Abstract
Contaminated environments can pose new challenges when new contaminants appear and can select organisms with new genetic and metabolic strategies. The increased presence of Te(IV) in the environment is becoming more important. This highlights how underexplored the investigation of how bacteria molecularly respond to less common environmental contaminants, such as tellurite when compared to other metals/ metalloids. Understanding what tools an organism uses from its genetic pool when responding to a new contaminant requires a multiple-technique approach, such as metabolic tests and differential omics analysis. These analyses provide a full metabolic and phenotypical map of stress response that can include new resistance mechanisms, whether specific or not. This study aimed to determine if Bacillus altitudinis strain 3W19, isolated from a Te(IV) contaminated site, presents specific changes at the proteomic level when exposed to the metalloid. In strain 3W19, growth in the presence of Te(IV) upregulated pathways of amino acid metabolism and membrane transport and downregulated pathways of carbohydrate metabolism. Growth in the presence of Te(IV) also induced the formation of reactive oxygen species and lowered the metabolic activity of the strain. This metal led to the overexpression of the proteins of the ter gene cluster. When compared with other strains, the ter system identified in this strain differed in genomic organization from related Bacillus sp. strains. Together, these strain-specificities can contribute to understanding its Te(IV) resistance phenotype.
Similar content being viewed by others
Introduction
Understanding the impact of metalloids on bacterial metabolism is a research field with potential applications in environmental restoration or manufacturing for high-tech sectors. One of the metalloids with increasing interest is tellurium (Te) and one with growing concerns for its environmental impacts. Te is a scarce element, amongst the least abundant on Earth’s crust1, with growing demand across industrial sectors, such as photovoltaic, thermoelectric production and metallurgy, in iron and lead alloys2. The increased demand for Te led to the increase of soluble forms of this metalloid in natural habitats3. Research on the biochemical aspects of microorganisms in the presence of Te or its ions is relatively scarce when compared to elements of the same chemical group. The limited literature shows that Te oxyanions possess toxicity similar to the most toxic heavy metals in bacteria and other microorganisms4. Most of the early research on Te-Bacteria interaction focused on Te(IV) and Te(VI) toxicity and resistance5, with a higher focus on the most bioavailable form Te(IV). Considering the high toxicity of tellurium ions to living organisms, it is highly relevant to explore the detoxification mechanisms of Te(IV) resistant organisms for the development of bioremediation strategies.
Comparative genomics gives new perspectives on genetic-based mechanisms of Te(IV) resistance by revealing new genetic elements, homologues for known genes, or new genetic organizations of known Te(IV) resistance genetic elements. Among several known genes involved in Te(IV) resistance, the mechanisms encoded by the terZABCDEF operon are the most studied and well-described. In this operon, proteins encoded by genes terC, terD and terE were identified to be directly responsible for the resistance of Escherichia coli4 of up to 1024 mg ml− 1 Te(IV). In Pseudomonas citronellolis SJTE-3, the terC and terD genes are the minimal functional combination for resistance and reduction6. Previous authors reported that E. coli containing a plasmid with the ter operon had more membrane-bound Te deposits and increased resistance to Te(IV) compared to the same strain without that plasmid7. This suggests that the resistance conferred by the ter gene cluster is linked to the reduction of Te(IV) to Te(0). Identifying new bacterial mechanisms for metal resistance, or the metabolic byproducts responsible for biotransformation, can provide insights for removing toxics from the environment, which is crucial for bioremediation purposes.
A previous work on Bacillus altitudinis strain B1S54.2 3W19, a Te(IV) resistant strain able to reduce Te(IV) efficiently forming Te-containing particles, isolated from a multi-metal contaminated site8 and used in the current study, identified two genes encoding TerD family proteins and one gene encoding a TerC family protein9. In recent years, new proteomic studies have focused on the impact of tellurium in strains of the genera Shewanella and Bacillus or Escherichia. In Shewanella sp. strain CNZ-1, in the presence of Te(IV), an increase of the proteins belonging to the type II secretion system, phage shock proteins, general secretion pathway and transportation system were observed10. In Bacillus sp. strain Y3, the sulfate metabolism and putative thioredoxin were closely associated with Se(IV) and Te(IV) reduction11. In E. coli BL21 carrying a Te(IV) resistance gene determinant, high Te(IV) concentrations induced a several-fold change of DnaK and GroEL proteins but not of proteins from the ter operon12.
The current study aimed to determine if B. altitudinis strain 3W19 possesses unique genetic potential for tellurite resistance and undergoes specific changes at the proteomic level when exposed to the metalloid. To achieve this objective, the strain’s metabolic response to growth in the presence of Te(IV) and the underlying cellular responses were studied and reported on a whole cell perspective. This strain was selected after demonstrating resistance to high concentrations of tellurium while, at the same time, being able to reduce 0.75 mg Te(IV) h-1. A first genetic analysis showed an arrangement of the ter gene cluster confirmed within the present work to be unlike other reported Bacillus strains with more putative genes for Te (IV) resistance and a new configuration of the biosynthetic module within. Finally, by combining genomic and differential proteomic analyses, we were able to understand the effect of Te(IV) on this organism at the proteomic level and describe how it responds to Te(IV) with specificity.
Results
Bacillus strain 3W19 isolated from mining sediment and resistance to Te(IV)
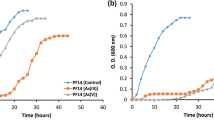
In the current study, we improved strain’s 3W19 phylogenetic identification with the inclusion of additional information from the strain’s genome sequence. The strain was identified as Bacillus altitudinis, with an average nucleotide identity (ANI) of 98.83% with the type strain Bacillus altitudinis DSM 21,631, referred hereafter as B. altitudinis 3W19. The maximum concentration of the metalloid Te(IV) at which the strain 3W19 can grow was determined in R2A broth (Fig. 1). The strain was able to grow up to 0.5 mM Te(IV) with no visible lag phase. At Te(IV) concentrations above 0.5 mM the specific growth rate (µ) decreased significantly.
