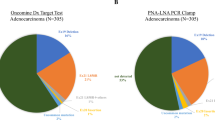
Abstract
Quickly identifying driver gene mutations in solid cancers is important. However, next-generation sequencing (NGS)-based mutation detection methods are time-consuming and expensive. Peptide nucleic acid (PNA) probe-based mutant mRNA detection systems are quick and inexpensive. We previously demonstrated that epidermal growth factor receptor (EGFR)-mutations were efficiently visualized in formalin-fixed paraffin-embedded (FFPE) specimens from transplanted non-small cell lung cancer (NSCLC) tumors using an EGFR mutation-specific PNA:DNA probe. Herein, the efficiency of PNA:DNA probes in detecting EGFR-mutations in FFPE specimens from patients with NSCLC and the colocalization of EGFR-mutations with tumor-infiltrating lymphocyte (TIL) status were determined. The EGFR mutation L858R-specific PNA:DNA probe detected heterogeneously localized mutations with a sensitivity similar to detection with the anti-L858R antibody. TIL analysis of L858R-mutated tumors revealed that CD8+PD-1+ T cells and CD68+ macrophages were scarce in tumors, but in the cytokeratin-positive intra-tumoral regions, CD4+, FoxP3+, and CD204+ cells tended to be more abundant in the L858R-positive tumor area than in the negative area. Thus, PNA:DNA probes specific for EGFR-mutations can detect areas with heterogeneous EGFR mutants in whole cancer tissues and can be used to evaluate the mutation-associated TIL status in EGFR-mutant cancer tissues.
Similar content being viewed by others
Introduction
A peptide nucleic acid (PNA)-based mutant mRNA detection system was developed by Nielsen et al., prompting many trials applying PNA probes to the molecular genetics field1,2,3,4,5. In addition to the speed and low-cost, many advantages of the PNA technology have been demonstrated, including high sensitivity and specificity for hybridization to complementary DNA or RNA, single base mismatch discrimination, highly stable binding to DNA or RNA due to the higher melting temperature, and resistance to nuclease digestion and low ionic strength conditions due to the uncharged backbone6,7,8.
Progress in next-generation sequencing technology has enabled us to make genetic diagnostic tools, such as target sequencing panel and cell-free DNA detection methods 9,10,11. The development of genetic diagnostics, including the diagnosis of epidermal growth factor receptor (EGFR), RAS, ROS1, and ALK gene mutations, allows the selection of suitable therapies for patients with non-small cell lung cancers (NSCLC) 12,13,14. Although NGS technology is accurate and covers a wide variety of target genes, the technology is time-consuming and expensive compared to conventional genetic mutation detection technologies15,16,17.
In the present study, we developed a system for detecting EGFR-mutations based on EGFR mutation-specific PNA:DNA probes. This system was successfully applied to the detection of EGFR-mutant tumors in formalin-fixed paraffin-embedded (FFPE) sections of tumors from nude mice transplanted with EGFR-mutant tumor cells18,19. The objective of the present study was to determine the detection efficiency of PNA:DNA probes for EGFR-mutations and the association of the localization of EGFR-mutations with tumor-infiltrating lymphocyte (TIL) status in patients with EGFR-mutant NSCLC.
Since the initiation of the Project High-tech Omics-based Patient Evaluation (HOPE) in 2014, more than 10,000 cancer patients have been enrolled. Cancer genomic profiling was performed in these patients using whole-exome sequencing (WES) and gene expression profiling (GEP). FFPE specimens from patients with EGFR-positive NSCLC in the Project HOPE were included in the present study.
Results
Clinical characteristics of 31 NSCLC patients enrolled in the project HOPE
The characteristics of the 31 NSCLC patients enrolled in the study are shown in Table 1. All patients were enrolled in the HOPE project, and EGFR-mutations were subjected to WES analysis. Twenty cases had EGFR gene mutations and 11 patients had no mutations. The mutations included 11 cases with L858R mutations and 9 cases with exon19 deletion mutations. No significant differences in age, tumor content, variant allele frequency (VAF), and survival time were detected between the mutation types, except that the EGFR gene-mutated cases included more female patients.
