Abstract
Secondary/persistent endodontic infections (SPEIs) result from failed root canal therapy, causing persistent apical periodontitis. Current diagnostic methods for SPEIs predominantly rely on clinical and radiographic indicators, which often lack adequate sensitivity and specificity. Consequently, there is an urgent need to effectively detect SPEIs or monitor their progression. The aim of this study was to compare and characterize the microbiota of root canals and gingival sulci of teeth affected by SPEI to identify keystone pathogens as potential diagnostic biomarkers through advanced next-generation sequencing (NGS) techniques. Ninety samples from 30 affected teeth in 25 patients undergoing nonsurgical retreatment were analyzed. Bacterial DNA was extracted, the V3–V4 region of the 16S rRNA gene was amplified, and sequencing was performed (Illumina MiSeq). Amplicon sequence variants (ASVs) identified 16 phyla, 182 genera, and 390 species. Microbiota in root canals differed from gingival sulci, with Acinetobacter and Veillonella prevalent in canals, and Streptococcus and Actinomyces dominant in sulci. Certain species, including Shuttleworthella satelles, Olsenella uli, Dialister invisus, Massilia timonae, and Klebsiella pneumoniae were detected in both sites, suggesting microbial migration via anatomical structures. Detecting these potential keystone pathogens of SPEI as biomarkers in readily accessible sulcus fluid could facilitate diagnoses and monitoring of progression and/or resolution. These insights provide a foundation for more accurate and targeted diagnostic and therapeutic strategies for management of SPEIs.
Similar content being viewed by others
Introduction
Secondary/persistent endodontic infections (SPEIs) develop within the root canal of a tooth when initial therapy fails to eliminate microbial infections and can lead to persistent extra-radicular inflammation and infection1,2. SPEIs are primarily caused by pathogenic microorganisms that survive the initial treatment and often form complex biofilm structures that are difficult to eradicate3. Their treatment is further complicated by the challenges of detecting and monitoring SPEIs using current diagnostic techniques4. Traditional diagnostic techniques lack the sensitivity and specificity to accurately identify the causative agents of SPEIs, leading to suboptimal treatment outcomes5. Therefore, enhanced diagnostic methods, treatment protocols, and prevention strategies are required to manage SPEIs and prevent relapse.
Current diagnoses are based on the emergence or persistence of clinical and radiographic signs and/or symptoms of apical periodontitis in endodontically treated teeth4. However, as the pathogenesis of SPEIs depends on endodontic microbial communities4, identification of keystone pathogens could provide a strategy for enhanced diagnoses6. Advanced microbiological techniques such as next-generation sequencing (NGS) provide comprehensive insights into microbiota and could be used to identify keystone pathogens4. This approach could enhance diagnoses and provide insights into the progression of endodontic disease for more effective treatment strategies.
The persistence of apical periodontitis with SPEIs is dependent on apical microbial penetration within root canals, which are usually inaccessible for sampling7. Instead, gingival sulci that probably communicate with root canals through anatomical structures, including lateral canals, apical foramina, and dentinal tubules, could provide a more readily accessible source8,9. Microorganisms transfer between the root canal and gingival sulcus through these anatomical pathways, particularly when periodontal disease has compromised the integrity of these structures8. The disruption of periodontal tissues in lateral canals connecting the periodontal pocket to the root canal facilitates bacterial migration from the canal to the sulcus10,11. As the gingival sulcus is an easily accessible site for sampling, it could serve as a surrogate source for identifying keystone pathogens implicated in endodontic infections, especially SPEIs11.
Accordingly, microbial samples from gingival sulci and root canals were analyzed by NGS technology in a preliminary analysis to characterize and compare the microbial communities of primary endodontic infections (PEIs) with those of SPEIs and significant differences in microbial diversities and compositions have been reported12. However, the previous study was limited by a small sample size (n = 30). In the present study, we collected 90 samples from 30 teeth in 25 patients and improved pre-processing and analysis techniques using more refined methods than previous studies. By focusing on specific pathogens commonly detected in the root canal and gingival sulcus of SPEI-affected teeth, we aim to identify disease-specific biomarkers for SPEI, gain deeper insights into the microbial ecology of endodontic infections, and ultimately demonstrate the feasibility of diagnosing and monitoring SPEI through gingival sulcus-based sampling.