Genome annotation and the potential for Te(IV) resistance reveals a new organization in a known Te(IV) resistance gene cluster in Bacillus Sp
The strain’s genome was sequenced and annotated using the NCBI genome annotation pipeline to identify its genetic potential for Te(IV) resistance. The draft genome of B. altitudinis 3W19 was obtained from Illumina sequencing resulting in a genome of 3.7 Mb, assembled into 54 contigs. Genome annotation yielded a total of 3719 identified coding sequences (CDS) regions, 3782 genes, 1 tmRNA, and 59 tRNAs (Supplementary Table S1). The analysis of the genome annotation revealed the genetic potential for Te(IV) resistance and reduction. Elements from the ter gene cluster, terA/B/C/D/E/Z were found in B. altitudinis 3W19 genome confirming the results found by PCR amplification9. In this organism, the ter gene cluster was structured with a terB Ba_CDS_3821, antisense, three terD paralog genes Ba_CDS_3822/3823/3824, hereafter terD1/ terD2/ terD3, and one terC gene Ba_CDS_3825 in a gene cluster like arrangement. This cluster was followed by genes coding for a biosynthetic module including a HpcH/HpaI aldolase/citrate lyase family protein Ba_CDS_3826 (Blastp identity of 100%, E = 0.0), a phosphoribosyltransferase (PRTase) family protein Ba_CDS_3827 (Blastp identity of 100%, E = 0.0), a cysteine protease StiP family protein Ba_CDS_3828 (Blastp identity of 99.73%, E = 0.0) and a HAD family hydrolase Ba_CDS_3829 (Blastp identity of 100%, E = 0.0). In this organized structure, two additional genes were found: Ba_CDS_3830 encoding the YceG - putative tellurium resistance protein (Blastp identity of 59%, E = 0.0), and the Ba_CDS_3831 encoding the YceH/ TelA - toxic anion resistance protein (Blastp identity of 83%, E = 0.0). In strains of the genus Bacillus, it is usually observed that when ter genes are functionally linked, the most common organizations are either continuous (cluster 1), containing 1 terA, 3 terD, 1 terF, 1 yceG and 1 yceH, or discontinuous (cluster 2), 3 terD, 1 terF, a biosynthetic module followed by 1 yceG and 1 yceH. Strain 3W19 ter gene cluster is organized in the cluster 2 form, similar to those of Bacillus glycinifermentans B-27, Bacillus atrophaeus 1942 or Bacillus pumillus NJ-M2, (Fig. 2).
Phylogenetic relationship between Bacillus strains containing ter gene clusters. Pictographic representation of ter gene clusters based on data from B. altitudinis strain 3W19 sequencing and annotation and from IMG/ JGI genomic database for the remaining Bacillus strains. Phylogenetic relationship of Bacillus strains was constructed by core genome alignment using UBCG2 pipeline.
Differential proteomics in the presence of Te(IV) and the regulation of major cellular mechanisms
To evaluate the differences in proteome between growth in the presence and absence of Te (IV) a reference proteome was constructed with all the CDS obtained from the annotation of B. altitudinis 3W19’s sequenced genome. The reference proteome was used to identify the proteins obtained in the five independent biological replicates of each test condition, with and without Te(IV). The annotation resulted in 51% (1988 CDSs) of the strain 3W19 reference proteome detected and identified. The analysis of the proteome of the strain grown with or without Te(IV) revealed differences in the metabolic profile of the strain in the presence of Te(IV). The proteome of the strain grown in the presence of Te(IV) showed 65 proteins with a positive significant change in abundance (SCA) and 97 with a negative SCA (Fig. 3a). Moreover, nine identified proteins were exclusively found when the strain was grown in the presence of Te(IV) and 361 proteins were undetected in these conditions (Fig. 3a). A full list of exclusive and SCA proteins can be found in the supplementary material (Supplementary Tables S2-S3).
For the construction of metabolic pathways, and the following determination of their over/ down representation, we annotated the strain’s genome using the KEGG’s annotation pipeline with the option of pathway assignment. One thousand six hundred proteins were assigned to, at least, one KEGG functional pathway13, which represented 80% of the annotated sequences of strain 3W19. In proteins with positive SCA, there were 46 matches to KEGG hierarchical level 2 pathways (KEGG L2 pathways) (Fig. S1a) and in proteins with negative SCA, there were 133 matches to KEGG L2 pathways (Fig. S1b). KEGG L2 pathways were used to calculate statistically significant metabolic variations, here represented as pathway over- and under-represented, resulting from Te(IV) treatment. Pathway representation considered the number of proteins with positive and negative SCA in each pathway and the size of the pathway, in the reference proteome, relative to the size of the annotated strain’s proteome. The growth of the strain in the presence of Te(IV) was associated with the over-representation of metabolic pathways of amino acids metabolism and membrane transport (Fig. 3b). Only the metabolism of carbohydrates was significantly down-represented in the presence of Te(IV) (Fig. 3b). As expected, despite the large relevant data obtained, a high percentage of proteins identified remained hypothetical or with unrecognized functions.
Differential proteomics in Bacillus altitudinis strain 3W19. (a) Venn diagram representing the total proteins obtained, exclusive and shared in the presence and absence of Te(IV). Volcano plot indicates the total shared proteins between tests (Welsh t.test; FDR-0.05; S0 > 1). Those with SCA are indicated by large red circles. (b) Pathway representation in the presence and absence of Te(IV). SCA proteins with KEGG assigned pathways, mapped with subsystem KEGG L2, were analyzed for regulation using Fisher’s exact test. FDR adjusted p-values are presented for each pathway, p-values equal or less than 0.05 were considered to determine significant pathways. Black bars display pathway size compared to the size of the reference proteome, which can be grouped in pathways. Blue bars display the ratio of SCA proteins in the pathway compared to the total amount of SCA proteins in pathways.
Te(IV) induced over-expression of elements of Ter gene cluster and caused changes in the abundance of proteins from distinct pathways
Apart from altering the representation of some complete metabolic pathways, Te(IV) induced SCA in proteins of interest in the response to oxidative damage and Te(IV) detoxification. The identified proteins with the highest positive SCA were Ba_CDS_2106 – ArsC2, arsenate reductase (thioredoxin) [EC:1.20.4.4] with fold-change (FC) of log2 3.0 and Ba_CDS_3824 – TerD1, Te(IV) resistance protein TerD; with FC of log2 2.9 (Table 1a, Fig. 4). None of these proteins was assigned to a KEGG L2 pathway. On the opposite spectrum, the highest negative SCA was observed for membrane transport proteins, Ba_CDS_939 OppA - oligopeptide transport system substrate-binding protein, with FC of log2 -5.40 and the folding, sorting and degradation protein, Ba_CDS_3377 SRP54 signal recognition particles subunit with FC of log2 -4.04, (Table 1b/ Fig. 4). The differential proteome analysis focused on proteins previously demonstrated experimentally to confer tellurite/ tellurate resistance14. In this group, we found the two aforementioned Ba_CDS_2106 - ArsC2 and Ba_CDS_3823 – TerD1 along with the proteins Ba_CDS_3824 – TerD2 and Ba_CDS_3825 – TerD3, also identified as a part of the Te resistance gene cluster ter.
Regulation and expression of Te(IV) resistance genes Ter
To validate the high SCA in proteins from the ter gene cluster we evaluated the corresponding gene expression levels in growth with and without Te(IV). This step was performed by quantitative real-time PCR (qRT-PCR). The expression of genes encoding proteins TerD1, TerD2 and TerD3 increased in the presence of 0.5 mM of Te(IV) with fold change ratios of 2.25, 1.95 and 1.91 respectively (Table 2). The variation in log2 transformation of gene expression values, for each gene, was statistically significant. Furthermore, the analysis of the predicted regulatory and promoter regions, using PromPredict and BPROM, revealed the presence of individual promoters per gene. Therefore, more information is needed to confirm the ter genes organization found in strain 3W19 as an operon.