Measuring cytokeratin and EGFR mutation-positive area using PNA:DNA probes
The immunofluorescence staining set included PNA, anti-cytokeratin antibody, and antibodies for TIL-staining, as shown in Table 2. Cytokeratin-positive areas indicate intra-tumoral regions and cytokeratin-negative areas indicate extra-tumoral or stromal regions. No differences in the ratios of cytokeratin-positive areas were detected between the L858R mutation, exon 19 deletion mutation, and no mutation groups (Fig. 1A). EGFR-mutated tumors exhibited positive staining with the PNA:DNA probe (19.5% for L858R mutation probe and 8.6% for exon 19 deletion mutation probe). In contrast, the tumors without EGFR-mutations showed lower positive rate for both PNA:DNA probe staining (Fig. 1B and Fig. 2). The L858R probe exhibited more positive staining than the exon 19 deletion probe.
Mutation-specific peptide nucleic acid (PNA)-positive staining in epidermal growth factor receptor (EGFR) mutation-positive lung cancer tissues. Formalin-fixed paraffin-embedded (FFPE) tissues from patients with non-small cell lung cancer were stained with EGFR mutation-specific PNA probes, anti-cytokeratin antibodies, and DAPI. PNA-positive staining rates in cytokeratin-stained cancer tissues were determined. (A) Cytokeratin-positive rate in DAPI-stained cancer tissues from the EGFR-mutated (L858R or exon19-deletion) and no mutation tumor groups. (B) EGFR mutation probe positive rates in cytokeratin-stained cancer tissues from the EGFR-mutated (L858R or exon19-deletion) and no mutation tumor groups. The positive staining rate for each EGFR mutation-specific PNA probe in the EGFR-mutated (L858R or exon19-deletion) and no mutation tumor groups were compared using the Mann–Whitney U test. **P < 0.01.
PNA:DNA probe efficacy for EGFR mutation detection
Receiver operating characteristic (ROC) curve analysis revealed that the L858R probe was more sensitive and specific than the exon 19 deletion probe in detecting tumors with EGFR-mutations (Fig. 3A, B).
A receiver operating characteristic (ROC) curve analysis and comparison of the efficacy of EGFR mutation-specific PNA probes. (A) ROC curve for PNA probe positivity (%) in L858R-mutated and exon19 deletion-mutated tumor groups. The area under the curve for each plot indicates the performance of the L858R-specific (0.97; 95% CI, 0.91–1) and exon19 deletion-specific (0.84; 95% CI, 0.64–1) PNA probes. (B) Comparison of the PNA probe efficacy between the EGFR mutation-positive and EGFR mutation-negative tumor groups. L858R mutation-positive tumor group (n = 11), exon19 deletion mutation-positive tumor group (n = 9), and no EGFR mutation group (n = 11).
Comparison of TIL status between L858R mutation-positive tumors and no EGFR mutation tumors
TIL numbers, such as CD4+, CD8+, and CD68+ cells, tended to be lower in the intra-tumoral regions in both the L858R mutation and no mutation group compared to the TIL numbers in the extra-tumoral regions. However, TIL cells were higher in the extra-tumoral region in the L858R mutation group compared with the no mutation group (Fig. 4, Supplementary Fig. S1, S2 and S3). PD-L1+, FoxP3+, and CD204+ cells tended to increase in the intra-tumoral region in the L858R mutation group.
Comparison of the tumor-infiltrating lymphocyte (TIL) status between the L858R mutation-positive and no EGFR mutation tumor groups. Comparison of TIL numbers in cytokeratin-positive intra-tumoral region and cytokeratin-negative extra-tumoral region between L858R mutation-positive tumors and no EGFR mutation tumors. Immune cells, including CD8+PD-1+ T cells, CD4+FoxP3+ T cells, and CD68+ or CD204+ macrophages, were counted using an in-house automatic counting system. The positive immune cell numbers per field in the EGFR-mutated (L858R) and no mutation tumor groups were compared using the Mann–Whitney U test. *P < 0.05, **P < 0.01.
Association of TIL status in L858R mutation-positive tumors with overall survival
The higher number of CD204+ immune cells (macrophages) in the extra-tumoral region in the L858R mutated tumors tended to correlate with poor survival (Supplementary Fig. S4) (r = − 0.519), but no significant correlation was found between CD204+ cell numbers and overall survival. The other TIL numbers did not show any association with overall survival time.
