Abstract
This study aims to investigate the impact of sarcopenia, osteopenia, and osteosarcopenia on the risk of developing osteoarthritis (OA) in individuals with prediabetes. We collected and analyzed data from five National Health and Nutrition Examination Surveys (NHANES) conducted between 2001 to 2006, 2013 to 2014, and 2017 to 2018. The study population consisted of participants who self-reported having OA and those who had bone mineral density and skeletal muscle mass index (SMI) measured using dual-energy X-ray absorptiometry (DXA). We employed weighted multinomial logistic regression models and restricted cubic splines to examine the relationship between osteosarcopenia and the risk of OA in prediabetic individuals. Individuals with prediabetes exhibited a greater susceptibility to osteosarcopenia compared to those with normal glucose tolerance (NGT). In adults with prediabetes, muscle mass index (SMI) was independently associated with bone mineral density (BMD). Furthermore, both sarcopenia and osteopenia were positively correlated with osteoarthritis (OA) within the prediabetic population. Notably, the presence of osteosarcopenia in prediabetes significantly increased the risk of developing OA compared to the presence of either sarcopenia or osteopenia alone. In adults with prediabetes, osteopenia and sarcopenia are risk factors for OA, and the combination of sarcopenia and osteopenia further increases the prevalence of OA.
Similar content being viewed by others
Introduction
Prediabetes is defined as an intermediate metabolic condition that lies between normal blood glucose and diabetes, which includes impaired fasting glucose, impaired glucose tolerance, and a mild elevation in hemoglobin A1c (HbA1c) levels1.While a clear diagnosis of prediabetes has not yet been established, a range of pathophysiological changes related to diabetes have already taken place2, and the prevalence of diabetes-related complications starts to increase in prediabetic individuals compared to patients with normal blood glucose levels. Studies have shown that prediabetes is linked to an elevated risk of early kidney disease and chronic kidney disease3, as well as an increased risk of idiopathic and polyneuropathic disorders4,5, retinopathy2,6,7, and macrovascular diseases8,9,10.
Osteopenia and osteoporosis are recognized as systemic bone disorders11, which are associated with reduced bone mass and microstructural damage that increase fracture risk and bone fragility12. Osteopenia is becoming more prevalent among women post-menopause at age 55 and men over the age of 65. Sarcopenia is now defined as a progressive and systemic disease of skeletal muscle, involving an accelerated loss of muscle mass and function13. Studies have shown that sarcopenia is linked to a heightened risk of multiple adverse outcomes, such as falls, functional deterioration, frailty, and increased mortality14,15. The incidence of osteopenia and sarcopenia is rising as the population ages. There is a clear correlation between osteopenia and sarcopenia across the lifespan, and their pathophysiological processes show significant consistency during the disease progression. Given the strong connection between the two conditions, the concept of osteosarcopenia has been introduced16,17, indicating the coexistence of osteopenia and sarcopenia.
Previous studies have indicated that patients with diabetes have a higher risk of developing sarcopenia and skeletal-related diseases such as fractures or osteoarthritis(OA)18,19,20,21,22,23, as well as osteopenia and sarcopenia are increasingly recognized as chronic complications of diabetes. However, in pre-diabetic patients, the risk of sarcopenia, osteopenia and osteosarcopenia reduction and their impact on OA are not known.
In this study, we analyzed relevant data from the NHANES database across five cycles from 2001 to 2006, 2013 to 2014, and 2017 to 2018 to examine the associations between sarcopenia, osteopenia, and prediabetes, as well as the impacts of sarcopenia, osteopenia, and osteosarcopenia on OA in adults with prediabetes in the United States.
Methods
Database and study population
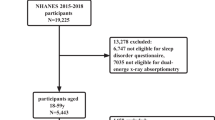
In this study, we utilized public data from the National Health and Nutrition Examination Survey (NHANES) across five cycles from 2001 to 2006, 2013 to 2014, and 2017 to 2018. This is a complex, multistage, stratified cross-sectional population survey conducted by the Centers for Disease Control and Prevention (CDC) to collect health and nutrition data on a nationally representative non-institutionalized U.S. population. Participants underwent household interviews to obtain information about their sociodemographic and socioeconomic backgrounds, followed by physiological measurements, laboratory tests, and questionnaires conducted at mobile examination centers (MEC). In this cross-sectional study, the selected database included a total of 50,938 participants. Based on the following exclusion criteria: (1) missing data on muscle mass and bone data; (2) missing data related to OA; (3) missing data for diagnosing prediabetes; (4) under 20 years of age; (5) Missing data on vitamin D and physical activity; (6) Missing data on indicators of inflammation; (7) History of hormone use, e.g., prednisolone, methylprednisolone, dexamethasone. Ultimately, the study included 8656 participants, as shown in Fig. 1.
