Abstract
The aim of this study was to investigate risk factors, develop, and assess the predictive nomogram for low appendicular skeletal muscle mass index (ASMI) in middle-aged and elderly populations. A total of 3,209 inpatients were divided into a Training Set (n = 2,407) and a Validation Set (n = 802). A nomogram was developed using R software for internal validation, and external validation was performed using the Validation Set. Gender (male), age, height, weight, triglyceride levels, alanine aminotransferase levels, alcohol consumption, and the triglyceride-glucose index to body-mass index ratio (TyG/BMI) were identified as predictors for the nomogram of low ASMI. In the Training Set, Q1-Q4 subgroups were performed for TyG/BMI, and logistic regression analysis showed that a TyG/BMI ratio greater than 0.37 was significantly associated with an increased risk of developing low ASMI (P < 0.001), with an area under the receiver operating characteristic curve (AUC) of 0.879 for the nomogram. In the Validation Set, the nomogram also demonstrated excellent calibration and discrimination, with an AUC of 0.881. Decision curve analysis (DCA) indicated excellent clinical utility of the nomogram. The study innovatively used TyG/BMI to predict low ASMI, which can reduce the impact of obesity on the diagnosis of sarcopenia. The nomogram can be effectively used to screen for possible sarcopenia in community settings. Due to the cross-sectional study design and unable to obtain complete data on the assessment of muscle strength, the predictive efficacy of our nomogram model requires further confirmation through external validation by large, multicenter prospective studies on sarcopenia population.
Similar content being viewed by others
Introduction
With the global aging phenomenon, the prevalence of chronic diseases among the elderly has risen. Sarcopenia, a systemic, progressive skeletal muscle disease associated with aging, is characterized by the loss of skeletal muscle mass (SMM). It significantly increases the risk of adverse outcomes, including unintentional falls, fractures, serious physical disability, and mortality, profoundly impacting individuals’ health1. Studies have shown that individuals with sarcopenia have twice the risk of disability and all-cause mortality compared to those without2. Furthermore, research suggests that insulin resistance influences the development of sarcopenia3. The triglyceride-glucose index (TyG) has been proposed as a marker of insulin resistance and has shown correlation with the skeletal muscle mass index (SMI)4. Both the European Working Group on Sarcopenia in Older People 2018 (EWGSOP2) and the Asian Working Group for Sarcopenia 2019 (AWGS2019) have established similar thresholds for assessing and diagnosing sarcopenia in older populations, considering low muscle mass, muscle strength, and physical performance5,6.
As sarcopenia research advances, there is increasing recognition of the importance of early identification of individuals at risk and the implementation of multidomain lifestyle interventions to improve patient outcomes7. It is well established that a low appendicular skeletal muscle mass index (ASMI) is a key criterion for diagnosing sarcopenia. Modalities such as magnetic resonance imaging (MRI), computed tomography (CT), dual-energy X-ray absorptiometry (DXA), and bioelectrical impedance analysis (BIA) are used to assess ASMI5,6However, the high cost or radiation exposure associated with MRI, CT, and DXA limits their generalizability. Additionally, BIA’s accuracy can be influenced by factors like hydration status, food intake, and fat distribution, making ASMI measurement and sarcopenia screening challenging. Currently, calf circumference and the SARC-F scale are recommended for self-screening in the community, while grip strength and the 5-time chair stand test are recommended for assessing muscular strength and physical performance. However, despite the SARC-F scale’s good reliability, its low to moderate sensitivity and moderate to high specificity make it suboptimal for sarcopenia screening8,9 This underscores the importance of incorporating ASMI into community screening and diagnosing sarcopenia.
There is a growing body of evidence supporting the use of predictive nomogram models to predict sarcopenia in a straightforward manner10,11 However, due to the small number of participants and the complexity and subjectivity of the data required to construct the nomogram, practical and accessible methods for diagnosing sarcopenia, particularly for individuals with low ASMI, remain insufficient. Therefore, this study aimed to investigate the association between TyG/BMI and low ASMI, with the goal of constructing a predictive nomogram model that is easy to use in a clinical setting by inclusion of objective data from more participants, and evaluating its utility in middle-aged and elderly populations.