Comparison of TIL status between PNA-positive area and PNA-negative area in the intra-tumoral region of L858R mutated tumors
PD-L1 expression levels tended to decrease in PNA-positive areas compared with PNA-negative areas of the intra-tumoral regions, but no significant difference in PD-L1 expression level was found between PNA-positive areas and PNA-negative areas (Fig. 5). In contrast, CD4+, FoxP3+, and CD204+ cells were more abundant in the L858R-positive tumor areas compared with the negative areas of the intra-tumoral regions in L858R mutated tumors.
Comparison of the tumor-infiltrating lymphocyte (TIL) status between the PNA-positive and PNA-negative areas in the intra-tumoral region of L858R-mutated tumors. The cytokeratin-positive intra-tumoral region (CK +) was classified into PNA-positive (PNA +) area and PNA-negative (PNA −) areas based on the L858R mutation-specific PNA probe staining results. To correctly compare TIL numbers between the PNA-positive and the PNA-negative areas, the immune cell numbers were normalized by dividing the actual immune cell numbers by the ratio of the PNA-positive or PNA-negative stained areas (normalized positive cell number per area). The positive cell numbers per area were compared between the PNA-positive and the PNA-negative areas using the Mann–Whitney U test.
Association of intra-tumoral immune cell status between PNA-positive area and PNA-negative area with overall survival
In the intra-tumoral regions of the L858R-mutated tumors, CD204+ cell numbers in PNA-negative areas were significantly and negatively associated with overall survival (r = − 0.6292) (Fig. 6). In addition, no significant correlation was found between PD-L1+ cell numbers in PNA-positive areas of intra-tumoral regions and overall survival (r = − 0.5154).
Association of intra-tumoral immune cell status between the PNA-positive and PNA-negative areas and overall survival. Immune or tumor cells, such as PD-L1+ cells, CD68+ cells, and CD204+ cells, were counted using an automatic counting system. The positive cell numbers per field were obtained from the EGFR-mutated (L858R) tumor group and classified into cytokeratin-positive intra-tumoral and cytokeratin-negative extra-tumoral regions. Correlation analysis of the normalized positive cell numbers per area with the overall survival time was performed using Pearson correlation test. *P < 0.05 was considered statistically significant.
Discussion
NSCLCs with EGFR-mutations exhibit low PD-L1 expression, TILs, and TMB status. Consequently, these tumors respond poorly to anti-PD-1/PD-L1 blockade and respond better to combination therapy containing cisplatin and EGFR-tyrosine kinase inhibitors (TKIs)20,21,22,23,24. However, the immunological tumor microenvironment (TME) status can change. Dynamic changes in biomarkers and immune cell markers occur after EGFR-TKI treatment. Increased PD-L1 expression and decreased FOXp3+ regulatory T cell number have been detected after EGFR-TKI treatment25,26,27,28. In some cases, the TMB number tended to increase after EGFR-TKI therapy28. Based on these changes in the immunological TME status, anti-PD-1 blockade therapy after EGFR-TKI treatment may be beneficial. Haratani et al. reported that patients with T790M-negative EGFR-mutated NSCLC are more likely to benefit from anti-PD-1 blockade after EGFR-TKI treatment than patients with T790M-positive tumors29.
The recovery of immunological parameters in EGFR-mutated tumors after EGFR-TKI therapy depends on the reduction of EGFR mutation-positive tumor clones. Therefore, rapid and efficient tools such as our PNA probe are very useful. In addition, we demonstrated that heterogeneous immune cells localize between PNA-positive and PNA-negative areas of intra-tumoral regions in L858R mutated tumors. Our PNA probe may be useful for evaluating the TIL status, according to the heterogeneous location of the EGFR mutation in the intra-tumoral region.