Assessment of prediabetes
In this study, participants were classified as diabetic if they met any of the following conditions24: (1) a confirmed history of diabetes in the questionnaire; (2) glycated hemoglobin (HbA1c) levels ≥ 6.5%; (3) fasting blood glucose levels ≥ 7.0 mmol/L. Participants who met all the following criteria were defined as having normal glucose tolerance (NGT): (1) denial of a history of diabetes or prediabetes in the questionnaire; (2) HbA1c levels < 5.7%; (3) fasting blood glucose levels < 5.6 mmol/L. The remaining participants were classified as prediabetic.
Assessment of osteopenia, sarcopenia, and osteosarcopenia
Bone mineral density (BMD) was assessed using dual-energy X-ray absorptiometry (DXA), and T-scores were calculated as (BMD of the respondent—mean BMD of the reference group)/SD of the reference group (SD represents standard deviation). A T value of less than − 1 and more than − 2.5 in the lumbar spine (L1–L4) or femoral neck, measured by DXA, is considered to be osteopenia, and a T value of less than − 2.5 is diagnostic of osteoporosis. Although osteopenia and osteoporosis represent distinct diagnostic categories, they were combined in this study to reflect the continuum of bone density loss in osteosarcopenia, as adopted in prior cohort studies16,17. Appendicular skeletal muscle mass (ASM) was defined as the sum of lean mass in the arms and legs. ASM was then divided by body mass index (BMI) to further define sarcopenia. As our study focused on U.S. adults from NHANES, we adopted the National Institutes of Health (NIH) definition of sarcopenia (men < 0.789 kg/BMI, women < 0.512 kg/BMI)25. Patients with both osteopenia and sarcopenia are defined as having osteosarcopenia16.
Osteoarthritis
The self-reported diagnosis of osteoarthritis (OA) was determined by asking: “Has a doctor or other health professional ever told you that you have arthritis?” If the response was “yes”, participants were further asked, “What type of arthritis is it?”. If participants answered “osteoarthritis or degenerative arthritis”, Based on the different responses to these two questions, participants were divided into an OA group (solely had OA), other arthritis participants (which were excluded from further analysis) and no-arthritis group. Self-reported doctor-diagnosed OA is the most predominantly used case definition in epidemiological studie26. Previous studies have shown that self-reported OA has an 85% concordance with clinically diagnosed OA26, indicating that self-reported OA results are highly reliable.
Covariates
In our analysis, a comprehensive set of covariates was included to control for potential confounding factors and ensure the credibility of our findings. These covariates included demographic factors such as gender and race; age, to account for the natural aging process; education level, to represent socioeconomic status and cognitive reserve; and the ratio of family income to poverty, to measure economic status. Additionally, health-related behaviors and conditions were considered: history of hypertension, waist circumference (as an indicator of central obesity), body mass index (BMI) (for assessing general obesity), and laboratory-related indicators such as blood calcium, albumin, serum iron, serum phosphorus, and serum creatinine.Inflammatory biomarkers in participating subjects were white blood cell (WBC) count, neutrophil ratio, lymphocyte ratio, neutrophil-to-lymphocyte ratio (NLR)27. Physical activity levels were stratified into three distinct categories following internationally recognized MET-minute cut-points derived from WHO guidelines28: 1. Insufficient: Characterized by suboptimal adherence to established physical activity recommendations, defined as irregular engagement in movement behaviors without achieving guideline-specified minimum thresholds; 2. Moderate: Meeting baseline requirements for health maintenance through sustained weekly energy expenditure (500–1000 MET-min/week), equivalent to 150–300 min of moderate-intensity exercise; 3. High: Demonstrating superior compliance exceeding 1,000 MET-min/week, reflecting an intensive exercise protocol associated with enhanced cardiometabolic risk reduction and neuroprotective benefits. By controlling for covariates, a more comprehensive assessment of the investigated relationships was ensured.
Statistical analysis
Multiple imputation was applied to data with less than 30% missing, and the Kolmogorov–Smirnov method was used to evaluate data distribution. Chi-square tests, Mann–Whitney U tests, or independent t-tests were performed as appropriate to compare differences between two groups. In this study, multicategorical logistic regression analyses were performed to investigate the association between osteomuscular decompensation in prediabetes and OA. Multicategorical logistic regression included demographic characteristics as well as traditional factors associated with OA, sarcopenia, and osteomalacia. Odds ratios (ORs) were calculated by three models: an unadjusted model (Model 1), an age, sex, BMI, and race/ethnicity-adjusted model (Model 2), and a fully adjusted model for potential confounders (Model 3). ASM and BMD were used as continuous variables in restricted cubic spline (RCS) plots to evaluate their nonlinear relationship with osteoarthritis risk, adjusting for confounding factors, while continuous variables among covariates were also plotted using RCS. These analyses were performed using R software (version 4.2.3, http://www.Rproject.org) and EmpowerStats (version 4.1, http://www.empowerstats.com), and p values less than 0.05 were considered statistically significant.
