and CHA2DS2-VASc scores. The proportional hazard assumption was evaluated using Schoenfeld residuals and Kaplan-Meier curves, and no violations were detected. A subgroup analysis was conducted to examine the association between the incidence of AF and various variables. Statistical significance was set at P < 0.05, and all analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC, USA).
Data availability
Data is provided within the manuscript or supplementary information files.
Abbreviations
- AF:
-
atrial fibrillation
- ALD:
-
alcohol-related liver disease
- ALT:
-
alanine aminotransferase
- AST:
-
aspartate aminotransferase
- BMI:
-
body mass index
- BP:
-
blood pressure
- CCI:
-
Charlson Comorbidity Index
- CHF:
-
congestive heart failure
- CI:
-
confidence interval
- CKD:
-
chronic kidney disease
- CRP:
-
C-reactive protein
- DBP:
-
diastolic blood pressure
- DM:
-
diabetes mellitus
- DPP:
-
dipeptidyl peptidase
- eGFR:
-
estimated glomerular filtration rate
- FLI:
-
fatty liver index
- GGT:
-
gamma-glutamyl transferase
- GLP:
-
glucagon-like peptide
- HbA1c:
-
glycated hemoglobin
- HDL:
-
high density lipoprotein
- HR:
-
hazard ratio
- HTN:
-
hypertension
- ICD-10:
-
international classification of diseases 10th revision
- IRB:
-
institutional review board
- LDL:
-
low density lipoprotein
- MA:
-
medical aid
- MAFLD:
-
metabolic dysfunction-associated fatty liver disease
- MASLD:
-
metabolic dysfunction-associated steatotic liver disease
- MetALD:
-
metabolic dysfunction and alcohol-related steatotic liver disease
- MI:
-
myocardial infarction
- MRI-PDFF:
-
magnetic resonance imaging derived proton density fat fraction
- NAFLD:
-
non-alcoholic fatty liver disease
- NHI:
-
national health insurance
- NHIS:
-
national health insurance service
- OHA:
-
oral hypoglycemic agent
- PAD:
-
peripheral arterial disease
- SBP:
-
systolic blood pressure
- SD:
-
standard deviation
- SLD:
-
steatotic liver disease
- TG:
-
triglyceride
- TIA:
-
transient ischemic attack
- T2DM:
-
type 2 diabetes mellitus
- TyG:
-
triglyceride-glucose index
- WC:
-
waist circumference
References
Kirchhof, P. The future of atrial fibrillation management: integrated care and stratified therapy. Lancet 390, 1873–1887. https://doi.org/10.1016/s0140-6736(17)31072-3 (2017).
Bordignon, S., Chiara Corti, M. & Bilato, C. Atrial Fibrillation Associated with Heart Failure, Stroke and Mortality. J. Atr. Fibrillation. 5, 467. https://doi.org/10.4022/jafib.467 (2012).
Miyasaka, Y. et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 114, 119–125. https://doi.org/10.1161/circulationaha.105.595140 (2006).
Harati, H. et al. No evidence of a causal association of type 2 diabetes and glucose metabolism with atrial fibrillation. Diabetologia 62, 800–804. https://doi.org/10.1007/s00125-019-4836-y (2019).
Watanabe, H. et al. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation 117, 1255–1260. https://doi.org/10.1161/circulationaha.107.744466 (2008).
Kornej, J., Börschel, C. S., Benjamin, E. J. & Schnabel, R. B. Epidemiology of Atrial Fibrillation in the 21st Century: Novel Methods and New Insights. Circ. Res. 127, 4–20. https://doi.org/10.1161/circresaha.120.316340 (2020).
Fauchier, G. et al. Metabolically healthy obesity and cardiovascular events: A nationwide cohort study. Diabetes Obes. Metab. 23, 2492–2501. https://doi.org/10.1111/dom.14492 (2021).
Ahn, H. J. et al. Cumulative burden of metabolic syndrome and its components on the risk of atrial fibrillation: a nationwide population-based study. Cardiovasc. Diabetol. 20, 20. https://doi.org/10.1186/s12933-021-01215-8 (2021).
Lee, S. Y. et al. Association Between Change in Metabolic Syndrome Status and Risk of Incident Atrial Fibrillation: A Nationwide Population-Based Study. J. Am. Heart Assoc. 10, e020901. https://doi.org/10.1161/jaha.121.020901 (2021).
Haghbin, H. et al. Nonalcoholic fatty liver disease and atrial fibrillation: possible pathophysiological links and therapeutic interventions. Ann. Gastroenterol. 33, 603–614. https://doi.org/10.20524/aog.2020.0550 (2020).
Qi, X., Li, J., Caussy, C., Teng, G. J. & Loomba, R. Epidemiology, screening, and co-management of type 2 diabetes mellitus and metabolic dysfunction-associated steatotic liver disease. Hepatology https://doi.org/10.1097/hep.0000000000000913 (2024).