B. altitudinis 3W19 solves intracellular Te(IV) impacts in reactive oxygen species production
To validate the observed results of proteins overexpression related to ROS mitigation, from the differential proteomic analysis, the ROS production was measured overtime in the presence and absence of Te (IV). Quantification of reactive oxygen species (ROS) showed that the addition of 0.5 mM of Te(IV) induced oxidative stress. Under that experimental condition, ROS formation increased by 2.1-fold when compared to the control situation. The continuous tracking of ROS formation can indicate if there is an active metabolism to reduce these toxic species. After 5 h of growth in the presence of Te(IV), the strain was able to reduce its intracellular ROS to levels equal to or lower than the control assay (Fig. 5a).
Tellurite impact in glucose consumption and dependence on nitrogen for growth in the presence of tellurite
To confirm the strain’s metabolic adaptation to Te(IV) which consist of an under-representation of carbohydrate metabolism and an over-representation of amino acid metabolism, first, the differences in glucose consumption with and without Te (IV) were measured, secondly, the strain was grown in the presence of increasing concentrations of casamino acids, increasing the available nitrogen content at each concentration. In the first assay, most glucose was consumed in all tested conditions in the first four hours of growth, with or without Te(IV) in the growth medium (Fig. 5b). When comparing glucose concentration present in media over time against time 0 h, in each condition, we observe that glucose is consumed faster in growth without Te(IV), nevertheless, more glucose is consumed in the presence of the metalloid until 8 h of growth, 81.8% against 76.5% in the control situation. We observed that in the presence of 0.5 mM of Te(IV) cells required a readily available carbon source for a longer time period. As to the effects of supplementation of N source, strain 3W19 growth rate decreased when Te(IV) was added at 0.5 mM and it recovered when, in the presence of Te(IV), the media was supplemented with 2 g.l− 1 of casamino acids. The addition of 5 g.l− 1 of casamino acids significantly increased the growth rate compared to the control situation and growth with 0.5 mM of Te(IV) (Fig. 5c).
Tellurite impact in bacterial stress response and glucose consumption and importance of nitrogen source for growth of Bacillus altitudinis strain 3W19 in the presence of Te(IV). (a) ROS production assay showing two incubation periods with H2DCFDA. Relative Fluorescence Units (RFU) or the ratio between the fluorescence of the sample (test) and the fluorescence of the control experiment. One-way ANOVA was used to compare each condition variation through time and statistical analysis is indicated as p-value ** p ≤ 0.01, (b) Glucose quantification over time, measured by DNS reagent oxidation, of strain 3W19 grown in the conditions: Control [no Te(IV)] and 0.5 mM Te(IV), 2wayANOVA multiple comparison analysis performed between each time and time 0 h in control and 0.5 mM Te(IV) and statistical analysis represented as p-value: a - p ≤ 0.05, b - p < 0.0001; ns - p > 0.05. (c) Bacterial growth in the control and 0.5 mM Te(IV) with variations of concentration of N sources in the growth media. Horizontal coloured bars indicate median value, 2wayANOVA multiple comparison analysis performed between all conditions and statistical analysis is indicated as p-values: * p ≤ 0.05, **** p < 0.0001. Three biological replicates (n = 3) were performed in each assay and in panels (a) and (b) error bars indicate standard deviation.
Discussion
Tellurite induces toxicity in Bacillus Sp. 3W19 with the production of reactive oxygen species
The Bacillus strain 3W19 is, according to a previous study by Farias and colleagues, 2022 9, an environmental bacteria with high resistance to Te(IV). When compared to other reported studies of Te (IV) resistance in environmental bacteria strain 3W19 shows high resistance levels15,16. These comparisons are always limited by the specific growth requirements needed by each bacteria strain. Decreasing growth rates were observed, in liquid medium spiked with increased concentrations of Te(IV) of 0.25 mM and higher (Fig. 1), which was expected due to the toxicity effects of Te(IV).
In prokaryotes, tellurite toxicity is mostly related to the metalloid´s interactions and substitution with sulphur-containing residues in thiol-containing enzymes, to the competition with selenium for essential biological functions, and to its strong oxidizing ability that may lead to general oxidative damage17,18. Detoxification mechanisms that confer direct resistance to Te(IV) generally involve enzymatic reduction of Te(IV) to Te(0). Among the proteins with previous experimentally demonstrated Te(IV) reducing ability, none was identified in strain 3W19 with SCA. Nevertheless, this reduction can occur by a specific mechanism or by unspecific molecules such as glutathione, and/or other reduced thiols, like cysteinyl, glycine or cysteine5. It was previously demonstrated that reduction by glutathione18 and a cysteine-containing desulfurase19 results in the formation of reactive oxygen species. Instead of employing a specific Te(IV) detoxification mechanism most bacteria use the reduction strategy coupled with ROS mitigation20,21. In strain 3W19, at the concentrations of Te(IV) tested, a reduction of ROS produced over time was observed, Fig. 2a, suggesting an active metabolism to reduce oxidative stress.
Bacillus Sp. 3W19 counteracts Te(IV) and its reduction byproducts through maintenance of membrane, protection of proteins and activation of proteins from the Ter gene cluster
Using differential proteomics two main actions are identified in the strain’s 3W19 response to oxidative stress: maintenance of membrane and proteins; and activation of a Te(IV) specific response. Maintenance of membrane and proteins is attributed to the SCA of proteins such as Ba_CDS_2843 – PspA - phage shock protein A and Ba_CDS_2214 – HslO - molecular chaperone Hsp33 (Table 1a, Fig. 5). In literature, in the studies of differential proteomic profiling, PspA is recognized as a marker protein for stress response, with the function of maintaining cytoplasmic membrane integrity and/or the proton-motive force22,23. In B. altitudinis 3W19, the PspA protein is encoded by a gene organized in an operon-like structure as part of the lia operon, a genetic mechanism that codes for proteins involved in cell envelope stress response24. Moreover, by protecting the transmembrane proton gradient, the cells’ ability to produce ATP is preserved, this effect was previously observed in E. coli strains21. The positive SCA of HslO (log2 2.21), a chaperone that protects proteins from thermally unfolding and oxidative damage, clearly indicates the strain’s response to oxidative stress, as observed in ROS assay. It is the first report of involvement of This chaperone in Te(IV) resistance. A previous study identified the protein PspA and HslU in the interactome of the Te(IV) resistance protein TerC, as these three are co-located in immunoprecipitation and blue native PAGE assays25. A String (v12.0) network analysis shows that HslU is not in the neighborhood nor co-occurs with HslO, even though both are molecular chaperones. Unlike HslO, HslU is an ATPase subunit of a proteasome-like degradation complex and is membrane-bound, which could explain why was not detected in the membrane fraction studied by Turkovicova and colleagues 201625. Further studies could confirm that strain 3W19 uses the molecular chaperone HslO in the TerC interactome rather than HslU.