Ethics approval and consent to participate
The NHANES protocol was ethically approved by the National Center for Health Statistics Research Ethics Review Board (NCHS, 2012) and written informed consent was obtained from all participants (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx). Therefore, no external ethical approval and informed consent were required. This study was conducted following the guideline of strengthening the reporting of cohort, cross-sectional and case–control studies in surgery (STROCSS 2021), Supplemental Digital Content 2, http://links.lww.com/JS9/C184 (Strengthening the Reporting of Cohort Studies in Surgery) criteria29. All analyses in this study were performed in accordance with the NHANES guidelines and regulations.
Results
Participant demographic information
This cross-sectional study enrolled 8656 adults, of whom 2557 (29.54%) were diagnosed with prediabetes based on standard glycemic criteria. Table 1 summarizes the baseline characteristics of the cohort. Compared to normoglycemic individuals, participants with prediabetes exhibited a higher prevalence of male sex, Hispanic ethnicity, osteopenia,osteosarcopenia and osteoarthritis (p < 0.05). Additionally, the prediabetes group demonstrated marginally elevated age, blood urea nitrogen (BUN), red blood cell distribution width (RDW) (p < 0.05).
As shown in Table 2, after adjusting for age, sex, race, body mass index, education, hypertension, waist circumference, urinary creatinine, urinary protein, blood calcium, blood iron, serum protein, white blood cell count, neutrophil percentage, lymphocyte percentage, NLR, RDW, MET, and vitamin D, patients with prediabetes were more likely to develop osteopenia and sarcopenia (OR 1. 326, 95% CI 1.047–1.678; OR 1.415, 95% CI 1.174–1.819), and prediabetes is an independent risk factor for osteopenia and sarcopenia.
Additionally, to investigate the impact of osteosarcopenia on OA within the prediabetic population, we categorized the prediabetic individuals into four groups: the normal group (without sarcopenia or osteopenia), the sarcopenia group, the osteopenia group, and the osteosarcopenia group (characterized by the presence of both sarcopenia and osteopenia), as illustrated in Table 3. Compared to the normal group, individuals in the osteosarcopenia group were significantly older, exhibited a lower proportion of males, had higher body mass index (BMI) and waist circumference (WC), and demonstrated lower poverty-income ratios (PIR) and educational attainment. This group also had a higher proportion of individuals identifying as white and black, while the proportion of Hispanics was lower in comparison to the Asian ethnic level. Furthermore, the osteosarcopenia group presented with a higher prevalence of hypertension, lower urinary creatinine levels, elevated urinary protein levels, reduced blood calcium and iron levels, lower blood protein concentrations, and higher levels of blood cholesterol, triglycerides, and blood urea nitrogen (BUN). Additionally, they exhibited slightly elevated uric acid levels, increased inflammatory markers, as well as low hemoglobin, vitamin D levels, and physical activity. Notably, subjects in the sarcopenia group were significantly more likely to develop OA compared to those in the normal group.
As shown in Table 4, lumbar spine bone density was independently associated with skeletal muscle mass in pre-diabetic adults, which is similar to the results of previous studies30. Also as shown in Fig. 2, among prediabetic subjects, those with osteosarcopenia had a significantly higher incidence of OA compared to those without sarcopenia.
As shown in Table 5, patients with osteosarcopenia had a higher prevalence of OA after adjusting for age, sex, race, body mass index, education level, vitamin D, activity level, biochemical markers, and inflammatory markers. In the NGT group, osteopenia, sarcopenia, and osteosarcopenia were all positively associated with the risk of developing OA (Model 3: OR 1.3514, 95% CI 1.3492–1.3535; OR 1.2555, 95% CI 1.2506–1.2604; OR 1.6106, 95% CI 1.6007–1.6205). In the group of pre-diabetic subjects, both sarcopenia and osteopenia were positively associated with OA in the fully adjusted model (Model 3: OR 1.6089, 95% CI 1.6063–1.6116; OR 1.5344, 95% CI 1.5304–1.5383). The prevalence of OA was significantly higher in the presence of osteosarcopenia (OR 2.7575; 95% CI 2.7474–2.7676), which was significantly higher than the prevalence in the osteosarcopenia group in the NGT group.Meanwhile, as shown in Fig. 2, the incidence of OA was higher in pre-diabetic subjects with osteosarcopenia compared to non-diabetic patients.
As shown in Fig. 3, in the prediabetic population, after adjusting for sex, age, race, education level, income-to-poverty ratio, BMI, hypertension, and biochemical indicators, it was found that ASM, bone density, age, BMI, waist circumference (WC), and blood urea nitrogen were linearly associated with the risk of osteoarthritis. Specifically, as ASM decreases, the incidence of osteoarthritis gradually declines; when ASM is less than 21.74 kg/m3, ASM becomes a risk factor for osteoarthritis. When ASM is greater than 21.74 kg/m3, the risk of osteoarthritis decreases. Additionally, when bone density is less than 1.086 g/cm3, bone density is significantly associated with the occurrence of osteoarthritis as a risk factor. With increasing age, the incidence of osteoarthritis gradually rises, and when age exceeds 49 years, age becomes a risk factor for osteoarthritis, which is similar to the age of menopause in women. Blood urea nitrogen is also positively correlated with the risk of osteoarthritis in prediabetes; when blood urea nitrogen exceeds 4.64 mmol/L, it becomes a risk factor for osteoarthritis. Both BMI and WC are related to obesity; when BMI exceeds 27.43 kg/m2 and WC exceeds 96.7 cm, both become risk factors for the occurrence of osteoarthritis.