Materials and methods
Study population
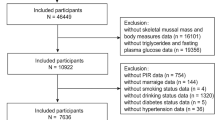
In this retrospective analysis, participants underwent BIA assessment and biochemical tests. Exclusion criteria comprised age < 45, acute infection, acute coronary syndrome and stroke, viral hepatitis and severe liver function abnormalities, hemodialysis for renal failure, diseases of the hematologic system, active rheumatic immune disease, hormone or antibiotic therapy, deformed or mutilated limbs, inability to cooperate with testing, and inadequate clinical information. The study included a total of 3,209 participants from Wuhan Union Hospital covering January 2020 to December 2022 (Fig. 1). The study fully complied with the Declaration of Helsinki and was approved by the ethics committee of Tongji Medical College, Huazhong University of Science and Technology (2024S045).
ASMI definition
Appendicular skeletal muscle mass (ASM) and ASMI (ASM (kg)/height (m2)) data were acquired using the BIA instrument (Inbody270, South Korea). According to the AWGS2019 recommended threshold values, ASMI below 7.0 kg/m2 in men and below 5.7 kg/m2in women are classified as low ASMI6.
Data collection
Basic demographic data, such as age, gender, height, weight, systolic blood pressure (SBP), diastolic blood pressure (DBP), smoking, and alcohol consumption, were recorded. Blood samples were collected from each participant during hospitalization after an overnight fast (Mindray BC-3000, China). Biochemical tests included white blood cell (WBC) count, red blood cell (RBC) count, platelet (PLT) count, hemoglobin (HB), fasting blood glucose (FBG), albumin (ALB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine (Cr), uric acid (UA), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C).
Calculation formula and population subgroups
BMI = weight [kg] / height [m2], and TyG = ln(TG [mg/dL]×FBG[mg/dL]/2)12. The Triglyceride-glucose-index to body-mass-index ratio (TyG/BMI) = ln(TG [mg/dL]×FBG[mg/dL]/2)÷ weight [kg] / height [m2]. TG: 1 mmol/L = 88.5 mg/dL and FBG: 1 mmol/L = 18 mg/dL. TG and FBG values converted were used to calculate TyG and TyG/BMI index.
(1) Participants were randomized into the Training Set (n = 2,407) and the Validation Set (n = 802) in a 3:1 ratio. (2) The Training Set was further divided into the Low ASMI/ASMI (L) group (n = 512) and the Normal ASMI/ASMI (N) group (n = 1,895). (3) Within the Training Set, participants were categorized into Q1-Q4 groups based on TyG/BMI value: Q1 (TyG/BMI ≤ 0.33, n = 602), Q2 (0.33 < TyG/BMI ≤ 0.37, n = 602), Q3 (0.37 < TyG/BMI ≤ 0.40, n = 602), and Q4 (TyG/BMI > 0.40, n = 601).
Statistical analysis
In this study, IBM SPSS Statistics software (version 25.0) was utilized for all analyses. The Shapiro-Wilk (S-W) test was employed to assess normality for continuous variables. Variables with P > 0.05 were deemed normally distributed and are presented as mean ± standard deviation (SD); otherwise, they are presented as median M (Q1-Q3). Two independent samples t-tests were applied for normally distributed data, while the Wilcoxon rank sum test was used for non-normally distributed data. Categorical variables were expressed as frequency (n) or percentage (%), and analyzed using chi-square tests or Fisher’s exact probability method. Logistic regression analysis was conducted to estimate the odds ratio (OR) and 95% confidence interval (CI) of low ASMI based on demographic data and biochemical tests. GraphPad Prism 9.5 software was employed for graphical representation. R 4.3.2 software was used to construct the nomogram, generate the receiver operating characteristic (ROC) curve, calculate the area under the ROC curve (AUC), and conduct decision curve analysis (DCA) to assess net benefits at different threshold probabilities. P values below 0.05 were considered statistically significant.
Results
Characteristics of the study population
A total of 3,209 subjects, aged 45–102 years with a mean age of 71.7 ± 5.6 years, were included in the study, comprising 2,132 males and 1,077 females. The incidence of low ASMI was 20.69% (664/3,209). Baseline data between the Training Set (n = 2,407) and the Validation Set (n = 802) were comparable, except for ASMI (P < 0.05) (Table 1). Compared to the normal ASMI group, the low ASMI group exhibited higher values of age, HDL-C, TyG/BMI, while lower values of ASMI, height, weight, body mass index (BMI), HB, ALB, UA, Cr, FBG, TG, ALT, AST, SBP, DBP, TyG, and proportion of males, smokers, and alcohol consumers (Fig. 2, Supplement 1). Significant differences were observed between the two groups in several parameters including ASMI, gender, age, height, weight, BMI, HB, ALB, UA, Cr, FBG, TG, HDL-C, ALT, AST, SBP, DBP, smoking status, alcohol consumption, TyG, and TyG/BMI (P < 0.05), while no significant differences were found in PLT, TC, and LDL-C (P > 0.05).