Activation of a Te(IV) specific response is viewed in the overexpression of ter gene cluster, terD1/ terD2 and terD3, with experimentally confirmed activity, as demonstrated by qRT-PCR. This result complements the observed LC-MS/MS result of positive SCA for TerD1, TerD2, encoded by the second and third genes in the observed ter gene cluster. Considering that TerC was found without SCA, we hypothesize that the strain does not require all the proteins in the known ter resistance system for Te(IV) resistance, or that some proteins are expressed at significantly lower levels than others. Similarly, in Pseudomonas citronellolis SJTE-3, only terD and terC are considered core genes in Te(IV) resistance6. Unlike the expression pattern observed for E. coli BL21(pLK18)12, in this study, the addition of sublethal concentrations of Te(IV) clearly induces overexpression of genes from the ter cluster. Compared to the E. coli BL21, in B. altitudinis 3W19, either has a lower threshold of Te(IV) concentration to trigger differential expression of ter genes, or this strain exhibit a greater dependence on Ter proteins for Te(IV) resistance. Furthermore, the presence of the complete ter gene cluster and the overexpression of some of its genes support the observed response to ROS. Previous studies highlighted the importance of the ter operon in E. coli KL53 in the mitigation of ROS induced cell damage26.
In strain 3W19 the Ter gene cluster has additional putative Te(IV) resistance determinants compared to bacteria of the same genera
The ter gene cluster in strain 3W19 comprises genes encoding for proteins with previous experimentally involvement in Te(IV) resistance: TerB Ba_CDS_3821, TerD (Ba_CDS_3822/3823/3824) and TerC Ba_CDS_3825. These genes conferring resistance to Te(IV)27 are followed by a biosynthetic module with four genes, along with two additional genes coding for proteins with putative function in Te(IV) resistance.
This genetic organization is similar to that observed in previously studied organisms but exhibits notable differences possibly relevant to Te(IV) resistance. When comparing the genetic organization of the ter gene cluster in strain 3W19 with phylogenetically related strains, two different arrangements were identified, cluster 1 and 2, that match the phylogenetic relationship of the Bacillus strains. The exception to this pattern is found in Bacillus amyloquefaciens ALB69. This deviation could result from a yet unidentified evolutionary mechanism. The ter gene cluster in strain 3W19 differs from the remaining cluster 2 genetic systems in the presence of a terB and a unique composition of the biosynthetic module. The terB gene encodes for the inner membrane bound TerB protein28 that forms a complex with TerC, considered a key protein in Te(IV) resistance29. The terB gene is almost exclusively present when the ter gene cluster includes a biosynthetic module. This characteristic supports the hypothesis that TerB plays a role in linking TerD and TerC either to the enzymes encoded by the biosynthetic module or to the diffusible product synthesized by them30. In the remaining strains from the same clade as strain 3W19, the terB is absent. The biosynthetic module can include genes encoding two or five enzymes. In both configurations, a PRTase and a HAD superfamily enzyme are always present. In the five-gene system, the additional enzymes include an additional PRTase, an ATP-grasp amide bond synthetase and a TIM barrel enzyme. Collectively, the enzymes in the biosynthetic module are involved in nucleotide biosynthesis, with the most likely product being a pyrimidine-derived ribonucleoside30. In strain 3W19, only three of the five genes from the enzyme biosynthetic module system are present: a phosphoribosyltransferase (PRTase) Ba_CDS_3827; a HAD family hydrolase Ba_CDS_3829 and a HpcH/HpaI aldolase/citrate lyase family protein Ba_CDS_3826, that belongs to the TIM barrel enzymes. According to the model proposed by Anantharaman, Iyer and Aravind, 201430 these proteins are sufficient for the production of a pyrimidine-derived ribonucleoside. Additionally, strain 3W19 possesses, within its biosynthetic module, a gene encoding a cysteine protease StiP family protein Ba_CDS_3828. This protein is not involved in nucleotide biosynthesis but is instead required for maintaining normal cell morphology and providing resistance to Te(IV) in Acinetobacter baylyi31. These two major differences in the ter genetic arrangement make us consider the role of the biosynthetic module more relevant for Te(IV) resistance in B. altitudinis strain 3W19 compared to other Bacillus strains. The additional genes Ba_CDS_3830 and Ba_CDS_3831, encoding YceG and YceH, are reported to enhance growth in Bacillus antraxis in the presence of Te(IV)32. Deleting yceG and yceH genes in B. anthracis increased cell susceptibility to the ROS-generating agent H2O232. ROS generation is a byproduct of Te(IV) reduction; therefore, these genes play a role in the general stress response associated with Te(IV) detoxification. Despite these genomic evidences of a gene cluster were all the elements are related to Te(IV) resistance only the TerD Ba_CDS_3823/3824 had SCA in the presence of Te(IV).
Strain 3W19 reduces energy consumption with the decrease in carbohydrate metabolism, activates enzymes responsible for thiol renewal content and over expresses proteins to protect the cell membrane from oxidative stress
The differential protein profiles observed revealed that this strain displays a unique and complex network of biological processes (Fig. 6). Significant metabolic changes were identified in carbohydrate and amino acids metabolism and membrane transport (Fig. 4b). The absence of 361 proteins in the presence of Te(IV) (Fig. 4a), accounting for 31% of the total identified proteins, with a 106 of those in Metabolism, 32 in carbohydrate metabolism and 8 in energy metabolism (Kegg Pathway), coupled with the down representation of proteins, with SCA, 35 in carbohydrate metabolism and 18 in energy metabolism (Fig. S1), suggests of a low-energy metabolic state.
The carbohydrate metabolism was significantly reduced, as evidenced by the negative FC in 35 SCA proteins. These proteins are involved in major pathways such as glycolysis or the TCA cycle, as well as specific metabolic processes of degradation of starch and sucrose, pyruvate, glyoxylate and dicarboxylate, propanoate and butanoate. This result is also observed in the decreased rate of glucose consumption during cell growth, Fig. 2b. Some pathways like glycolysis/gluconeogenesis exhibit pronounced changes across the pathway. Here, a metabolic shift was observed, with 11 proteins either showing negative SCA or being undetected in the presence of Te(IV) (Tables S2/ S3). This metabolic shift from glycolysis to pathways such as the pentose phosphate pathway is a hallmark of stress response33. Although significant variations were found in the representation of entire pathways, this result did not align exactly with the proteins exhibiting the highest positive or negative SCA. This is observed in proteins grouped under carbohydrate metabolism, Table 1b, where, despite six proteins showing FC between log2 -2 and − 3.5, none of them share the same metabolic pathway within the carbohydrate metabolism.