Results
Alpha and beta diversity of microbiota
To identify the microbial diversity and community composition that are unique or shared between the root canal and gingival sulcus, numbers of amplicon sequence variants (ASVs) in the samples and the alpha diversity Shannon index were compared. Both the number of ASVs and Shannon index of root canals showed significant differences compared to Sulcus-C and Sulcus-E, though there were no differences between Sulcus-C and Sulcus-E (Fig. 1A,B). Notably, the root canals of teeth with SPEIs had distinct numbers and diversity of microbiota from the sulcus, whereas separate areas of the gingival sulcus did not show any differences.
Alpha diversity of the root canal and gingival sulcus with secondary/persistent endodontic infections (SPEIs). Bacterial alpha diversity assessed using observed ASVs (A) and Shannon indices (B) of Sulcus-C, Sulcus-E, and Canal samples in SPEI patients. The boxplot illustrates the interquartile range (IQR), with the bottom edge representing the first quartile (Q1), the lower whisker extending to 1.5 times the IQR below Q1, a median line inside, the upper edge as the third quartile (Q3), and the upper whisker reaching the highest value within 1.5 times the IQR above Q3. Points outside the boxplot represent outliers. Triple asterisks (***) indicate statistically significant differences (q-value < 0.001).
To estimate the relevance of microbial community patterns for comparisons between the root canal and gingival sulcus of teeth with SPEIs, principal coordinate analysis (PCoA) based on unweighted and weighted UniFrac was performed. In both unweighted and weighted UniFrac PCoA plots, Sulcus-C and Sulcus-E exhibited no differences in bacterial communities. However, Canal showed significant differences compared to Sulcus-C and Sulcus-E (q < 0.05) (Fig. 2A,B). Overall, beta diversity analysis indicated that the microbial communities of root canals had noticeable characteristics from those of gingival sulci.
Principal coordinates analysis (PCoA) and beta diversity of bacterial communities. PCoA plot based on unweighted UniFrac distance (A) and weighted UniFrac distance (B) showing bacterial community structures of Sulcus-C, Sulcus-E, and Canal samples from SPEI patients. Statistical significance was assessed using pairwise PERMANOVA.
Taxonomic analysis of microbiota
Taxonomic distributions at the phylum, genus, and species levels were analyzed in the Canal, Sulcus-C, and Sulcus-E. All ASV sequences were annotated using the Nucleotide Basic Local Alignment Search Tool (BLASTN) provided by the National Center for Biotechnology Information (NCBI); and 16 phyla, 182 genera, and 390 species were identified. At the phylum level, Bacillota, Pseudomonadota, Actinomycetota, Bacteroidota, and Fusobacteriota were abundant in both root canals and gingival sulci (Fig. 3A). Bacillota was the most dominant phylum in Canal, accounting for 38.93% of the microbial population, followed by Pseudomonadota (19.69%), Bacteroidota (15.88%), Actinomycetota (11.93%), and Fusobacteriota (7.44%). Similarly, Bacillota was the most dominant phylum in both Sulcus-C and Sulcus-E (32.70% in Sulcus-C, 35.08% in Sulcus-E), followed by Pseudomonadota (28.61% in Sulcus-C, 20.73% in Sulcus-E), Actinomycetota (21.97% in Sulcus-C, 19.19% in Sulcus-E), Bacteroidota (7.30% in Sulcus-C, 11.95% in Sulcus-E), and Fusobacteriota (6.48% in Sulcus-C, 6.66% in Sulcus-E). Interestingly, Synergistota was more abundant in Canal (3.17%) than in either Sulcus-C or Sulcus-E (< 1.00% in Sulcus-C/-E). In contrast, Campylobacterota was not observed in Canal (< 1.00%) but was present in Sulcus-C (1.73%) and Sulcus-E (1.21%). Candidatus_Saccharibacteria and Spirochaetota were found mainly in Sulcus-E (2.43% and 1.89%, respectively), with less than 1% observed in either Canal or Sulcus-C. Although there were specific differences in microbial composition at the phylum level, the overall sampling sites showed similar distributions.