Discussion
Prediabetes and osteopenia
Multiple studies have demonstrated a significant association between type 2 diabetes and reduced BMD across various body sites, increasing the risk of osteopenia or osteoporotic fractures in these areas31,32. However, studies exploring the relationship between prediabetes and bone health are limited, and the results are contradictory30,33,34,35, with some studies showing no significant difference in BMD between prediabetic and non-diabetic individuals30,34, while others indicate that prediabetes can lead to bone loss, increasing the risk of osteoporosis and osteoporotic fractures35. This study found that the incidence of osteopenia in the prediabetic population was lower compared to the normal population. Prediabetes is associated with chronic inflammation36 and is closely linked to endothelial damage37, thus prediabetes can influence BMD through inflammatory factors such as TNF-α and hs-CRP, as well as endothelial damage factors like vWF and ET-1, stimulating osteoclast formation38.
Prediabetes and sarcopenia
Previous studies have shown that sarcopenia is positively correlated with the prevalence of prediabetes39. Type 2 diabetes has been identified as an independent risk factor for sarcopenia40. In terms of sarcopenia, grip strength adjusted for BMI or weight has been found to be associated with prediabetes41. In this study, after adjusting statistical models, prediabetes was found to be an independent risk factor for sarcopenia in a multi-ethnic population. Previous studies have indicated that skeletal insulin resistance is a significant link between sarcopenia and glucose abnormalities42. The mechanisms may involve insulin resistance, skeletal muscle mitochondrial dysfunction, enhanced oxidative stress response, dysregulated secretion of myokines, and chronic low-grade inflammation43,44,45. Elevated blood glucose is a major factor leading to impaired skeletal muscle stem cell regenerative function, resulting in muscle mass loss and progressive decline in the glycogen-lipid metabolism function of skeletal muscle, which accelerates the progression of diabetes. Therefore, it is necessary for healthcare providers to pay more attention to the development of sarcopenia in prediabetes and diabetes.
Sarcopenia and osteopenia
Sarcopenia-osteopenia (OS) is also referred to as the "sarcopenic bone syndrome." In this study, osteopenia was closely associated with the SMI of prediabetic subjects. To our knowledge, this study is the first attempt to provide an association between SMI and osteopenia in adults with prediabetes in the United States. Previous research has shown that muscle and bone functions are closely related46, sharing common mechanical and molecular mechanisms, and are bidirectionally regulated through these mechanisms47. Muscle strength is the primary mechanical component that generates strain in bones, and a decline in muscle function and performance can lead to decreased bone loading and reduced bone density47. Additionally, skeletal muscle functions as an endocrine organ that secretes various myokines, including myostatin, leukemia inhibitory factor (LIF), fibroblast growth factor 2 (FGF2), brain-derived neurotrophic factor (BDNF), and irisin, which directly influence bone remodeling48,49. Bones also secrete osteokines, such as osteocalcin, to regulate muscle growth. Current studies suggest that deficiencies in several hormones, including vitamin D, may play a critical role in the pathogenesis of osteosarcopenia50,51.
Osteosarcopenia and osteoarthritis
A large cross-sectional study conducted in Germany previously indicated that the prevalence of sarcopenia in elderly women over 70 with OA is 1.60 times that of women without OA52. Up to now, multiple studies53,54,55 have indicated that the incidence of OA is positively associated with the incidence of sarcopenia. Firstly, decreased muscle strength can alter mechanical stress, reduce joint stability, and accelerate cartilage degeneration and subchondral bone changes. Xu et al.’s56 rat muscle atrophy model demonstrated that, compared to the control group, the group with reduced muscle strength was more likely to exhibit OA characteristic changes, such as cartilage surface wear and thinning, and trabecular bone transforming into a plate-like structure, confirming that decreased muscle strength can induce OA. Additionally, inflammation acts as a “bridge” in linking the pathogenesis of sarcopenia and OA. The increase in pro-inflammatory cytokines leads to an imbalance in the inflammatory system, inducing dysregulation of muscle and joint cartilage protein synthesis and degradation, ultimately causing synovial inflammation, muscle loss, and cartilage destruction. Silva et al.57 found in a rat model that the gastrocnemius muscle on the knee OA side expressed more IL-1β, with a 10% reduction in cross-sectional area (increased myostatin, decreased myogenin), indicating a correlation between inflammatory signaling and gastrocnemius atrophy, which is associated with OA.