Risk factors associated with ASMI based on logistic regression analysis
In the Training Set, univariate logistic analysis was conducted to determine the risk factors for ASMI. Table 2 indicates that gender, age, height, weight, BMI, HB, ALB, UA, Cr, FBG, TG, HDL-C, ALT, SBP, DBP, smoking, alcohol consumption, TyG, and TyG/BMI were all significant risk factors for low ASMI (P < 0.01). Based on these results and the clinical significance of each variable, multivariate logistic analysis included 15 risk factors, revealing that gender, age, height, weight, TG, ALT, alcohol consumption, and TyG/BMI were independent variables for low ASMI. Specifically, being male, older age, greater height, alcohol consumption, and higher TyG/BMI were associated with an increased risk of low ASMI (P < 0.05).
Furthermore, multivariate logistic regression analysis was used to assess the association between TyG/BMI and low ASMI (Table 3). Model 1, adjusted for age, smoking, alcohol consumption, SBP, and DBP, demonstrated that the risk of low ASMI was significantly elevated in the Q3 and Q4 groups compared to the Q1 group (P < 0.001). However, the difference between the Q2 and Q1 groups was not significant (P > 0.05). In Model 2, which included additional adjustments for ALT, AST, ALB, HB, PLT, UA, Cr, TC, HDL-C, and LDL-C, the risk of low ASMI remained higher in the Q3 and Q4 groups compared to the Q1 group (P < 0.001), while the difference between the Q2 and Q1 groups was not significant (P > 0.05). This study suggests that individuals with a TyG/BMI value greater than 0.37 have a significantly increased risk of developing low ASMI.
Establishment, validation and evaluation of the predictive nomogram model
Gender, age, height, weight, TG, ALT, alcohol consumption, and TyG/BMI were utilized to construct a predictive nomogram model. The results revealed that being male, increasing age, greater height, lower weight, normal range TG and ALT levels, higher alcohol consumption, and elevated TyG/BMI were associated with an increased risk of low ASMI (Fig. 2). To assess the nomogram’s performance (Fig. 3), calibration curves were generated, demonstrating a strong fit between actual and predicted values across the 0 to 1.0 range, indicating robust calibration (Fig. 4A and B). For low ASMI, the AUC in the Training Set was 0.879 (95% CI: 0.860–0.899), with a sensitivity and specificity of 89.0% and 78.5%, respectively, and a Youden index of 0.68. In the Validation Set, the AUC was 0.881 (95% CI: 0.848–0.913), with a sensitivity of 85.4% and specificity of 81.4%, indicating excellent discrimination (Fig. 5A and B). DCA demonstrated that the net benefit probability ranged from 20 to 80% in the Training Set and from 10 to 90% in the Validation Set, suggesting that the nomogram could provide substantial benefits for predicting low ASMI (Fig. 6A and B).
Nomogram for predicting low ASMI. The nomogram uses patient gender, age, height, weight, alcohol consumption, ALT, TG, and TyG/BMI to calculate total scores, providing the probability of low ASMI. Explanation is as follows: (1) Find the height of the patient. (2) Draw a line on the “Points” axis. (3) Record the height-related score. (4) Repeated the process for other predictors. (5) Calculate and find the corresponding scores on the “Total Points” axis. Scores > 200 indicate a risk of low ASMI exceeding 90%.