The relative abundances of bacteria in the root canal and gingival sulcus infected with SPEIs. Relative abundance of bacterial composition in Sulcus-C, Sulcus-E, and Canal samples from SPEI patients at the phylum (A), genus (B), and species (C) levels. Taxa with a relative abundance less than 1% in each sample were grouped as ‘< 1%.’
At the genus level, significant differences were observed between the root canal and the gingival sulcus, whereas no significant differences were observed between Sulcus-C and Sulcus-E (Fig. 3B). In Canal, Acinetobacter was the most abundant genus, accounting for 10.28%, followed by Veillonella (7.97%), Fusobacterium (7.40%), Streptococcus (7.26%), Enterococcus (5.21%), Phocaeicola (4.51%), and Porphyromonas (4.48%). In gingival sulci, Streptococcus was the most common genus (25.78% in Sulcus-C, 22.98% in Sulcus-E), followed by Actinomyces (9.72% in Sulcus-C, 10.78% in Sulcus-E), both of which were significantly less abundant in Canal (1.01%). Furthermore, Fusobacterium (4.62% in Sulcus-C, 5.58% in Sulcus-E), Acinetobacter (7.09% in Sulcus-C, 4.64% in Sulcus-E), and Veillonella (3.02% in Sulcus-C, 3.68% in Sulcus-E) were relatively less prevalent in gingival sulci than root canals. In contrast, Neisseria (5.73% in Sulcus-C, 4.92% in Sulcus-E), Haemophilus (7.62% in Sulcus-C, 4.21% in Sulcus-E), Rothia (6.30% in Sulcus-C, 3.38% in Sulcus-E), and Lautropia (3.36% in Sulcus-C, 3.01% in Sulcus-E) were more abundant in gingival sulci than root canals (< 1.00%).
At the species level (Fig. 3C), Acinetobacter baumannii was the most abundant species in the root canal (9.78%), followed by Enterococcus faecalis (5.21%), Veillonella dispar (5.19%), Fusobacterium animalis (4.58%), Phocaeicola abscessus (4.51%), Arachnia propionica (3.32%), Porphyromonas gingivalis (3.07%), Pseudoramibacter alactolyticus (3.00%), Enterobacter mori (2.89%), Peptostreptococcus stomatis (2.80%), and Streptococcus sanguinis (2.61%). S. sanguinis was the predominant species in both Sulcus-C (7.98%) and Sulcus-E (6.61%), followed by S. mitis (7.69% in Sulcus-C, 6.60% in Sulcus-E). A. baumannii (7.69% in Sulcus-C, 6.60% in Sulcus-E) and V. dispar (7.69% in Sulcus-C, 6.60% in Sulcus-E) were less abundant in gingival sulcus compared to root canal. Despite these distinctions between the microbial communities of root canals and gingival sulci, they were not distinct from each other and shared several taxonomic groups.
Differential abundance and enrichment of microbiota
Microbiome Multivariable Association with Linear Models (MaAsLin) 2 analysis was performed to compare the abundances of bacterial genera and species among the three sampling sites. There were 32 genera dominant in Canal compared to Sulcus-C, and 15 genera were more abundant in Sulcus-E than Sulcus-C (q < 0.05) (Fig. 4A). Interestingly, Olsenella, Shuttleworthella, Mogibacterium, Dialister, and Family XIII UCG-001 were the most abundant genera in Canal and Sulcus-E compared to Sulcus-C, suggesting that they are associated with SPEIs. At the species level, 40 species were predominant in Canal, and 15 species showed greater abundance in Sulcus-E than in Sulcus-C (q < 0.05) (Fig. 4B). Among these, [Eubacterium] nodatum, Shuttleworthella satelles, Olsenella uli, [Eubacterium] brachy, Family XIII UCG-001 species, and Dialister invisus were increased in abundance in Sulcus-E compared to Sulcus-C. These results indicate that S. satelles, O. uli, and D. invisus, which were more abundant in Canal and Sulcus-E compared to Sulcus-C according to the MaAsLin2 analysis, could be keystone pathogens in the pathogenesis of SPEIs.