Osteopenia/osteoporosis and OA are both prevalent bone diseases.OA accelerates bone loss. Existing studies58 have suggested a causal relationship between osteopenia/osteoporosis and increased risk of OA,. A meta-analysis59 has shown that bone loss progressively increases with age and may contribute to OA through various mechanisms. Another study60 indicated that oxidative stress and mitochondrial dysfunction are common pathophysiological mechanisms in OA and osteopenia/osteoporosis, and they are also shared therapeutic targets. A prospective study61 showed that patients with OA on imaging had a higher total hip bone loss rate over 2.6 years. Another animal study62 indicated that in a rabbit model of OA, osteopenia was proven to worsen cartilage lesions. This study demonstrates that osteopenia raises the incidence of OA, and that the incidence of OA is higher in patients who also have osteosarcopenia compared with patients who have osteopenia or sarcopenia alone, and that patients with osteosarcopenia are at higher risk for OA, especially in the pre-diabetic period.
For the treatment of OA, total joint arthroplasty (TJA) is the preferred option for treating end-stage OA, but both osteopenia/osteoporosis and sarcopenia can lead to reduced quality of recovery after TJA63,64, and increase the likelihood of postoperative adverse events. Bone loss around the prosthesis after TJA can lead to early loosening of the prosthesis, thereby increasing the possibility of revision operation. Mau-Moeller et al.65 found through analyzing changes in peri-prosthetic bone density in OA patients after TKA that reduced bone density around the distal femoral prosthesis is directly related to decreased muscle mass in the affected limb, indicating that sarcopenia can lead to reduced peri-prosthetic bone density after TKA66, which in turn can lead to prosthesis loosening and revision. Meanwhile, prediabetes can also increase the risk of postoperative infection. This also explains from another perspective the importance of conducting bone density testing and sarcopenia assessment during the perioperative period of TJA.
Advantages and limitations
This study is based on the NHANES database, with a large sample size, using a nationally representative sample, and controlling for a multitude of confounding factors such as gender, race, age, income, and biochemical indicators. However, this study has some limitations. First, even with multiple adjustments, we cannot completely rule out the possibility that important variables not included in the NHANES database may affect the results. Second, Since the diagnosis of OA was derived from an interview questionnaire in the NHANES database, there was a lack of extensive imaging data and a lack of clinical assessment of the severity and location of OA, despite the strong concordance between self-reported OA and examination confirmations26. This limitation makes it challenging to obtain a more detailed understanding of how osteopenia affects different joints and stages of OA. Third, Our analysis did not stratify osteosarcopenia by osteopenia versus osteoporosis due to insufficient subgroup sample sizes. Future studies should explore potential differences between these populations. Finally, given the cross-sectional nature of this study, it does not directly reflect causality, and extensive prospective research is still needed to determine this.
Conclusion
In conclusion, this study is the first to use a cross-sectional examination to investigate the association between osteosarcopenia and OA in prediabetes. Compared to NGT patients, those with prediabetes have an increased risk of both sarcopenia and osteopenia. In the prediabetic group, patients with osteosarcopenia have a higher risk of developing OA compared to those with sarcopenia or osteopenia alone. For prediabetic patients, to prevent OA, it is important to focus on the prevention and treatment of osteosarcopenia, especially for those undergoing TJA for OA who also have osteosarcopenia, emphasis should be placed on perioperative care, muscle rehabilitation training, and anti-osteopenia/anti-osteoporosis treatment.
Data availability
This study analyzed publicly available datasets. These data can be found at: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
References
Ádám, G. T., Christian, H., Wolfgang, R., Eric, J. B. & Mika, K. Prediabetes: A high-risk state for diabetes development. Lancet https://doi.org/10.1016/s0140-6736(12)60283-9 (2012).
Khan, R. M. M. et al. From pre-diabetes to diabetes: Diagnosis, treatments and translational research. Medicina (Kaunas) https://doi.org/10.3390/medicina55090546 (2019).
Stefan, N. & Cusi, K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 10, 284–296. https://doi.org/10.1016/s2213-8587(22)00003-1 (2022).
Ziegler, D., Herder, C. & Papanas, N. Neuropathy in prediabetes. Diabetes Metab. Res. Rev. 39, e3693. https://doi.org/10.1002/dmrr.3693 (2023).
Lai, Y. R. et al. Leptin mediate central obesity on the severity of cardiovascular autonomic neuropathy in well-controlled type 2 diabetes and prediabetes. J. Transl. Med. 18, 396. https://doi.org/10.1186/s12967-020-02559-7 (2020).
Jin, J. & Lu, P. Association between prediabetes and retinopathy: A meta-analysis. Horm. Metab. Res. 53, 801–809. https://doi.org/10.1055/a-1678-7092 (2021).
Kirthi, V. et al. Prevalence of retinopathy in prediabetes: Protocol for a systematic review and meta-analysis. BMJ Open 11, e040997. https://doi.org/10.1136/bmjopen-2020-040997 (2021).