Discussion
China is home to the world’s largest elderly population and is undergoing a transition to an aging society. A 2010 study conservatively estimated that over 50 million people worldwide are affected by sarcopenia, a number projected to reach 200 million in the next 40 years13. The direct costs of sarcopenia already amount to 1.5% of overall healthcare costs annually14. Sarcopenia not only prolongs hospital stays and increases the risk of postoperative complications but also predisposes individuals to frailty, mobility issues, and other chronic conditions15,16. Early identification of individuals at risk for sarcopenia and timely interventions such as physical activity or nutritional supplementation can effectively mitigate the risks of weakness, mobility issues, disability, and hospitalization, leading to potential cost savings in healthcare17. Sarcopenia’s incidence is increasing, and its prevalence rises with age as muscle mass diminishes, leading to weakness and mobility challenges18. Many individuals with undiagnosed mild-to-moderate sarcopenia attribute their low muscle strength to normal aging, overlooking the possibility of underlying disease and thus delaying seeking medical attention. This poses a significant barrier to early sarcopenia identification19. Currently, there is no fully standardized definition or diagnostic criteria for sarcopenia. In this study involving Chinese subjects, the Asian Working Group for Sarcopenia 2019 (AWGS2019) diagnostic criteria were applied. Given the difficulty of accurately assessing ASM and ASMI in community settings due to resource constraints, identifying an alternative index or model for low ASMI is crucial. This study offers a practical auxiliary diagnostic approach to address the limitations of current diagnostic criteria for possible sarcopenia in community settings.
The nomogram demonstrates a degree of generalizability. Data on demographics, laboratory tests, and ASMI from body composition analysis were gathered. Demographic information and laboratory tests can be readily conducted in community settings, facilitating the use of the nomogram in such environments. The study population was randomly divided into a Training Set and a Validation Set in a 3:1 ratio, with no significant differences observed between the populations in terms of various data points. Following logistic regression and clinical significance, eight risk factors for low ASMI were identified.
(1) Gender (male) and age.Studies have consistently shown a higher prevalence of sarcopenia in elderly men compared to women in Chinese communities20,21,22, while the evidence regarding gender differences in Western countries is controversial23. For hospitalized elderly adults, the prevalence was 29.7% in men and 23.0% in women, consistent with our study. We also found that the risk of sarcopenia in Hong Kong and Taiwan was almost half that of elderly men living on the mainland of China, although this difference was not significant in elderly women24. This underscores the importance of analyzing gender-specific differences in sarcopenia prevalence and risk factors among populations in mainland China, Taiwan, and Hong Kong. Age is a well-recognized risk factor for sarcopenia23and was associated with low ASMI in our study. Other studies have also found a significant association between sarcopenia and severe sarcopenia in Chinese elderly adults and advanced age25,26.
(2) Alcohol consumption.Our study identified alcohol consumption as a potential risk factor for low ASMI, aligning with a prior cross-sectional study on a Chinese elderly population27. However, another study suggested that alcohol consumption may not be directly associated with sarcopenia28. Therefore, further prospective studies are warranted to elucidate the relationship between alcohol consumption and the risk of sarcopenia.
(3) TG.The study revealed an association between lower TG levels and low ASMI. Previous research suggests that higher lipid metabolism levels within the normal range are protective against sarcopenia29. Conversely, lipid accumulation in and around muscle cells can lead to mitochondrial dysfunction, interference with fatty acid β-oxidation, and increased production of reactive oxygen species, resulting in lipotoxicity and insulin resistance30.
(4) ALT.Alanine aminotransferase is a crucial enzyme that plays a pivotal role in the intermediary metabolism of glucose and amino acids31. Our study demonstrated that ALT acts as a protective factor against sarcopenia, with higher levels of ALT within the normal physiological range being associated with a reduced risk of sarcopenia. Furthermore, prior studies also have indicated that low ALT levels are significantly correlated with decreased skeletal muscle mass (SMM), a higher prevalence of sarcopenia, and increased long-term mortality, particularly in elderly populations32,33.
(5) Height, weight, and TyG/BMI.In our study, we observed that height and weight may play a role in sarcopenia, as individuals with low ASMI tended to have higher height and lower weight. This relationship is often reflected in BMI. However, our multivariate logistic regression analysis indicated that BMI was not statistically significant. This could be due to the inclusion of height, weight, and TyG/BMI in the analysis, which may have affected the independent contribution of BMI. It is possible that BMI is less effective in discriminating low ASMI compared to height and weight alone, suggesting that some patients with sarcopenia may not be accurately identified using BMI alone. TyG is currently recognized as a reliable indicator of insulin resistance, and studies have shown a positive correlation between TyG and low SMI in the extremities4,34. Sarcopenic obesity, characterized by the coexistence of obesity and low muscle mass, is associated with glucose metabolism disorders3536This condition has more severe consequences and higher medical costs compared to sarcopenia or obesity alone37. A cross-sectional study found that the TyG index is associated with the risk of developing sarcopenic obesity38. ASM is influenced by body size, with larger individuals generally having greater muscle mass39. Therefore, relying solely on height, weight, or BMI to predict sarcopenia may exclude individuals with sarcopenic obesity, reducing the diagnostic accuracy of the model. Our study confirmed a significant positive correlation between TyG/BMI and low ASMI. Furthermore, our study innovatively used the TyG/BMI index to predict possible sarcopenia, effectively identifying patients with sarcopenic obesity.