The development of other PNA-based EGFR mutation detection methods has been reported, including real-time PCR clamping technology30,31. In a study by Kim et al., genomic EGFR-mutations are detected in 34.6% of non-squamous NSCLC patients using DNA extracted from FFPE specimens, whereas DNA direct sequencing showed a positive rate of 26.3%31. These results indicate that PCR clamping is more effective than DNA sequencing in detecting EGFR-mutations. The sensitivity of our PNA probe for L858R EGFR-mutations was 100% in 11 cases harboring EGFR mutation verified by WES sequencing. This result indicates that the efficiency of the PNA probe is comparable to direct DNA sequencing.
Intra-tumoral genomic heterogeneity is a crucial problem in the clinical field. Various heterogeneous patterns of genomic differences have been detected, including the differences between primary and metastasis lesions32 and the spatiotemporal differences in multiple biopsies33,34. For example, after acquired resistance to the EGFR-TKI against T790M, many genomic mutations, local copy number amplifications, chromosomal instability, and neuroendocrine differentiation are acquired, resulting in the development of osimertinib resistance35,36. We demonstrated that heterogeneous immune cell localization and PD-L1 expression in the PNA-positive and PNA-negative areas of intra-tumoral regions in L858R-mutated tumors can be evaluated. These results suggest that PNA:DNA probes specific for EGFR-mutations can be a useful tool for detecting areas with heterogeneous EGFR-mutations.
Although our results are encouraging, several technical problems and limitations need to be solved. The sensitivity of the PNA probe specific for the L858R mutation is comparable to the sensitivity of direct DNA sequencing, but the PNA probe specific for the exon19-deletion mutation is less sensitive. Thus, the development of a better PNA probe for the exon19-deletion mutation is urgently needed. In addition, the present study had potential biases, including the use of FFPE specimens, the small sample size, and limited data from only one database.
Despite these limitations, we verified the detection efficiency of our PNA:DNA probes and the association of EGFR mutation localization with TIL status and prognosis using FFPE specimens from NSCLC patients with EGFR-mutations. These results suggest that PNA:DNA probes specific for EGFR-mutations are promising tools for detecting tumor heterogeneity and TIL status before and after EGFR-TKI treatment.
Conclusion
In summary, our PNA:DNA probe specific for EGFR L858R mutation detection exhibited a sensitivity of 100% and a specificity of 90% in clinical NSCLC FFPE specimens. Thus, the efficacy of the L858R mutation-specific PNA probe is comparable to the efficacy of direct DNA sequencing. The PNA:DNA probes specific for EGFR-mutations can be useful tools for evaluating tumor heterogeneity and TIL status to facilitate treatment decision-making.
Materials and methods
Ethical statement
All protocols in the study were approved by the Institutional Review Board of the Shizuoka Cancer Center (Authorization Number: 26-62). Written informed consent was obtained from all study participants. All experiments using clinical samples were conducted in accordance with the approved guidelines. This study was conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Human Genome and Genetic Analysis Research.
Patient characteristics
Eighty-one non-small cell lung cancer patients, who were enrolled in the cancer genome project named HOPE at Shizuoka Cancer Center in 2014, were screened for the study. The enrolled patients included 20 cases with EGFR gene mutations (9 Exon19 deletion cases and 11 L858R cases) and 11 cases with no EGFR-mutations. FFPE specimens derived from the 31 NSCLC patients were obtained and used for PNA:DNA probe staining experiments specific for EGFR-mutations.
DNA microarray-based GEP and WES using next-generation sequencing
GEP and WES analyses were described previously37. Briefly, the ratio of the expression intensity between the tumor tissue and the surrounding normal tissue was calculated from normalized values for GEP. In WES, all variants called by the variant caller were filtered using the following parameters: quality < 30, VAF < 10%, and depth of coverage < 20× . WES was performed using a NextSeq 6000 System (Illumina, San Diego, CA, USA), and transcriptome profiling was performed using a SurePrint G3 Human Gene Expression 8 × 60K v2 Microarray (Agilent Technologies, Canta Clara, CA, USA).
Variant calling and annotation
Small variants included small nucleotide variants (SNVs) and indels (≤ 50 bp), and SVs were greater than 50 bp. For SNVs and indel mutations, somatic and germline variants were identified using the DRAGEN Somatic Variant Caller and Small Variant Caller (Illumina), respectively. Variants with a low sequence depth in normal and/or tumor tissues, low variant depth, and/or low VAF in tumors were discarded from the downstream analysis. Variants were annotated using Ensembl Variant Effect Predictor ver. 104. The drivability of the variants was analyzed using an in-house pipeline.