The amino acid metabolism was significantly increased by statistical analysis (Fig. 4b). In this pathway, the proteins with the highest SCA were Ba_CDS_402, DapB − 4-hydroxy-tetrahydrodipicolinate reductase and Ba_CDS_3332, CarA - carbamoyl-phosphate synthase small subunit, both with FC ≤ log2 2, (Table S2). These enzymes are involved in the biosynthesis of lysine and the biosynthesis of alanine, aspartate, and glutamate, respectively. The negative SCA in Ba_ CDS_1815 AspA FC = log2 -2.49, an enzyme that catabolizes L-aspartate into fumarate and ammonia is also important to maintain the products of the CarA biosynthesis. Metabolism of amino acids plays an important role in stress response by contributing to energy production, synthesis of new building blocks, and the generation of amino acids with metal-binding properties, such as cysteine, and anti-oxidant molecules34. As shown in Fig. 2c, supplementing the medium with additional amino acids led to a marginal but significant increase in bacterial growth in the presence of Te(IV). Unlike what was observed in previous studies11,12 no specific activation of sulphur metabolism was detected in strain 3W19, as revealed by SCA. In particular, cysteine synthesis, which is critical for replenishing reduced thiol content, was not active. This conclusion is supported by the negative SCA observed for the essential protein for this function, the CDS_2216 – CysK - cysteine synthase, with a decreased FC = log2 -1.1. Instead, the maintenance of a reduced thiol pool, considered essential for cells growth in the presence of Te(IV), rely on the overregulation of oxidoreductases. These included Ba_CDS_1920 – TrxB - thioredoxin reductase with FC of 1.1; Ba_CDS_688 – ArsC1 - arsenate reductase (glutaredoxin) with FC of 1.3 and the previously mentioned Ba_CDS_2106 - ArsC2 - arsenate reductase (thioredoxin). Of these, only the Ba_CDS_1920 - TrxB had high similarity to a protein with demonstrated activity in mitigating Te(IV) toxicity11. These three protein action resembles the one described in Bacillus subtilis by Bennet and colleagues where their mechanism of disulphide bonds formation reverses damages from ROS oxidation35.
Membrane transport metabolism was overrepresented as indicated by high SCA values in proteins with high FC such as Ba_CDS_3267 – RbsD - D-ribose pyranase (Fig. 5) and Ba_CDS_1628 – YtrE - acetoin utilization transport system ATP-binding protein (Table S2). YtreE, in conjunction with Ba_CDS_3295 – MurG - UDP-n-acetylglucosamine-N-acetylmuramyl-(pentapeptide) pyrophosphoryl-undecaprenol n-acetylglucosamine transferase, were identified as essential in stress response. This aligns with observations in the stress-induced modulation of the cell wall biosynthesis, or the lantibiotic stress response23. YtrE also emerges as a reliable marker protein for stress responses involving interference with cell wall biosynthesis23. MurG, which is a transferase responsible for the conversion of lipid I to lipid II and a key enzyme in the synthesis of peptidoglycan monomers36, was exclusively detected in strain 3W19 in the presence of Te(IV). This enzyme is assigned to the KEGG L2 pathway of membrane transport. Similar to the previously mentioned Ba_CDS_2843 - PspA, YtrE has been identified multiple times in proteomic profiling of stress response because of its role in the maintenance of cell wall integrity during stress23,37.
Schematic representation of main changes observed by Te (IV) exposure. The image represents a bacilli-like cell labelled well well-defined geometric shapes representing proteins listed in Table S2; red arrows represent negative effects on the cell and green arrows represent positive effects on the cell. Non-labelled undefined geometric blue or purple shapes represent generic cellular proteins. Outside the cell, large arrows represent the metabolic pathway with significant over/ down representation.
Conclusions
Overall, the genetic organization of B. altitudinis strain 3W19 includes all the essential elements required for Te(IV) resistance, along with additional elements that are beneficial additions for this function, absent in closely related Bacillus strains.
Proteins with the highest positive SCA included the arsenate reductase ArsC and the Te resistance protein TerD, which are clear indicators of a specific response to Te(IV). The existence of proteins with similar activities in different enzymatic pathways highlights this strain’s high level of adaptation and functional redundancy to overcome metalloid stress. Nevertheless, there was no clear indication of what protein/s are directly responsible for Te(IV) reduction.
The stress response in strain 3W19 under Te(IV) exposure involves mechanisms that preserve cell wall integrity. Proteins such as YtrE, MurG, and PspA are likely to contribute to the preservation of cell wall integrity and proton-motive force which is essential for the maintenance of cellular metabolic activity. The metabolic adjustments observed in B. altitudinis 3W19 include increased amino acid metabolism and decreased carbohydrate metabolisms, consistent with common responses to metal exposure already described in the literature33,34. Taken together, the proteomic profile and the cell response to ROS formation show that this strain activates an effective cell response to the known damages produced by the toxic Te(IV). It achieves this with minimal energy consumption while efficiently reducing Te(IV). Combining with the previous study9 that demonstrated the high resistance and Te (IV) removal from liquid these characteristics establish B. altitudinis 3W19 as an optimal model organism for bioremediation applications.
Methods
Bacterial strain tellurite resistance determination, growth and tellurite induced stress response
Bacillus altitudinis B1S54.2 3W19 (3W19) was isolated from the mining sediment from the Panasqueira mining site, Barroca Grande, Portugal (40°07’32.71"N, 7°42’51.18"W)9.
The specific growth rates of strain 3W19 of B. altitudinis, in R2A medium with and without Te(IV) at 0.1 mM, 0.25 mM, 0.5 mM and 1 mM, were calculated based on the variation of optical density (OD) with incubation at 25 ºC for 24 h. The growth assays, in solid and liquid media, were repeated in triplicate to ensure the reproducibility of the experiments. The same growth conditions in liquid R2A medium were used for the determination of growth rate in the presence of different concentrations of nitrogen. Variations in the amount of N were given in increasing concentrations of casamino acids (Oxoid) of 2 g.l− 1 and 5 g.l− 1.
General metabolic activity was measured by glucose. For glucose consumption, reduced glucose was quantified in cell-free media, in a 10 h growth period, from inoculates doped with 0.5 mM of Te(IV) compared to the condition without Te(IV). Dinitrosalicilic acid was used as an indicator of reduced glucose following the protocol used by Miller, 194738, with results presented as available glucose in media. General oxidative stress was measured with the use of 2,7-dichlorofluorescein‐diacetate (H2DCFDA)39, inferring the production of reactive oxidative species (ROS). For the last two assays, cells were grown in R2A broth without metal (control) and in 0.5 mM Te(IV). For general oxidative stress assay, the protocol used was the same as the ones used by the authors for the strain Paenibacillus pabuli ALJ109b40, with the exception that for B. altitudinis strain 3W19 the cultures were incubated in the test conditions up till an OD Abs 600 nm of 0.2.
The values presented show the ratio between the values in the Te(IV) conditions and values in the control condition. All assays were conducted in triplicates. In all assays with representative replicates statistical analysis was performed using either one-way ANOVA or two-way ANOVA using either Tukey’s or Dunnet’s, multiple comparison tests, respectively.
In all assays, tellurite was provided in the form of sodium tellurite, Na2TeO4 (Sigma-Aldrich).