Bacterial abundance in the root canal and gingival sulcus lesion at genus and species levels. Heatmap illustration of differentially abundant bacterial genera (A) and species (B) identified by MaAsLin2 analysis. Values were calculated by dividing the coefficient (coef) by the standard error (stderr). Significance levels are denoted as follows: * for q < 0.05, ** for q < 0.01, and *** for q < 0.001. Symbols ‘+’ and ‘−’ indicate not significant but increasing or decreasing tendency, respectively. Blank cells represent taxa that were not detected.
Correlations between the root canal and gingival sulcus of SPEI teeth
To identify the relationships between Canal and Sulcus-E, further comprehensive analysis was conducted using Spearman correlations (Fig. 5). At the genus level, Asticcacaulis, Pelospora, Fastidiosipila, Olegusella, and Pseudopropionibacterium showed strong correlation between Canal and Sulcus-E (Fig. 5A). Moreover, the 10 species Paracoccus marinus, Massilia timonae, Schaalia meyeri, Limosilactobacillus oris, Klebsiella pneumoniae, Methylobacterium aquaticum, Sphingomonas adhaesiva, Olegusella massiliensis, Kocuria salina, and Peptoniphilus lacydonensis were the only shared bacteria between Canal and Sulcus-E, displaying robust correlations among fully classified taxonomies at the species level (Fig. 5B). In addition, 9 species including E. faecalis, E. infirmum, O. uli, Methanobrevibacter oralis, and Treponema socranskii were more abundant in Canal than Sulcus-C but showed no differences between Canal and Sulcus-E (Supplementary Fig. 1). Among them, M. oralis was not detected in Sulcus-C samples. These results suggest that bacteria S. meyeri, M. timonae, K. pneumoniae, and K. salina, which were specifically present in the Canal and Sulcus-E but absent from Sulcus-C, are suitable diagnostic biomarkers of SPEI.
Correlations of centered log-ratio (clr)-transformed relative abundances of uniquely shared bacterial taxa between the root canal and gingival sulcus lesions. Bacterial taxa uniquely shared between lesion sites were identified at the genus (A) and species level (B), and the correlations of their clr-transformed relative abundance at the two sites were assessed using Spearman’s correlation analysis. Only taxa with statistically significant correlations (rho > 5, q < 0.05) are presented.
Discussion
Analyzing the microbiota associated with SPEIs could reveal key pathogens and microbial interactions that contribute to the persistence of infection. This could lead to the development of biomarkers for SPEIs. Understanding their microbial community compositions and dynamics will be essential for developing targeted therapeutic strategies13. Accordingly, this investigation studied the distinct or uniquely shared microbial communities between the root canal and gingival sulcus in teeth with SPEIs. Cutting-edge NGS technology was successfully used to analyze 90 root canals and gingival sulcus samples from 25 patients with 30 affected teeth undergoing nonsurgical endodontic re-treatment. Using this approach, 16 phyla, 182 genera, and 390 species were identified. Bacteria such as S. satelles, O. uli, and D. invisus are relatively abundant within SPEIs and could be keystone pathogens. Certain bacterial species including P. marinus, M. timonae, and S. meyeri are exclusively associated with root canals (Canal) and gingival sulci (Sulcus-E) of teeth with SPEIs and have potential to serve as biomarkers for diagnosis of SPEIs.