PNA:DNA probes specific for EGFR mutations
The development of PNA:DNA probes specific for the EGFR mutant mRNA sequences were previously reported18. Briefly, for making L858R mutation probe, fluorescein isothiocyanate (FITC)-conjugated PNA (FITC-PNA; fluorescein-O-Linker-PNA (TTTGGCCCGCCCAA)-k-k; Panagene, Daejeon, Korea) and quencher-conjugated DNA (Q-DNA; GCGGGCCA-Dabcyl; Integrated DNA Technologies, Coralville, IA, USA) were prepared. PNA:DNA probes were constructed by hybridizing the synthesized FITC-PNA and Q-DNA. The probes used in the present study were designed specifically for L858R and del E746-A750 EGFR-mutations.
Immunofluorescence staining using a multicolor staining kit
The following antibody were used for immunostaining: anti-human cytokeratin antibody (AE1 and AE3, Nichirei, Tokyo, Japan), anti-human CD4 antibody (4B12, Thermo Fisher Scientific, Waltham, MA, USA), anti-CD8 antibody (C8/144b, GeneTex Inc., Irvine, CA, USA), anti-human PD-1 antibody (NAT105, Abcam, Cambridge, UK), anti-human PD-L1 antibody (28-8, Abcam), anti-human FoxP3 antibody (236A/E7, Abcam), and-human CD68 antibody (3A9A7, Proteintech Inc., Rosemont, IL), and anti-human CD204 antibody (KT118, Transgenic Inc., Kobe, Japan). The immunofluorescence staining set is shown in Table 2. Staining was performed using the Opal 7-color IHC kit (Akoya Biosciences, Inc., Menlo Park, California, USA) and a Zeiss Imager Z1 fluorescence microscope equipped with the ZEN software system (Carl Zeiss, Oberkochen, Germany) as reported previously19. Briefly, after deparaffinization and hydration of FFPE sections, activation by autoclaving in citrate buffer (pH 6.0) was performed. After refixation with formaldehyde, sections were blocked with serum-free DAKO protein block reagent (Agilent Technologies, Santa Clara, CA, USA). Endogenous peroxidase was quenched with 3% H2O2/MeOH. After incubation with primary and secondary antibodies, fluorescence reaction was carried out using an Opal IHC kit. Next, PNA:DNA coupling was carried out and used for probe staining.
Establishment of the EGFR mutation detection and TIL automatic counting system
The method for measuring the cytokeratin and PNA probe positive area and TIL numbers was shown previously38. In brief, five of 10 acquired images were selected using a random table. WinROOF (Mitani Shoji, Tokyo, Japan)38, ImageJ (ver.1.53g58), and R software (ver. 3.4.0) were used to measure the fluorescence of positively-stained areas and for the TIL automatic counting system.
Statistical analysis
Positive staining rate and TIL numbers were compared using the Mann–Whitney U test. Correlation analysis of the positive staining rate of the PNA:DNA probe and TIL staining with other parameters was performed using Pearson correlation test. ROC curve analysis was used for a statistical analysis of PNA probe positivity for EGFR mutations. P < 0.05 were considered statistically significant.
Data availability
The datasets generated and/or analysed during the current study are available in the NBDC Human Database repository (https://humandbs.dbcls.jp, Accession number JGAS000274).
References
Pellestor, F. & Paulasova, P. The peptide nucleic acids (PNAs), powerful tools for molecular genetics and cytogenetics. Eur. J. Hum. Genet. 12, 694–700 (2004).
Vilaivan, T. Fluorogenic PNA probes. Beilstein J. Org. Chem. 14, 253–281 (2018).
Sadamoto, S. et al. Histopathological study on the prevalence of trichosporonosis in formalin-fixed and paraffin-embedded tissue autopsy sections by in situ hybridization with peptide nucleic acid probe. Med. Mycol. 58, 460–468 (2020).
Nielsen, P. E. PNA technology. Mol. Biotechnol. 26, 233–248 (2004).