Genome sequencing, annotation, strain identification and promoter search
Strain 3W19 was grown in liquid media R2A broth, streaked from a single colony. Cells were collected and DNA was extracted using a DNeasy PowerSoil Kit (Quiagen), according to the manufacturer’s instructions. Preparation of libraries of total genomic DNA and sequencing on an Illumina MiSeq System, 2 × 300 bp chemistry (MiSeq Reagent Kit v3), were performed according to the protocol carried out in40. Pairing, trimming and assembly based on Bruijn graphs were performed using CLC genomics workbench v9.5.4 (Qiagen) using default parameters. The resulting contigs were submitted to GhostKOALA (KEGG Orthology And Links Annotation) annotated genomes as reference proteome41. In GhostKOALA, KEGG identifiers (K numbers) were assigned to the sequence data by GHOSTX searches, against a nonredundant set of KEGG GENES.
Genome phylogeny was determined by using rMLST42 and phylophlan43 analyses and refined using GTDB-Tk v.0.1.3 toolkit44. GTDB-Tk v.0.1.3 toolkit enabled us to find single-copy bacterial marker genes in the assembled genome of strain 3W19 and to construct a multiple alignment of concatenated single-copy gene sequences, comprising all species from the GTDB database44.
To determine whether genes of interest were under the action of one or more promoters, the Prom Predict algorithm45 and the online software BPROM46 were used on specific contigs where genes of interest were found. Both results were jointly analysed.
The phylogenetic relationship of Bacillus strains was reconstructed using UBCG2 with calculation of Gene Support Index from concatenated gene sequences from core genomes of closely related type strains of Bacillus genus with ter operons annotated in their genomes47. Pictographic representation of ter gene clusters was designed with data from RAST (Seed server) for Bacillus altitudinis strain 3W19 and NCBI gene database and IMG/ JGI genomic database for the remaining Bacillus strains.
Protein extraction, digestion, and mass spectrometry
Total protein content was extracted from exponential growth cultures, at the same growth phase, of 3W19 strains grown in both Te(IV) treated and untreated conditions. Log phase cells were centrifuged at 4000 g for four minutes and washed twice in cold PBS. Cells were lysed, by resuspension in lysis buffer (guanidinium hydrochloride 6 M, tris(2-carboxyethyl) phosphine (TCEP) 10 mM, 2-chloroacetamide (CAA) 40 mM, HEPES 50 mM pH 8.5), vortexed and boiled at 95 °C for 5 min. Additionally, samples were subjected to sonication with continued on/off cycles of 10 s with a Q500 sonicator (Qsonica, Newtown, USA) for 4 min, on an ice water mixture. The sonicated samples were centrifuged at 10,000 g before proceeding with digestion. Before trypsin digestion, protein concentration was measured in all samples using Bradford (BioRad) according to the manufacturer’s description. Thirty µg of protein material was used for digestion. The samples were four-fold diluted in digestion buffer [acetonitrile (ACN) 10%, HEPES 50 mM pH8.5] and then incubated for 4 h with trypsin (1:100 trypsin-to-protein ratio) (Sigma T6567) at room temperature with horizontal shaking at 500 rpm. Trypsin inactivation was achieved by adding trifluoroacetic acid (TFA) at 2% and debris were removed by centrifugation (10000 g, 10 min). Tryptic peptides were fractionated using a Stage tip protocol as described by Rappsilber and colleagues48. A total of three C18 plugs were gently punched out from the filter disk with the help of the sampling tool syringe. Plugs were placed at the tip of a 200 µL pipette tip with a plunger and activated with 30 µL methanol by centrifugation at 1000 g for 2 min, followed by 30 µL 100% ACN, and finally 2 × 30 µL of 3% ACN with 1% TFA. Peptides were loaded onto the filter unit by centrifugation at 1000 g. Bound peptides were washed twice using 30 µL of 0.1% formic acid (FA). Peptides were eluted using two rounds of 30 µL 60% ACN in 0.1% FA, with centrifugation between each round. The liquid was evaporated, and the peptides were redissolved in 2% ACN with 1% TFA. Peptide concentration in the samples was estimated with NanoDrop, and 1.5 µg peptide was loaded for analysis on a Q-Exactive instrument (Thermo Scientific, Bremen, Germany). All protocol steps and analysis parameters for mass spectrometry analysis were derived from the authors’ previous methods for total protein analysis of P. pabuli ALJ109b40.
Protein annotation and statistical analysis
The acquired raw data was analysed using MaxQuant version 1.5.5.155, using the standard settings. Oxidation (M) and Acetyl (Protein N-term) were set as variable modifications and Carbamidomethyl (C) was set as a fixed modification. Match-Between-Runs function was applied to enhance protein identification in the simple experimental setup. The label-free quantification algorithm was applied for quantification with an LFQ min ratio count of 249 a maximum of 2 missed tryptic cleavages were permitted. A minimum length of seven amino acids per peptide was required. The standard mass tolerance settings for the Orbitrap were used. A target-decoy search approach with the default MaxQuant setting of 1% false discovery rate (FDR) was applied for identification at both peptide and protein levels using the built-in Andromeda peptide search engine49,50 and GhostKOALA annotated genomes as reference proteome.
Differential abundance analysis was performed using a Welsh t-test, with S0 = 1, while only including proteins observed in 3 out of the 5 biological replicates in both groups. P-values were adjusted for multiple hypothesis testing by FDR correction, with q < 0.05 as the significance cut-off.
Regulated pathways were identified using Fisher’s exact test and Storey FDR correction51 was used to correct for multiple hypothesis testing. The comparative analyses of the metabolism were constructed with annotated proteins, with K numbers assignedto all 3 KEGG Metabolic Pathway hierarchical levels.
RNA extraction purification and qRT-PCR
Total RNA extractions from log-grown cells (OD600 ≈ 0.5) of control and Te(IV) (0.5 mM) conditions were carried out using the GeneJet RNA Purification kit (Thermo Scientific) according to the manufacturer’s protocol and stored at -80 °C until further use. The purity and integrity of the isolated total RNA were analysed both by agarose gel electrophoresis and by Nanodrop spectrophotometer (Thermo Scientific). The presence of any DNA contamination in isolated total RNA was removed by using RNase free-DNase (Invitrogen, Thermo Fisher Scientific). First-strand cDNAs were synthesized from 5 µg of DNAse treated RNA with the SuperScript™ II RT cDNA Synthesis Kit (Invitrogen, Thermo Fisher Scientific) using oligo dT primer (NZYTech) as per the recommended protocol. The transcription of Te(IV) resistance terD paralogues was analysed by quantitative RT-PCR. For each treatment, three different cDNA samples were considered, and each sample included three technical triplicates. Specific primers for terD1, terD2 and terD3 and the housekeeping gene (16 S rRNA), designed on the nucleotide sequences previously obtained with genome sequencing, were used (Supplementary Table S4). Validation of primer amplification efficiency was visualised by PCR and confirmed on a 1% agarose gel (results not shown). Quantitative RT-PCR was performed on 10 µL-volume of SYBR Green Master mix (Biotool™) containing 50 ng of cDNA, using the Bio-Rad CFX96™ real-time PCR system, with the following cycling parameters: 95 °C for 2 min (denaturation), 38 cycles at 95 °C for 20 s and 60 °C for 1 min (annealing), and a last phase at 95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s and 60 °C for 15 s (extension). No genomic contamination was detected by the dissociation curve. Relative quantification values (fold change ratios) were obtained using the Pfaffl mathematical model (2−ΔΔCt calculation)52 where values obtained for treated samples were compared with those obtained for untreated samples. The fold change ratios were normalized with respect to the housekeeping gene, to compensate for variations in the amounts of cDNA. Log2 transformation of target gene expression levels, Control vs. Te(IV) conditions, was compared with paired Student′s t-test, using software GraphPad Prism5; GraphPad Software, Inc. For the statistical test, differences were considered significant if the p‐value was ˂ 0.05.