We observed strong correlations among 10 bacterial species, including P. marinus, M. timonae, and S. meyeri, which are exclusively shared between the canal and Sulcus-E. This suggests that these species play a crucial role in the pathogenesis of SPEIs and the persistence of endodontic infections that extend from the canal to surrounding periodontal tissues. K. pneumoniae shows increased associations with periodontal pockets, causing chronic periodontitis14. S. meyeri, M. timonae, and K. salina are found in the oral cavity and can contribute to infections under certain circumstances15,16,17. Simon et al. reported that root canal infections can drain through periodontal tissues, leading to chronic apical abscesses, or conversely, bacteria can enter the root canal through exposed cementum or lateral canals during periodontitis18. In addition, endodontic lesions can drain through the gingival fissure, and cases have been reported where endodontic microorganisms migrated to the periodontal tissues, causing bone destruction19. This anatomical and microbiological link between the root canal and the gingival sulcus suggests a clinically significant relationship. The presence of shared pathogens further implies that gingival sulcus samples may serve as an indirect method for detecting residual endodontic infections or recurrences. These unique microbial patterns present an opportunity to simplify the detection of keystone pathogens by analyzing readily accessible sulcus fluid instead of inaccessible apical root canal contents.
In the current study, the abundance of microbial communities at the phylum level was similar to that found in a previous study, but differences were observed at the genus level. The most abundant genus in Canal was Acinetobacter, followed by Veillonella, Fusobacterium, Streptococcus, Enterococcus, Phocaeicola, and Porphyromonas. In a previous study, the most dominant phyla in Canal were Bacillota (synonym of Firmicutes) and Pseudomonadota (synonym of Proteobacteria), and the most abundant genus was Streptococcus, followed by Actinobacter, Exiguobacterium, Fusobacterium, Campylobacter, and Propionibacterium12. Although Fusobacterium species were abundant, overall differences between the current and previous studies may be due to several factors including bacterial DNA extraction methods, sample size, analytical techniques, negative controls, and other distinctions. According to Shantelle et al., different types of bacterial DNA extraction kits have differences in efficiency, DNA purity, and yield20. The 50% increase in sample size from 60 to 90 SPEIs analyzed could have affected the results21. Also, the present study used enhanced analytic techniques including MaAsLin2 and negative controls in this study. The presence of PCR bands on untreated paper points and in pure reagents were selected as negative controls22, and contaminants were removed during the analytic process using a decontamination package. However, both previous and current studies utilized paper point sampling, which may not adequately retrieve whole microbial cells from biofilm sequestered within the intricate apical root canal anatomy7. Alves et al. proposed cryopulverization as an alternative sampling technique, which can circumvent many of the limitations of the conventional paper point method23. An upcoming study will utilize such a cryopulverization technique on apical root segments of teeth with SPEIs obtained through periradicular surgery. Ultimately, standardization and optimization of techniques are essential to compare the microbiological results obtained from different studies.
Interestingly, the abundance of O. uli was increased in root canals (Canal) and gingival sulci (Sulcus-E) of teeth with SPEIs compared to the gingival sulci (Sulcus-C) of healthy contralateral teeth, consistent with previous studies. Recently, Sheela et al. reported that the dominant genera in post-treatment infection (SPEI) compared to PEI were Prevotella, Treponema, Lactobacillus, Streptococcus, Leptotrichia, Aggregatibacter, Bifidobacterium, Proprionibacterium and Mycoplasma24. They reported that Olsenella showed significant variation in SPEI bacteria compared to those in PEIs. Another study using PCR found that D. invisus were present in 23.3% of PEIs and 80% of SPEIs, and O. uli was present in 36.6% of PEIs and 30% of SPEIs25. The high prevalence of these species in both primary and persistent infections, as demonstrated by numerous studies utilizing advanced molecular techniques like PCR and 16S rRNA gene sequencing, indicates their importance as keystone pathogens. While direct evidence of S. satelles related to endodontic infections is insufficient, and further in vitro or in vivo research is needed, these findings suggest that these specific bacteria are appropriate candidates for keystone pathogens of SPEI.