Lee, B. P., Dodge, J. L. & Terrault, N. A. National prevalence estimates for steatotic liver disease and subclassifications using consensus nomenclature. Hepatology 79, 666–673. https://doi.org/10.1097/hep.0000000000000604 (2024).
Mantovani, A., Byrne, C. D., Bonora, E. & Targher, G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes Care. 41, 372–382. https://doi.org/10.2337/dc17-1902 (2018).
Barrera, F. et al. The Janus of a disease: Diabetes and metabolic dysfunction-associated fatty liver disease. Ann. Hepatol. 29, 101501. https://doi.org/10.1016/j.aohep.2024.101501 (2024).
Mantovani, A. et al. MASLD Is Associated With an Increased Long-Term Risk of Atrial Fibrillation: An Updated Systematic Review and Meta-Analysis. Liver Int. 45, e16128. https://doi.org/10.1111/liv.16218 (2025).
Fernández-Garibay, V. M. & Chavez-Tapia, N. C. The rationale for the aggressive progression of MASLD in patients with type 2 diabetes. Ann. Hepatol. 30, 101778. https://doi.org/10.1016/j.aohep.2025.101778 (2025).
Hagström, H., Vessby, J., Ekstedt, M. & Shang, Y. 99% of patients with NAFLD meet MASLD criteria and natural history is therefore identical. J. Hepatol. 80, e76–e77. https://doi.org/10.1016/j.jhep.2023.08.026 (2024).
Chan, W. K. et al. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 32, 197–213. https://doi.org/10.7570/jomes23052 (2023).
Moon, J. H., Jeong, S., Jang, H., Koo, B. K. & Kim, W. Metabolic dysfunction-associated steatotic liver disease increases the risk of incident cardiovascular disease: a nationwide cohort study. EClinicalMedicine 65, 102292. https://doi.org/10.1016/j.eclinm.2023.102292 (2023).
Han, E. et al. Fatty Liver & Diabetes Statistics in Korea: Nationwide Data 2009 to 2017. Diabetes Metab. J. 47, 347–355. https://doi.org/10.4093/dmj.2022.0444 (2023).
Younossi, Z. M. et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 77, 1335–1347. https://doi.org/10.1097/hep.0000000000000004 (2023).
Mantovani, A. et al. Association between non-alcoholic fatty liver disease and risk of atrial fibrillation in adult individuals: An updated meta-analysis. Liver Int. 39, 758–769. https://doi.org/10.1111/liv.14044 (2019).
Lei, F. et al. The prevalence of MAFLD and its association with atrial fibrillation in a nationwide health check-up population in China. Front. Endocrinol. (Lausanne). 13, 1007171. https://doi.org/10.3389/fendo.2022.1007171 (2022).
Mantovani, A. et al. Association between metabolic dysfunction-associated fatty liver disease and supraventricular and ventricular tachyarrhythmias in patients with type 2 diabetes. Diabetes Metab. 49, 101416. https://doi.org/10.1016/j.diabet.2022.101416 (2023).
Lee, H. et al. Cirrhosis is a risk factor for atrial fibrillation: A nationwide, population-based study. Liver Int. 37, 1660–1667. https://doi.org/10.1111/liv.13459 (2017).
Choi, J. et al. Non-alcoholic Fatty Liver Disease and the Risk of Incident Atrial Fibrillation in Young Adults: A Nationwide Population-Based Cohort Study. Front. Cardiovasc. Med. 9, 832023. https://doi.org/10.3389/fcvm.2022.832023 (2022).
Simon, T. G. et al. Incident cardiac arrhythmias associated with metabolic dysfunction-associated steatotic liver disease: a nationwide histology cohort study. Cardiovasc. Diabetol. 22, 343. https://doi.org/10.1186/s12933-023-02070-5 (2023).
Akhtar, K. H. et al. The effect of weight loss on recurrence of atrial fibrillation after catheter ablation: A systematic review and meta-analysis. J. Cardiovasc. Electrophysiol. 34, 2514–2526. https://doi.org/10.1111/jce.16090 (2023).
Zhang, Y. et al. Correlation between the triglyceride-glucose index and the onset of atrial fibrillation in patients with non-alcoholic fatty liver disease. Diabetol. Metab. Syndr. 15, 94. https://doi.org/10.1186/s13098-023-01012-1 (2023).
Moon, J. H. et al. Triglyceride-Glucose Index Predicts Future Atherosclerotic Cardiovascular Diseases: A 16-Year Follow-up in a Prospective, Community-Dwelling Cohort Study. Endocrinol. Metab. (Seoul). 38, 406–417. https://doi.org/10.3803/EnM.2023.1703 (2023).
Fenger-Grøn, M., Overvad, K., Tjønneland, A. & Frost, L. Lean Body Mass Is the Predominant Anthropometric Risk Factor for Atrial Fibrillation. J. Am. Coll. Cardiol. 69, 2488–2497. https://doi.org/10.1016/j.jacc.2017.03.558 (2017).