Pellestor, F. & Paulasova, P. The peptide nucleic acids, efficient tools for molecular diagnosis (review). Int. J. Mol. Med. 13, 521–525 (2004).
Nielsen, P. E., Egholm, M., Berg, R. H. & Buchardt, O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 254, 1497–1500 (1991).
Paulasova, P. & Pellestor, F. P. The peptide nucleic acid (PNAs): a new generation of probes for genetic and cytogenetic analyses. Ann. Genet. 47, 349–358 (2004).
Ray, A. & Nordén, B. Peptide nucleic acid (PNA): its medical and biotechnical applications and promise for the future. FASEB J. 14, 1041–1060 (2000).
Abate, R. E. et al. Next generation sequencing-based profiling of cell free DNA in patients with advanced non-small cell lung cancer: advantages and pitfalls. Cancers 12, 3804. https://doi.org/10.3390/cancers12123804 (2020).
Wu, T. M. et al. Power and promise of next-generation sequencing in liquid biopsies and cancer control. Cancer Control 27, 1073274820934805. https://doi.org/10.1177/1073274820934805 (2020).
Vestergaard, L. K., Oliveira, D. N. P., Høgdall, C. K. & Høgdall, E. V. Next generation sequencing technology in the clinic and its challenges. Cancers 13, 1751. https://doi.org/10.3390/cancers13081751 (2021).
Nong, L. et al. Comparison of next-generation sequencing and immunohistochemistry analysis for targeted therapy-related genomic status in lung cancer patients. J. Thorac. Dis. 254, 4992–5003 (2019).
Lee, J. et al. Landscape of EGFR mutations in lung adenocarcinoma: a single institute experience with comparison of PANAMutyper testing and targeted next-generation sequencing. J. Pathol. Transl. Med. 56, 249–259 (2022).
Casula, M. et al. Comparison between three different techniques for the detection of EGFR mutations in liquid biopsies of patients with advanced stage lung adenocarcinoma. Int. J. Mol. Sci. 24, 6410. https://doi.org/10.3390/ijms24076410 (2023).
Kang, S., Woo, J. & Kim, S. A systematic review of comparison diagnostic tests by immunohistochemistry for the screening of Alectinib-treated patients in ALK-positive non-small cell lung cancer. Diagnostics 12, 1297. https://doi.org/10.3390/diagnostics12051297 (2022).
Tsao, M. S. & Yatabe, Y. Old soldiers never die: is there still a role for immunohistochemistry in the era of next-generation sequencing panel testing?. J. Thorac. Oncol. 14, 2035–2038 (2019).
Fan, X. et al. Comparison detection methods for EGFR in formalin-fixed paraffin-embedded tissues of patients with NSCLC. Pathol. Res. Pract. 216, 152783. https://doi.org/10.1016/j.prp2019.152783 (2020).
Shigeto, H., Ohtsuki, T., Iizuka, A., Akiyama, Y. & Yamamura, S. Imaging analysis of EGFR mutated cancer cells using peptide nucleic acid (PNA)-DNA probes. Analyst 144, 4613–4621 (2019).
Shigeto, H. et al. Localization of EGFR mutations in non-small-cell lung cancer tissues using mutation-specific PNA-DNA probes. Cancer Genom. Proteom. 20, 375–382 (2023).
Le, X. et al. Characterization of the immune landscape of EGFR-mutant NSCLC identifies CD73/adenosine pathway as a potential therapeutic target. J. Thorac. Oncol. 16, 583–600 (2021).
To, K. K. W., Fong, W. & Cho, W. C. S. Immunotherapy in treating EGFR-mutant lung cancer: current challenges and new strategies. Front. Oncol. 11, 635007. https://doi.org/10.3389/fonc.2021.635007 (2021).
Bai, Y. et al. PD-L1 expression and its effect on clinical outcomes of EGFR-mutant NSCLC patients treated with EGFR-TKIs. Cancer Biol. Med. 15, 434–442 (2018).
Wu, Q. et al. First-generation EGFR-TKI plus chemotherapy versus EGFR-TKI alone as first-line treatment in advanced NSCLC with EGFR activating mutation: a systematic review and meta-analysis of randomized controlled trials. Front. Oncol. 11, 598265. https://doi.org/10.3389/fonc.2021.598265 (2021).