Huang, D. et al. Macrovascular complications in patients with diabetes and prediabetes. Biomed. Res. Int. 2017, 7839101. https://doi.org/10.1155/2017/7839101 (2017).
Shi, Y. & Wen, M. Sex-specific differences in the effect of the atherogenic index of plasma on prediabetes and diabetes in the NHANES 2011–2018 population. Cardiovasc. Diabetol. 22, 19. https://doi.org/10.1186/s12933-023-01740-8 (2023).
Atar, A. I., Altuner, T. K., Bozbas, H., Atar, İ & Korkmaz, M. E. Endothelial dysfunction in prediabetes and diabetes mellitus in patients with normal coronary arteries. BMC Cardiovasc Disord 24, 622. https://doi.org/10.1186/s12872-024-04314-y (2024).
Marcella, D. W. & Elizabeth, S. Postmenopausal osteoporosis. N. Engl. J. Med. https://doi.org/10.1056/nejmcp2307353 (2023).
Peck, W. M. Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. https://doi.org/10.1016/0002-9343(93)90218-e (1993).
Cruz-Jentoft, A. J. & Sayer, A. A. Sarcopenia. Lancet 393, 2636–2646. https://doi.org/10.1016/s0140-6736(19)31138-9 (2019).
Gielen, E., Dupont, J., Dejaeger, M. & Laurent, M. R. Sarcopenia, osteoporosis and frailty. Metabolism 145, 155638. https://doi.org/10.1016/j.metabol.2023.155638 (2023).
Damluji, A. A. et al. Sarcopenia and cardiovascular diseases. Circulation 147, 1534–1553. https://doi.org/10.1161/circulationaha.123.064071 (2023).
Joy, E. A., Briesacher, M. & Wiegand, B. Musculoskeletal failure. Am. J. Lifestyle Med. 18, 826–829. https://doi.org/10.1177/15598276241256878 (2024).
Zhu, Y. et al. Association between sarcopenia and osteoporosis: The cross-sectional study from NHANES 1999–2020 and a bi-directions Mendelian randomization study. Front. Endocrinol. (Lausanne) 15, 1399936. https://doi.org/10.3389/fendo.2024.1399936 (2024).
Kalyani, R. R., Corriere, M. & Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2, 819–829. https://doi.org/10.1016/s2213-8587(14)70034-8 (2014).
Davie, G. S., Pal, K., Orton, E., Tyrrell, E. G. & Petersen, I. Incident Type 2 diabetes and risk of fracture: A Comparative cohort analysis using U.K. primary care records. Diabetes Care 44, 58–66. https://doi.org/10.2337/dc20-1220 (2021).
Hashimoto, Y., Takahashi, F., Okamura, T., Hamaguchi, M. & Fukui, M. Diet, exercise, and pharmacotherapy for sarcopenia in people with diabetes. Metabolism 144, 155585. https://doi.org/10.1016/j.metabol.2023.155585 (2023).
Fan, Y., Wei, F., Lang, Y. & Liu, Y. Diabetes mellitus and risk of hip fractures: a meta-analysis. Osteoporos. Int. 27, 219–228. https://doi.org/10.1007/s00198-015-3279-7 (2016).
Arruda, A. L. et al. Genetic underpinning of the comorbidity between type 2 diabetes and osteoarthritis. Am. J. Hum. Genet. 110, 1304–1318. https://doi.org/10.1016/j.ajhg.2023.06.010 (2023).
Xing, X., Wang, Y., Pan, F. & Cai, G. Osteoarthritis and risk of type 2 diabetes: A two-sample Mendelian randomization analysis. J. Diabetes 15, 987–993. https://doi.org/10.1111/1753-0407.13451 (2023).
Classification and Diagnosis of Diabetes. Standards of medical care in diabetes-2019. Diabetes Care 42, S13-s28. https://doi.org/10.2337/dc19-S002 (2019).
Studenski, S. A. et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 69, 547–558. https://doi.org/10.1093/gerona/glu010 (2014).
Wu, A. et al. Association of blood vitamin A with osteoarthritis: a nationally representative cross-sectional study. Front Nutr 11, 1459332. https://doi.org/10.3389/fnut.2024.1459332 (2024).
Dong, G. et al. The neutrophil-lymphocyte ratio as a risk factor for all-cause and cardiovascular mortality among individuals with diabetes: Evidence from the NHANES 2003-2016. Cardiovasc. Diabetol. 22(1), 267. https://doi.org/10.1186/s12933-023-01998-y (2023).
Tucker, L. A. Physical activity and telomere length in U.S. men and women: An NHANES investigation. Prev. Med. 100, 145–151. https://doi.org/10.1016/j.ypmed.2017.04.027 (2017).
Mathew, G. et al. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case–control studies in surgery. Int. J. Surg. 96, 106165 (2021).