Other species such as E. faecalis, E. infirmum, and T. socranskii were more abundant in Canal compared to Sulcus-C, despite showing no significant difference between Canal and Sulcus-E, which suggests a potential role for these microorganisms in sustaining infections. E. faecalis is well-known for its association with oral infectious diseases, especially endodontic infection and apical periodontitis, and is frequently implicated in endodontic treatment failures due to biofilm formation and resistance to endodontic medicaments26,27,28. Uniquely abundant species of both Canal and Sulcus-E, such as E. infirmum, M. oralis, S. exigua, and T. socranskii, have been associated with periodontal diseases in previous studies. E. infirmum is a member of the oral microbiome that is often found in subgingival plaque and is involved in the pathogenesis of periodontal disease29. M. oralis and S. exigua were in higher abundance within periodontal lesion sites compared to healthy regions30,31, and T. socranskii was associated with advanced periodontitis32. The presence of specific bacteria in both infected sites without significant variation suggests that these species are integral to the pathogenesis of SPEIs, suggesting them as biomarkers or keystone pathogens that maintain the infectious process across oral environments.
We identified specific bacterial species commonly distributed between the root canal and gingival sulcus and confirmed their potential as disease-specific biomarkers. Although teeth with radiographic evidence of open apices, root fractures, root perforations, endodontic-periodontal combined lesions, and progressive periodontal disease (Stage III or IV) were excluded from recruitment and also compared to the healthy contralateral tooth samples, a limitation of this study is its cross-sectional design. This prevents definitive conclusions about whether the infection spreads from the root canal to the gingival sulcus or vice versa. Future longitudinal studies with time-series data are needed to elucidate the actual transmission route and temporal dynamics33,34. Additionally, further experiments should investigate the infectious environment in greater detail using immunological and molecular biological techniques to track and validate bacterial migration and inflammatory response processes between the root canal and gingival sulcus. Such follow-up studies would contribute to a comprehensive understanding of the mechanisms underlying the development and persistence of SPEIs, the complex interactions between these sites, and the advancement of more effective diagnostic and therapeutic strategies.
Overall, this study demonstrated a correlation between the microbiota of root canals and their gingival sulci in teeth with SPEI, and that they differed from those of healthy sulci. The uniquely shared bacterial species included S. satelles, O. uli, D. invisus, P. marinus, M. timonae, and S. meyeri, which could potentially serve as biomarkers of SPEI. These findings contribute toward a comprehensive understanding of the interactions between the root canal and gingival sulcus in endodontic infections. Further in-depth investigations of these uniquely shared distinct bacterial species will be crucial for the development of rapid chairside diagnostic techniques and novel anti-biofilm therapeutic strategies against keystone pathogens13,35, which may include innovative endodontic disinfection therapies for SPEIs.
Materials and methods
Subjects and sampling
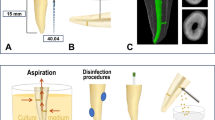
This study was conducted in accordance with the principle of the Declaration of Helsinki. All clinical procedures were approved by the Institutional Review Board (IRB) of Seoul National University Dental Hospital (IRB No. CRI21005), and signed informed consent forms were obtained from 25 patients (mean age 56.6 years) assigned for nonsurgical endodontic re-treatment. These patients had 30 teeth (11 incisors, 3 premolars, and 16 molars) that had received nonsurgical endodontic treatment at least 2 years prior and now exhibited periradicular radiolucencies consistent with SPEIs and post-treatment apical periodontitis. All teeth had intact coronal restorations without any exposure of obturating materials, and their canals were filled within 4 mm of the radiographic apex without any overfill. Patients who were pregnant or heavy smokers; those with systemic disorders including uncontrolled diabetes, hypertension, or autoimmune disease; those receiving radiation therapy to the head and neck area or who had received antibiotic therapy within the last 3 months were excluded from the study. In addition, teeth with radiographic evidence of open apices, root fractures, root perforations, endodontic-periodontal combined lesions, progressive periodontal disease (Stage III or IV), or that could not be adequately isolated by rubber dam were excluded. Samples were collected as previously described12. Briefly, under strict aseptic conditions, separate sterile paper points (DiaDent, Cheongju-si, Korea) were inserted into the root canal of the tooth with SPEI (Canal), the gingival sulcus of the tooth with SPEI (Sulcus-E), and the gingival sulcus of a healthy contralateral tooth (Sulcus-C), each for 30 s, and then placed into separate Nunc cryotube vials (Thermo Fisher, USA). All samples were transferred in liquid nitrogen and then stored at −80 °C.