Seong, H., Kim, S. H., Kim, M. H., Kim, J. & Eom, J. S. Additional local therapy before disease progression for EGFR-mutated advanced lung cancer: a systematic review and meta-analysis. Transl. Lung Cancer Res. 13, 491–502 (2024).
Toki, M. et al. Immune marker profiling and programmed death ligand 1 expression across NSCLC mutations. J. Thorac. Oncol. 13, 1884–1896 (2018).
Biton, J. et al. TP53, STK11, and EGFR mutations predict tumor immune profile and the response to anti-PD-1 in lung adenocarcinoma. Clin. Cancer Res. 24, 5710–5723 (2018).
Miyazaki, A. et al. Durable response of pembrolizmab for EGFR mutation-positive lung adenocarcinoma with early progression to osimertinib in first-line treatment. Intern. Med. https://doi.org/10.2169/internalmedicine.3784-24 (2024).
Isomoto, K. et al. Impact of EGFR-TKI treatment on the tumor immune microenvironment in EGFR mutation-positive non-small cell lung cancer. Clin. Cancer Res. 26, 2037–2046 (2020).
Haratani, K. et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann. Oncol. 28, 1532–1539 (2017).
Kim, H. J. et al. Detection and comparison of peptide nucleic acid-mediated real-time polymerase chain reaction clamping and direct gene sequencing for epidermal growth factor receptor mutations in patients with non-small cell lung cancer. Lung Cancer 75, 321–325 (2012).
Chen, Y. L. et al. Clinical implications of PNA-sequencing as a complementary test for EGFR mutation analysis in human lung cancer. Oncol. Lett. 26, 539. https://doi.org/10.3892/ol.2023.14126 (2023).
Shimizu, K. et al. Heterogeneity of the EGFR mutation status between the primary tumor and metastatic lymph node and the sensitivity to EGFR tyrosine kinase inhibitor in non-small cell lung cancer. Target Oncol. 8, 237–242 (2013).
Kobayashi, K. & Tan, A. C. Unraveling the impact of intratumoral heterogeneity on EGFR tyrosine kinase inhibitor resistance in EGFR-mutated NSCLC. Int. J. Mol. Sci. 24, 4126. https://doi.org/10.3390/ijms24044126 (2023).
Chabon, J. J. et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanism in lung cancer patients. Nat. Commun. 7, 11815. https://doi.org/10.1038/ncomms11815 (2016).
Hata, A. et al. Spatiotemporal T790M heterogeneity in individual patients with EGFR-mutant non-small-cell lung cancer after acquired resistance to EGFR-TKI. J. Thorac. Oncol. 10, 1553–1559 (2015).
Roper, N. et al. Clonal evolution and heterogeneity of osimertinib acquired resistance mechanisms in EGFR mutant lung cancer. Cell Rep. Med. 1, 100007. https://doi.org/10.1016/j.xcrm.2020.100007 (2020).
Nagashima, T. et al. Japanese version of The Cancer Genome Atlas, JCGA, established using fresh frozen tumors obtained from 5143 cancer patients. Cancer Sci. 111, 687–699 (2020).
Miyata, H. et al. Development of an automatic measurement method for CD8 and PD-1 positive T cells using image analysis software. Anticancer Res. 42, 419–427 (2022).
Author information
Authors and Affiliations
Contributions
H.M. organized all experiments and wrote the manuscript. Y.A., H.S., S.Y. performed planning, design, and supervision of experiments. Y.O., M.I. contributed to sample collection. T.S. performed pathological evaluation. Y.S., T.N. K.U. participated in whole genome sequencing. K.O. participated in transcriptome analysis. T.I., T.A., A.K. performed immunological experiments. A.I., Y.K., C.M., Ka.Y., K.M., H.K., T.T., and Ke.Y. were involved in data analysis and technical assistance. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Miyata, H., Shigeto, H., Ikeya, T. et al. Localization of epidermal growth factor receptor-mutations using PNA:DNA probes in clinical specimens from patients with non-small cell lung cancer. Sci Rep 15, 11314 (2025). https://doi.org/10.1038/s41598-025-95081-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-95081-z