Liu, Y., Chai, S. & Zhang, X. Effect of sarcopenia, osteoporosis, and osteosarcopenia on spine fracture in American adults with prediabetes. Front. Endocrinol. (Lausanne) 14, 1163029. https://doi.org/10.3389/fendo.2023.1163029 (2023).
Li, M., Sun, H., Chen, H., Ma, W. & Li, Y. Type 2 diabetes and bone mineral density: A meta-analysis and systematic review. Medicine (Baltimore) 103, e40468. https://doi.org/10.1097/md.0000000000040468 (2024).
Koromani, F. et al. Type 2 diabetes mellitus and vertebral fracture risk. Curr. Osteoporos. Rep. 19, 50–57. https://doi.org/10.1007/s11914-020-00646-8 (2021).
Liu, Z., Asuzu, P., Patel, A., Wan, J. & Dagogo-Jack, S. Association of bone mineral density with prediabetes risk among African-American and European-American adult offspring of parents with type 2 diabetes. Front. Endocrinol. (Lausanne) 13, 1065527. https://doi.org/10.3389/fendo.2022.1065527 (2022).
Lee, J. H. et al. Bone mineral density in prediabetic men. Korean Diabetes J. 34, 294–302. https://doi.org/10.4093/kdj.2010.34.5.294 (2010).
Tramontana, F. et al. More rapid bone mineral density loss in older men with diabetes: The osteoporotic fractures in men (MrOS) study. J. Clin. Endocrinol. Metab. https://doi.org/10.1210/clinem/dgae045 (2024).
Silverman, N. E., Nicklas, B. J. & Ryan, A. S. Addition of aerobic exercise to a weight loss program increases BMD, with an associated reduction in inflammation in overweight postmenopausal women. Calcif. Tissue Int. 84, 257–265. https://doi.org/10.1007/s00223-009-9232-z (2009).
Chen, X. et al. Protective effects of phenolic acid extract from ginseng on vascular endothelial cell injury induced by palmitate via activation of PI3K/Akt/eNOS pathway. J. Food Sci. 85, 576–581. https://doi.org/10.1111/1750-3841.15071 (2020).
Li, M. et al. Nonlinear relationship between untraditional lipid parameters and the risk of prediabetes: A large retrospective study based on Chinese adults. Cardiovasc. Diabetol. 23, 12. https://doi.org/10.1186/s12933-023-02103-z (2024).
Xu, J. et al. Association between sarcopenia and prediabetes among non-elderly US adults. J. Endocrinol. Invest. 46, 1815–1824. https://doi.org/10.1007/s40618-023-02038-y (2023).
Qiu, S. et al. Muscle strength and prediabetes progression and regression in middle-aged and older adults: A prospective cohort study. J. Cachexia Sarcopenia Muscle 13, 909–918. https://doi.org/10.1002/jcsm.12905 (2022).
Jang, B. N., Nari, F., Kim, S. & Park, E. C. Association between relative handgrip strength and prediabetes among South Korean adults. PLoS ONE 15, e0240027. https://doi.org/10.1371/journal.pone.0240027 (2020).
DeFronzo, R. A. & Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32(Suppl 2), S157-163. https://doi.org/10.2337/dc09-S302 (2009).
Gan, Z., Fu, T., Kelly, D. P. & Vega, R. B. Skeletal muscle mitochondrial remodeling in exercise and diseases. Cell Res. 28, 969–980. https://doi.org/10.1038/s41422-018-0078-7 (2018).
Chang, W. T. et al. Serum vitamin D, intact parathyroid hormone, and Fetuin A concentrations were associated with geriatric sarcopenia and cardiac hypertrophy. Sci. Rep. 7, 40996. https://doi.org/10.1038/srep40996 (2017).
Haeri, N. S., Perera, S., Nadkarni, N. K. & Greenspan, S. L. Association of inflammatory markers with muscle and cognitive function in early and late-aging older adults. J. Nutr. Health Aging 28, 100207. https://doi.org/10.1016/j.jnha.2024.100207 (2024).
Clynes, M. A., Gregson, C. L., Bruyère, O., Cooper, C. & Dennison, E. M. Osteosarcopenia: Where osteoporosis and sarcopenia collide. Rheumatology (Oxford) 60, 529–537. https://doi.org/10.1093/rheumatology/keaa755 (2021).
Yang, Y. J. & Kim, D. J. An overview of the molecular mechanisms contributing to musculoskeletal disorders in chronic liver disease: Osteoporosis, sarcopenia, and osteoporotic sarcopenia. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22052604 (2021).
Hamrick, M. W. The skeletal muscle secretome: An emerging player in muscle-bone crosstalk. Bonekey Rep. 1, 60. https://doi.org/10.1038/bonekey.2012.60 (2012).
Giacomo Giuseppina, D. et al. Irisin effects on catabolic and pro-inflammatory genes in human osteoarthritic chondrocytes. In Orthopaedic Proceedings (2020).