A nomogram for assessing the risk of low ASMI was developed to facilitate early identification of sarcopenia risk by primary and community physicians, as well as to enhance understanding among older adults. The study included eight independent factors (gender, age, height, weight, TG, ALT, alcohol consumption, and the TyG/BMI ratio, which were analyzed using multivariate logistic regression. Results from the nomogram prediction model indicated that gender, age, and alcohol consumption had minimal impact and may not significantly contribute to the diagnosis of low ASMI. Height, TG, ALT, and TyG/BMI showed intermediate impacts, while weight had the greatest impact and likely contributed the most to the diagnosis. Several studies have developed nomogram prediction models for sarcopenia1011Our nomogram, however, specifically targets low ASM, a prerequisite for diagnosing sarcopenia, and is designed to be more accessible, easier to implement, and more generalizable. Moreover, the AUC of our nomogram is comparable to those of previous models10,11, indicating excellent accuracy in predicting low ASM.
Limitations. Nevertheless, our study has several limitations. Although TG levels and TyG/BMI were identified as predictors of low ASMI nomograms, lipid-lowering medications may have had an impact on the study results. Due to the cross-sectional study design, we were not able to obtain complete data of muscle strength, medications used, and learn about the MNA scores, dietary habits, physical activity, and others potential confounding of these low ASMI patients. While our nomogram can effectively identify and diagnose possible sarcopenia in the community, it cannot directly diagnose sarcopenia. It is based on cross-sectional data from an observational survey, limiting our ability to establish causality and only allowing us to determine associations. Therefore, the predictive efficacy of our nomogram model requires further validation and confirmation through external validation and large, multicenter prospective studies.
Conclusions
This study introduced an innovative approach by incorporating the TyG/BMI ratio. Gender (male), age, height, weight, TG, ALT, alcohol consumption, and TyG/BMI were identified as risk factors for low ASM and used to develop a nomogram. The analysis revealed that gender, age, and alcohol consumption made modest contributions to the model’s diagnostic ability, while height, TG, ALT, and TyG/BMI made substantial contributions, with weight contributing the most. These findings suggest that our study may offer an effective means of accurately screening for possible sarcopenia in community settings.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Larsson, L. et al. Sarcopenia: Aging-Related loss of muscle mass and function. Physiol. Rev. 99 (1), 427–511. https://doi.org/10.1152/physrev.00061.2017 (2019).
Kitamura, A. et al. Sarcopenia: prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J. Cachexia Sarcopenia Muscle. 12 (1), 30–38. https://doi.org/10.1002/jcsm.12651 (2021).
Cruz-Jentoft, A. J., Sayer, A. A. & Sarcopenia Lancet ;393(10191):2636–2646 doi: https://doi.org/10.1016/s0140-6736(19)31138-9. (2019).
Hu, W., Ma, Y. & Xing, D. Association of triglyceride-glucose index and the presence of low muscle mass in type 2 diabetes patients. Clin. Exp. Med. 23 (3), 943–949. https://doi.org/10.1007/s10238-022-00834-z (2023).
Cruz-Jentoft, A. J. et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 48 (1), 16–31. https://doi.org/10.1093/ageing/afy169 (2019).
Chen, L. K. et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 21 (3), 300–07e2. https://doi.org/10.1016/j.jamda.2019.12.012 (2020).
Lu, Y. et al. Assessment of sarcopenia among Community-Dwelling At-Risk frail adults aged 65 years and older who received multidomain lifestyle interventions: A secondary analysis of a randomized clinical trial. JAMA Netw. Open. 2 (10), e1913346. https://doi.org/10.1001/jamanetworkopen.2019.13346 (2019).