Genomic DNA extraction, amplification, and sequencing
Bacterial genomic DNA was extracted from the paper points using a DNeasy PowerSoil Pro Kit (QIAGEN, cat# 47014), according to the manufacturer’s instructions. The quantity of genomic DNA was measured by a Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, cat# P7589). Then extracted bacterial gDNA was amplified by two-step polymerase chain reaction (PCR). The V3-V4 variable regions of the bacterial 16S ribosomal RNA gene were amplified using 341F and 805R primer sets, and PCR products were purified using AMPure XP beads (Beckman Coulter). Then, PCR was performed to add adapter and index to the amplicons for sequencing, and the products were purified using a QIAquick PCR Purification Kit (QIAGEN), followed by quantification with a Quant-iT™ PicoGreen™ dsDNA Assay Kit (Invitrogen). PCR product sizes were confirmed by gel electrophoresis. Equal amounts of total PCR products were pooled for sequencing on an Illumina MiSeq System (2 × 300 bp, paired-end sequencing).
Sequencing data analysis
Raw sequences were assigned to their respective samples through barcode-based demultiplexing, followed by quality checks using FastQC and MultiQC. The data were analyzed using QIIME2 plugins (version 2023.2). Cutadapt was used to trim adapter and primer sequences from the reads. Host genomic contaminants were removed using Bowtie2 with the human reference genome GRCh38. ASVs were derived after eliminating low-quality sequences and chimeras using DADA2. Additional filtering procedures were performed based on sequence frequency, prevalence, and length. Sequences shorter than 300 bp and those detected in two or fewer samples were excluded. Negative controls were included throughout the entire process to monitor and avoid contamination. Furthermore, contaminant sequences were identified and removed during data analysis using the R decontam package based on the frequency and prevalence of sequence features. Taxonomic assignment was conducted using a Naive Bayes classifier, which was pretrained on the V3-V4 regions of the SILVA 138.1 database. The taxonomic annotations were validated using NCBI BLAST+ software against the 16 S ribosomal RNA database, with a sequence similarity threshold of 99%. Taxonomic names assigned by the SILVA database were checked and manually revised. Statistical analysis and data visualization were performed in R packages.
Statistical analysis
Alpha and beta diversities of bacterial communities were compared between sampling sites using the Kruskal-Wallis test (with the Dunn test for pairwise multiple comparisons; rstatix package) and pairwise permutational multivariate analysis of variance (PERMANOVA; vegan package), respectively. Differentially abundant taxa were identified using MaAsLin2 (Maaslin2 package) with the compound Poisson linear model method and total sum scaling normalization. Spearman’s rank correlation coefficients were calculated using the R rstatix package. Bacterial relative abundances between sampling sites were compared using Wilcoxon rank sum tests (rstatix package). P-values were adjusted by the Benjamini-Hochberg method for multiple comparisons. Results were considered statistically significant if adjusted P-value (referred to as q-value) was less than 0.05.
Data availability
The raw sequencing data was uploaded in Fastq format on NCBI Short Read Archive (BioProject accession number PRJNA1161209).