Data availability
Draft genome of strain B1S54.2 3W19 was deposited at DDBJ/ENA/GenBank under the BioProject number PRJNA606037, the BioSample number SAMN14083405, and the accession number PRJNA606037. Raw sequences have been deposited in the Sequence Read Archive (SRA) database under the accession number SRR11095950. The 16 S rRNA sequence of strain B1S54.2 3W19 is deposited in NCBI database under accession number OK644222.1. Strain B1S54.2 3W19 was deposited at the University of Coimbra Bacterial Culture Collection (UCBCC) under accession number UCCCB 87 (https://ucccb.uc.pt/strain-details/?detail=UCCCB87). The mass spectrometry proteomics raw data and results from analysis by MaxQuant have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD017546.
References
Hein, J. R., Koschinsky, A. & Halliday, A. N. Global occurrence of tellurium-rich ferromanganese crusts and a model for the enrichment of tellurium. Geochim. Cosmochim. Acta. 67, 1117–1127 (2003).
Anderson, C. S. Mineral Commodity Summaries: TELLURIUM. (2021).
Missen, O. P. et al. Love is in the Earth: A review of tellurium (bio)geochemistry in surface environments. Earth Sci. Rev. 204, 103150 (2020).
Whelan, K. F., Sherburne, R. K. & Taylor, D. E. Characterization of a region of the IncHI2 plasmid R478 which protects Escherichia coli from toxic effects specified by components of the tellurite, phage, and colicin resistance cluster. J. Bacteriol. 179, 63–71 (1997).
Chasteen, T. G., Fuentes, D. E., Tantaleán, J. C. & Vásquez, C. C. Tellurite: history, oxidative stress, and molecular mechanisms of resistance: review Article. FEMS Microbiol. Rev. 33, 820–832 (2009).
Peng, W. et al. Characterization of the tellurite-resistance properties and identification of the core function genes for tellurite resistance in pseudomonas citronellolis sjte-3. Microorganisms 10, (2022).
Llyod-Jones, G. et al. Accumulation and intracellular fate of tellurite in tellurite-resistant Escherichia coli: A model for the mechanism of resistance. FEMS Microbiol. Lett. 118, 113–119 (1994).
Chung, A. P. et al. Tailings microbial community profile and prediction of its functionality in basins of tungsten mine. Sci. Rep. 9, 19596 (2019).
Farias, P., Francisco, R. & Morais, P. V. Potential of tellurite resistance in heterotrophic bacteria from mining environments. iScience 25, 104566 (2022).
Cheng, M., Sun, Y., Sui, X. & Zhang, H. Characterization of the differentiated reduction of selenite and tellurite by a halotolerant bacterium: process and mechanism. J. Water Process. Eng. 47, 102809 (2022).
Yasir, M., Zhang, Y., Xu, Z., Luo, M. & Wang, G. NAD(P)H-dependent thioredoxin-disulfide reductase TrxR is essential for tellurite and selenite reduction and resistance in Bacillus Sp. Y3. FEMS Microbiol. Ecol. 96, 1–11 (2020).
Aradská, J., Šmidák, R., Turkovičová, L., Turňa, J. & Lubec, G. Proteomic differences between tellurite-sensitive and tellurite-resistant e.coli. PLoS One 8 (2013).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592 (2023).
Pal, C., Bengtsson-Palme, J., Rensing, C., Kristiansson, E. & Larsson, D. G. J. BacMet: antibacterial biocide and metal resistance genes database. Nucleic Acids Res. 42, D737–D743 (2014).
Arenas, F. A. et al. Isolation, identification and characterization of highly tellurite- resistant, tellurite-reducing bacteria from Antarctica. Polar Sci. 8, 40–52 (2014).
Xie, H. G., Xia, W., Chen, M., Wu, L. C. & Tong, J. Isolation and characterization of the Tellurite-Reducing photosynthetic bacterium, Rhodopseudomonas palustris strain TX618. Water Air Soil. Pollut. 229, 158 (2018).
Ba, L. A., Mandy, D., Jamier, V. & Jacob, C. Tellurium: an element with great biological potency and potential. Org. Biomol. Chem. 8, 4203–4216 (2010).
Tremaroli, V., Fedi, S. & Zannoni, D. Evidence for a tellurite-dependent generation of reactive oxygen species and absence of a tellurite-mediated adaptive response to oxidative stress in cells of Pseudomonas pseudoalcaligenes KF707. Arch. Microbiol. 187, 127–135 (2007).
Tantaleán, J. C. et al. The Geobacillus stearothermophilus V IscS gene, encoding cysteine desulfurase, confers resistance to potassium tellurite in Escherichia coli K-12. J. Bacteriol. 185, 5831–5837 (2003).
Chasteen, T., Fuentes, D., Tantalean, J. & Vásquez, C. Tellurite: history, oxitative stress, and molecular mechanisms of resistance. FEMS Microbiol. Rev. 33, 820–832 (2009).
Maltman, C. & Yurkov, V. Extreme environments and high-level bacterial tellurite resistance. Microorganisms 7, 1–24 (2019).
Tsai, W. C., Kuo, T. Y., Lin, C. Y., Lin, J. C. & Chen, W. J. Photobacterium damselae subsp. Piscicida responds to antimicrobial peptides through phage-shock-protein A (PspA)-related extracytoplasmic stress response system. J. Appl. Microbiol. 118, 27–38 (2015).
Wenzel, M. et al. Proteomic response of Bacillus subtilis to lantibiotics reflects differences in interaction with the cytoplasmic membrane. Antimicrob. Agents Chemother. 56, 5749–5757 (2012).
Suntharalingam, P., Senadheera, M. D., Mair, R. W., Lévesque, C. M. & Cvitkovitch, D. G. The LiaFSR system regulates the cell envelope stress response in Streptococcus mutans. J. Bacteriol. 191, 2973–2984 (2009).