Voskoboinik, A. et al. Alcohol Abstinence in Drinkers with Atrial Fibrillation. N Engl. J. Med. 382, 20–28. https://doi.org/10.1056/NEJMoa1817591 (2020).
Choi, Y. J. et al. Alcohol Abstinence and the Risk of Atrial Fibrillation in Patients With Newly Diagnosed Type 2 Diabetes Mellitus: A Nationwide Population-Based Study. Diabetes Care. 44, 1393–1401. https://doi.org/10.2337/dc20-2607 (2021).
Jiang, H. et al. Alcohol consumption and atrial fibrillation risk: An updated dose-response meta-analysis of over 10 million participants. Front. Cardiovasc. Med. 9, 979982. https://doi.org/10.3389/fcvm.2022.979982 (2022).
Cha, M. J. et al. Alcohol consumption and risk of atrial fibrillation in asymptomatic healthy adults. Heart Rhythm. 17, 2086–2092. https://doi.org/10.1016/j.hrthm.2020.07.010 (2020).
Voskoboinik, A., Prabhu, S., Ling, L. H., Kalman, J. M. & Kistler, P. M. Alcohol and Atrial Fibrillation: A Sobering Review. J. Am. Coll. Cardiol. 68, 2567–2576. https://doi.org/10.1016/j.jacc.2016.08.074 (2016).
Kaul, R. et al. Alcohol and Atrial Fibrillation: A Pathophysiologic Perspective. Cardiol. Rev. 31, 177–184. https://doi.org/10.1097/crd.0000000000000479 (2023).
Russo, I. & Frangogiannis, N. G. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. J. Mol. Cell. Cardiol. 90, 84–93. https://doi.org/10.1016/j.yjmcc.2015.12.011 (2016).
Faria, A. & Persaud, S. J. Cardiac oxidative stress in diabetes: Mechanisms and therapeutic potential. Pharmacol. Ther. 172, 50–62. https://doi.org/10.1016/j.pharmthera.2016.11.013 (2017).
Tadic, M. & Cuspidi, C. Type 2 diabetes mellitus and atrial fibrillation: From mechanisms to clinical practice. Arch. Cardiovasc. Dis. 108, 269–276. https://doi.org/10.1016/j.acvd.2015.01.009 (2015).
Sun, Y. & Hu, D. The link between diabetes and atrial fibrillation: cause or correlation? J. Cardiovasc. Dis. Res. 1, 10–11. https://doi.org/10.4103/0975-3583.59978 (2010).
Kwon, S. et al. Association Between Atrial Fibrillation and Diabetes-Related Complications: A Nationwide Cohort Study. Diabetes Care. 46, 2240–2248. https://doi.org/10.2337/dc23-0931 (2023).
Echouffo-Tcheugui, J. B. et al. Care Patterns and Outcomes in Atrial Fibrillation Patients With and Without Diabetes: ORBIT-AF Registry. J. Am. Coll. Cardiol. 70, 1325–1335. https://doi.org/10.1016/j.jacc.2017.07.755 (2017).
Du, X. et al. Risks of cardiovascular events and effects of routine blood pressure lowering among patients with type 2 diabetes and atrial fibrillation: results of the ADVANCE study. Eur. Heart J. 30, 1128–1135. https://doi.org/10.1093/eurheartj/ehp055 (2009).
Zhang, Q. et al. Diagnostic accuracy assessment of molecular prediction model for the risk of NAFLD based on MRI-PDFF diagnosed Chinese Han population. BMC Gastroenterol. 21, 88. https://doi.org/10.1186/s12876-021-01675-y (2021).
Bedogni, G. et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 6, 33. https://doi.org/10.1186/1471-230x-6-33 (2006).
Harrison, S. A., Oliver, D., Arnold, H. L., Gogia, S. & Neuschwander-Tetri, B. A. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 57, 1441–1447. https://doi.org/10.1136/gut.2007.146019 (2008).
Acknowledgements
The authors declare no competing interests.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
G.K., K.H., and J.H.K conceived and designed the study. S.H.C. and K.L. collected and analyzed the data. S.H.C. interpreted the results and wrote the manuscript. G.K., K.H., and J.H.K edited the manuscript and contributed to the discussion. R.O., J.Y.K., M.J., Y.B.L., S.M.J., and K.Y.H. reviewed the manuscript and provided feedback. All authors contributed to the discussion and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Availability of data and materials:
All relevant data are available in this article and the supplementary files.
Ethics approval and consent to participate:
All procedures involving human participants were conducted in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The requirement for informed consent was waived because the data are publicly available and deidentified.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
†So Hyun Cho and Gyuri Kim contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cho, S.H., Kim, G., Lee, Kn. et al. Impact of steatotic liver disease categories on atrial fibrillation in type 2 diabetes: a nationwide study. Sci Rep 15, 11430 (2025). https://doi.org/10.1038/s41598-025-94783-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94783-8