Lim, J. Y., Low, N. A. & Merchant, R. A. Prevalence of sarcopenia in pre-frail community dwelling older adult and utility of SARC-F, SARC-CalF and calf circumference in case finding. J. Frailty Sarcopenia Falls. 5 (3), 53–56. https://doi.org/10.22540/jfsf-05-053 (2020).
Voelker, S. N., Michalopoulos, N., Maier, A. B. & Reijnierse, E. M. Reliability and concurrent validity of the SARC-F and its modified versions: A systematic review and Meta-Analysis. J. Am. Med. Dir. Assoc. 22 (9), 1864–76e16. https://doi.org/10.1016/j.jamda.2021.05.011 (2021).
Mo, Y. H. et al. Development and validation of a nomogram for predicting sarcopenia in Community-Dwelling older adults. J. Am. Med. Dir. Assoc. 23 (5), 715–21e5. https://doi.org/10.1016/j.jamda.2021.11.023 (2022).
Huang, S. W. et al. A nomogram for optimizing sarcopenia screening in Community-dwelling older adults: AB3C model. J. Am. Med. Dir. Assoc. 24 (4), 497–503. https://doi.org/10.1016/j.jamda.2023.02.001 (2023).
Xing, Y. et al. Stronger associations of TyG index with diabetes than TyG-Obesity-Related parameters: more pronounced in young, Middle-Aged, and women. Diabetes Metab. Syndr. Obes. 16, 3795–3805. https://doi.org/10.2147/dmso.S433493 (2023).
Cruz-Jentoft, A. J. et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 39 (4), 412–423. https://doi.org/10.1093/ageing/afq034 (2010).
Veronese, N. et al. Sarcopenia reduces quality of life in the long-term: longitudinal analyses from the english longitudinal study of ageing. Eur. Geriatr. Med. 13 (3), 633–639. https://doi.org/10.1007/s41999-022-00627-3 (2022).
Norman, K. & Otten, L. Financial impact of sarcopenia or low muscle mass-A short review. Clin. Nutr. 38 (4), 1489–1495. https://doi.org/10.1016/j.clnu.2018.09.026 (2019).
DeVitoLM et al. Extending human healthspan and longevity: a symposium report. Ann. N Y Acad. Sci. 1507 (1), 70–83. https://doi.org/10.1111/nyas.14681 (2022).
Tagliafico, A. S., Bignotti, B., Torri, L. & Rossi, F. Sarcopenia: how to measure, when and why. Radiol. Med. 127 (3), 228–237. https://doi.org/10.1007/s11547-022-01450-3 (2022).
Senescence, S. Crime. Jama 323(8), 795. https://doi.org/10.1001/jama.2019.13323 (2020).
Cho, M. R., Lee, S. & Song, S. K. A review of sarcopenia pathophysiology, diagnosis, treatment and future direction. J. Korean Med. Sci. 37 (18), e146. https://doi.org/10.3346/jkms.2022.37.e146 (2022).
Du, Y. et al. Sex differences in the prevalence and adverse outcomes of sarcopenia and sarcopenic obesity in community dwelling elderly in East China using the AWGS criteria. BMC Endo Dis. 19 (1), 109. https://doi.org/10.1186/s12902-019-0432-x (2019).
Liu, X. et al. Prevalence of sarcopenia in multi ethnics adults and the association with cognitive impairment: findings from West-China health and aging trend study. BMC Geriatr. 20 (1), 63. https://doi.org/10.1186/s12877-020-1468-5 (2020).
Ren, X. et al. Prevalence of sarcopenia in Chinese community-dwelling elderly: a systematic review. BMC Public. Health. 22 (1), 1702. https://doi.org/10.1186/s12889-022-13909-z (2022).
Fielding, R. A. et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 12 (4), 249–256. https://doi.org/10.1016/j.jamda.2011.01.003 (2011).
Chen, Z., Li, W. Y., Ho, M. & Chau, P. H. The prevalence of sarcopenia in Chinese older adults: Meta-Analysis and Meta-Regression. Nutrients 13 (5). https://doi.org/10.3390/nu13051441 (2021).
Wu, C. H. et al. Prevalence and associated factors of sarcopenia and severe sarcopenia in older Taiwanese living in rural community: the Tianliao old people study 04. Geriatr. Gerontol. Int. 14 (Suppl 1), 69–75. https://doi.org/10.1111/ggi.12233 (2014).