Di Filippo, L., De Lorenzo, R., Giustina, A., Rovere-Querini, P. & Conte, C. Vitamin D in osteosarcopenic obesity. Nutrients https://doi.org/10.3390/nu14091816 (2022).
Hosoi, T., Yakabe, M., Hashimoto, S., Akishita, M. & Ogawa, S. The roles of sex hormones in the pathophysiology of age-related sarcopenia and frailty. Reprod. Med. Biol. 23, e12569. https://doi.org/10.1002/rmb2.12569 (2024).
Kemmler, W. et al. Prevalence of sarcopenia in Germany and the corresponding effect of osteoarthritis in females 70 years and older living in the community: Results of the FORMoSA study. Clin. Interv. Aging 10, 1565–1573. https://doi.org/10.2147/cia.S89585 (2015).
Chen, S., Han, H., Jin, J., Zhou, G. & Li, Z. Osteoarthritis and sarcopenia-related traits: the cross-sectional study from NHANES 2011–2014 and Mendelian randomization study. J. Orthop. Surg. Res. 18, 502. https://doi.org/10.1186/s13018-023-03960-w (2023).
Zhang, L. et al. A bidirectional Mendelian randomization study of sarcopenia-related traits and knee osteoarthritis. Clin. Interv. Aging 18, 1577–1586. https://doi.org/10.2147/cia.S424633 (2023).
Culvenor, A. G. et al. Thigh muscle specific-strength and the risk of incident knee osteoarthritis: The influence of sex and greater body mass index. Arthritis Care Res. (Hoboken) 69, 1266–1270. https://doi.org/10.1002/acr.23182 (2017).
Xu, J. et al. Knee muscle atrophy is a risk factor for development of knee osteoarthritis in a rat model. J. Orthop. Translat. 22, 67–72. https://doi.org/10.1016/j.jot.2019.10.003 (2020).
Silva, J. M. S. et al. Muscle wasting in osteoarthritis model induced by anterior cruciate ligament transection. PLoS ONE 13, e0196682. https://doi.org/10.1371/journal.pone.0196682 (2018).
Qu, Y. et al. Osteoporosis and osteoarthritis: A bi-directional Mendelian randomization study. Arthritis Res. Ther. 25, 242. https://doi.org/10.1186/s13075-023-03213-5 (2023).
Salari, N. et al. Global prevalence of osteoporosis among the world older adults: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 16, 669. https://doi.org/10.1186/s13018-021-02821-8 (2021).
Riegger, J., Schoppa, A., Ruths, L., Haffner-Luntzer, M. & Ignatius, A. Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: A narrative review. Cell. Mol. Biol. Lett. 28, 76. https://doi.org/10.1186/s11658-023-00489-y (2023).
Ding, C. et al. Knee and hip radiographic osteoarthritis predict total hip bone loss in older adults: A prospective study. J. Bone Miner. Res. 25, 858–865. https://doi.org/10.1359/jbmr.091012 (2010).
Zhang, J. et al. Ultrastructural change of the subchondral bone increases the severity of cartilage damage in osteoporotic osteoarthritis of the knee in rabbits. Pathol. Res. Pract. 214, 38–43. https://doi.org/10.1016/j.prp.2017.11.018 (2018).
Daher, M., Mekhael, E. & El-Othmani, M. M. Osteoporosis in the setting of knee arthroplasty: A narrative review. Arthroplasty 6, 50. https://doi.org/10.1186/s42836-024-00273-z (2024).
Ardeljan, A. D., Polisetty, T. S., Palmer, J., Vakharia, R. M. & Roche, M. W. Comparative analysis on the effects of sarcopenia following primary total knee arthroplasty: A retrospective matched-control analysis. J. Knee Surg. 35, 128–134. https://doi.org/10.1055/s-0040-1713355 (2022).
Mau-Moeller, A. et al. Modulation and predictors of periprosthetic bone mineral density following total knee arthroplasty. Biomed. Res. Int. 2015, 418168. https://doi.org/10.1155/2015/418168 (2015).
Babu, J. M. et al. Sarcopenia as a risk factor for prosthetic infection after total hip or knee arthroplasty. J. Arthroplasty 34, 116–122. https://doi.org/10.1016/j.arth.2018.09.037 (2019).
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
Shanbin Zheng conceived and designed the experiments and contributed significantly to this paper as first author, and Liansheng Shao did the same as co-first author. Zhiyuan Chen and Xun Cao performed the data analysis. Jiahao Sun and Jiaqing Zhu wrote the manuscript. Chao Zhang revised the manuscript, Tianwei Xia and Jirong Shen provided guidance for the study, Jirong Shen is the corresponding author, with Tianwei Xia and Chao Zhang as the co-corresponding author. All authors contributed to the manuscript and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zheng, S., Shao, L., Zhu, J. et al. Exploring the link between osteosarcopenia and osteoarthritis in prediabetic adults using NHANES data. Sci Rep 15, 19747 (2025). https://doi.org/10.1038/s41598-025-95344-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-95344-9