Turkovicova, L. et al. Proteomic analysis of the TerC interactome: novel links to tellurite resistance and pathogenicity. J. Proteom. 136, 167–173 (2016).
Vávrová, S., Grones, J., Šoltys, K., Celec, P. & Turňa, J. The tellurite resistance gene cluster of pathogenic bacteria and its effect on oxidative stress response. Folia Microbiol. (Praha). https://doi.org/10.1007/s12223-024-01133-8 (2024).
Taylor, D. E. Bacterial tellurite resistance. Trends Microbiol. 7, 111–115 (1999).
Alekhina, O., Valkovicova, L. & Turna, J. Study of membrane attachment and in vivo co-localization of TerB protein from uropathogenic Escherichia coli KL53. Gen. Physiol. Biophys. 30, 286–292 (2011).
Vávrová, S., Grones, J., Šoltys, K., Celec, P. & Turňa, J. The tellurite resistance gene cluster of pathogenic bacteria and its effect on oxidative stress response. Folia Microbiol. (Praha). 69, 433–444 (2024).
Anantharaman, V., Iyer, L. M. & Aravind, L. Ter-dependent stress response systems: novel pathways related to metal sensing, production of a nucleoside-like metabolite, and DNA-processing. Mol. Biosyst. 8, 3142–3165 (2014).
Reichert, B. et al. Acinetobacter baylyi long-term stationary-phase protein stip is a protease required for normal cell morphology and resistance to tellurite. Can. J. Microbiol. 59, 726–736 (2013).
Franks, S. E. et al. Novel role for the YceGH tellurite resistance genes in the pathogenesis of Bacillus anthracis. Infect. Immun. 82, 1132–1140 (2014).
Christodoulou, D. et al. Reserve flux capacity in the Pentose phosphate pathway enables Escherichia coli’s rapid response to oxidative stress. Cell. Syst. 6, 569–578e7 (2018).
Izrael-Živković, L. et al. Cadmium specific proteomic responses of a highly resistant: Pseudomonas aeruginosa San Ai. RSC Adv. 8, 10549–10560 (2018).
Bennett, M. S., Guan, Z., Laurberg, M. & Su, X. D. Bacillus subtilis arsenate reductase is structurally and functionally similar to low molecular weight protein tyrosine phosphatases. Proc. Natl. Acad. Sci. 98, 13577–13582 (2001).
van Heijenoort, J. Lipid intermediates in the biosynthesis of bacterial peptidoglycan. Microbiol. Mol. Biol. Rev. 71, 620–635 (2007).
Beckering, C. L., Steil, L., Weber, M. H. W., Völker, U. & Marahiel, M. A. Genomewide transcriptional analysis of the cold shock response in Bacillus subtilis. J. Bacteriol. 184, 6395–6402 (2002).
Miller, G. L. Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959).
Jakubowski, W. 2,7-dichlorofluorescein oxidation and reactive oxigen species: what does it measure? Cell. Biol. Int. 24, 757–760 (2000).
Farias, P. et al. Impact of tellurite on the metabolism of Paenibacillus Pabuli AL109b with Flagellin production explaining high reduction capacity. Front. Microbiol. 12, 1–15 (2021).
Kanehisa, M., Sato, Y. & Morishima, K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J. Mol. Biol. 428, 726–731 (2016).
Jolley, K. A. et al. Ribosomal multilocus sequence typing: universal characterization of bacteria from domain to strain. Microbiology 158, 1005–1015 (2012).
Segata, N., Börnigen, D., Morgan, X. C. & Huttenhower, C. PhyloPhlAn is a new method for improved phylogenetic and taxonomic placement of microbes. Nat. Commun. 4, 2304 (2013).
Parks, D. H. & Beiko, R. G. Identifying biologically relevant differences between metagenomic communities. Bioinformatics 26, 715–721 (2010).
Rangannan, V. & Bansal, M. High-quality annotation of promoter regions for 913 bacterial genomes. Bioinformatics 26, 3043–3050 (2010).
Solovyev, V. & Salamov, A. Automatic annotation of microbial genomes and metagenomic sequences. In Metagenomics and its Applications in Agriculture, Biomedicine and Environmental Studies, 61–78 (Nova Science, 2010).
Kim, J., Na, S. I., Kim, D. & Chun, J. UBCG2: Up-to-date bacterial core genes and pipeline for phylogenomic analysis. J. Microbiol. 59, 609–615 (2021).
Rappsilber, J., Mann, M. & Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using stagetips. Nat. Protoc. 2, 1896–1906 (2007).
Cox, J. et al. Accurate Proteome-wide Label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteom. 13, 2513–2526 (2014).
Cox, J. et al. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 10, 1794–1805 (2011).
Storey, J. D. A direct approach to false discovery rates. J. R Stat. Soc. Ser. B Stat. Methodol. 64, 479–498 (2002).
Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, 45e – 45 (2001).
Acknowledgements
This work was supported by the project Agenda Microeletrónica (reference: C644916358-00000028) under the PRR - Recovery and Resilience Plan and by the Next Generation EU Funds, following NOTICE N.º 02/C05-i01/2022, Component 5 – Capitalization and Business Innovation - Mobilizing Agendas for Business Innovation. This research is sponsored by FEDER funds through the program COMPETE and by national funds through FCT, under the project UID/00285 - Center for Mechanical Engineering Materials and Processes and LA/P/0112/2020. Pedro Farias was supported by the AM2R project “Mobilizing Agenda for business innovation in the Two Wheels sector” reference: C644866475-00000012 | 7253 under the PRR - Recovery and Resilience Plan and by the Next Generation EU Funds, following NOTICE N.º 02/C05-i01/2022, Component 5 – Capitalization and Business Innovation - Mobilizing Agendas for Business Innovation. Mass spectrometry analysis was performed at the DTU Proteomics Core, Technical University of Denmark.
Author information
Authors and Affiliations
Contributions
PF: Data curation performed all benchwork and analyzed all data using bioinformatic and statistical analyses. Wrote the original draft. RF: Heading field sampling and processing of some analyses in the laboratory, assisting in strain isolations and growth and reduction assays. Reviewed the statistical analyses. Reviewed and edited the manuscript.LM: Headed in genome sequencing and analyses of sequencing data. Reviewed and edited the manuscript.JH: Headed proteomic assays and data resulting from proteomic analyses. Reviewed and edited the manuscript.SS: Conceptualized part of the experiment. Supervised laboratory and bioinformatic analyses on genome sequencing and proteomics. Reviewed and edited the manuscript.PVM: Conceptualized the whole experiment and secured funding. Supervised the laboratory, bioinformatics, and statistical analyses. Contribute to the original draft and revised manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Farias, P., Francisco, R., Maccario, L. et al. Metabolic response of tellurite resistant Bacillus altitudinis strain 3W19 highlights the potential as a model organism for bioremediation. Sci Rep 15, 12745 (2025). https://doi.org/10.1038/s41598-025-95321-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-95321-2