Han, P. et al. Prevalence and factors associated with sarcopenia in Suburb-dwelling older Chinese using the Asian working group for sarcopenia definition. J. Gerontol. Biol. Sci. Med. Sci. 71 (4), 529–535. https://doi.org/10.1093/gerona/glv108 (2016).
Lee, J. S. et al. Associated factors and health impact of sarcopenia in older Chinese men and women: a cross-sectional study. Gerontology 53 (6), 404–410. https://doi.org/10.1159/000107355 (2007).
Wu, X. et al. Sarcopenia prevalence and associated factors among older Chinese population: findings from the China health and retirement longitudinal study. PLoS One. 16 (3), e0247617. https://doi.org/10.1371/journal.pone.0247617 (2021).
Xu, Z. et al. Single-cell RNA sequencing and lipidomics reveal cell and lipid dynamics of fat infiltration in skeletal muscle. J. Cachexia Sarcopenia Muscle. 12 (1), 109–129. https://doi.org/10.1002/jcsm.12643 (2021).
Li, C. W. et al. Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review. J. Cachexia Sarcopenia Muscle. 13 (2), 781–794. https://doi.org/10.1002/jcsm.12901 (2022).
Senior, J. R. Alanine aminotransferase: a clinical and regulatory tool for detecting liver injury-past, present, and future. Clin. Pharmacol. Ther. 92 (3), 332–339. https://doi.org/10.1038/clpt.2012.108 (2012).
Le Couteur, D. G. et al. The association of Alanine transaminase with aging, frailty, and mortality. J. Gerontol. Biol. Sci. Med. Sci. 65 (7), 712–717. https://doi.org/10.1093/gerona/glq082 (2010).
Uliel, N. et al. Low ALT, a marker of sarcopenia and frailty, is associated with shortened survival amongst myelodysplastic syndrome patients: A retrospective study. Med. (Baltim). 102 (17), e33659. https://doi.org/10.1097/md.0000000000033659 (2023).
Ahn, S. H., Lee, J. H. & Lee, J. W. Inverse association between triglyceride glucose index and muscle mass in Korean adults: 2008–2011 KNHANES. Lipids Health Dis. 19 (1), 243. https://doi.org/10.1186/s12944-020-01414-4 (2020).
Stenholm, S. et al. Sarcopenic obesity: definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care. 11 (6), 693–700. https://doi.org/10.1097/MCO.0b013e328312c37d (2008).
Khadra, D. et al. Association between sarcopenic obesity and higher risk of type 2 diabetes in adults: A systematic review and meta-analysis. World J. Diabetes. 10 (5), 311–323. https://doi.org/10.4239/wjd.v10.i5.311 (2019).
Ahmadi, B. et al. Multimorbidity: epidemiology and risk factors in the Golestan cohort study, Iran: A Cross-Sectional analysis. Med. (Baltim). 95 (7), e2756. https://doi.org/10.1097/md.0000000000002756 (2016).
Kim, B. et al. Triglyceride-Glucose index as a potential indicator of sarcopenic obesity in older people. Nutrients 15 (3). https://doi.org/10.3390/nu15030555 (2023).
Jimenez-Gutierrez, G. E. et al. Molecular mechanisms of inflammation in sarcopenia: diagnosis and therapeutic update. Cells 11 (15). https://doi.org/10.3390/cells11152359 (2022).
Acknowledgements
We thank all the participants for their contributions to the study.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors meet criteria for authorship as stated in the Uniform Requirements for Manuscripts Submitted to Biomedical Journals. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study fully complied with the Declaration of Helsinki and was approved by the ethics committee of Tongji Medical College, Huazhong University of Science and Technology (2024S045). The ethics committee of Tongji Medical College, Huazhong University of Science and Technology approved the request for a waiver of informed consent. Informed consent was not required because all medical data were retrospectively reviewed and analyzed anonymously. The participants’ information was kept anonymous, was noninvasive, and will not cause harm to them.
Competing interests
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zou, J., Zhou, N., Li, S. et al. A predictive nomogram based on triglyceride glucose index to body mass index ratio for low appendicular skeletal muscle mass. Sci Rep 15, 11366 (2025). https://doi.org/10.1038/s41598-025-94823-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94823-3