References
Gunduz, G. et al. Endotoxin of Porphyromonas gingivalis amplifies the inflammatory response in hyperglycemia-induced zebrafish through a mechanism involving chitinase-like protein YKL-40 analogs. Toxicol. Res. 39, 625–636. https://doi.org/10.1007/s43188-023-00190-4 (2023).
Siqueira, J. F. Jr., Rocas, I. N., Ricucci, D. & Hulsmann, M. Causes and management of post-treatment apical periodontitis. Br. Dent. J. 216, 305–312. https://doi.org/10.1038/sj.bdj.2014.200 (2014).
Velazquez-Moreno, S. et al. Use of a cellulase from Trichoderma reesei as an adjuvant for Enterococcus faecalis biofilm disruption in combination with antibiotics as an alternative treatment in secondary endodontic infection. Pharmaceutics 15. https://doi.org/10.3390/pharmaceutics15031010 (2023).
Kesim, B. et al. Amplicon-based next-generation sequencing for comparative analysis of root canal microbiome of teeth with primary and persistent/secondary endodontic infections. Clin. Oral Investig. 27, 995–1004. https://doi.org/10.1007/s00784-023-04882-x (2023).
Keskin, C., Demiryurek, E. O. & Onuk, E. E. Pyrosequencing analysis of cryogenically ground samples from primary and secondary/persistent endodontic infections. J. Endod. 43, 1309–1316. https://doi.org/10.1016/j.joen.2017.03.019 (2017).
Zargar, N., Marashi, M. A., Ashraf, H., Hakopian, R. & Beigi, P. Identification of microorganisms in persistent/secondary endodontic infections with respect to clinical and radiographic findings: Bacterial culture and molecular detection. Iran. J. Microbiol. 11, 120–128 (2019).
Siqueira, J. F. Jr., Antunes, H. S., Rocas, I. N., Rachid, C. T. & Alves, F. R. Microbiome in the apical root canal system of teeth with post-treatment apical periodontitis. PLoS One 11, e0162887. https://doi.org/10.1371/journal.pone.0162887 (2016).
Sunitha, V. R., Emmadi, P., Namasivayam, A., Thyegarajan, R. & Rajaraman, V. The periodontal-endodontic continuum: A review. J. Conserv. Dent. 11, 54–62. https://doi.org/10.4103/0972-0707.44046 (2008).
Ran, S., Wang, J., Jiang, W., Zhu, C. & Liang, J. Assessment of dentinal tubule invasion capacity of Enterococcus faecalis under stress conditions ex vivo. Int. Endod J. 48, 362–372. https://doi.org/10.1111/iej.12322 (2015).
Rupf, S. et al. Comparison of profiles of key periodontal pathogens in periodontium and endodontium. Endod. Dent. Traumatol. 16, 269–275. https://doi.org/10.1034/j.1600-9657.2000.016006269.x (2000).
Fatima, T. et al. Gingival crevicular fluid (GCF): A diagnostic tool for the detection of periodontal health and diseases. Molecules 26. https://doi.org/10.3390/molecules26051208 (2021).
Park, D. H. et al. Microbiota association and profiling of gingival sulci and root canals of teeth with primary or secondary/persistent endodontic infections. J. Endod. 50, 1124–1133. https://doi.org/10.1016/j.joen.2024.04.016 (2024).
Wong, J., Manoil, D., Nasman, P., Belibasakis, G. N. & Neelakantan, P. Microbiological aspects of root canal infections and disinfection strategies: An update review on the current knowledge and challenges. Front. Oral Health 2, 672887. https://doi.org/10.3389/froh.2021.672887 (2021).
Gamboa, F. et al. Presence and antimicrobial profile of gram-negative facultative anaerobe rods in patients with chronic periodontitis and gingivitis. Acta Odontol. Latinoam. 26, 24–30 (2013).
Kononen, E. Polymicrobial infections with specific Actinomyces and related organisms, using the current taxonomy. J. Oral Microbiol. 16, 2354148. https://doi.org/10.1080/20002297.2024.2354148 (2024).
Van Craenenbroeck, A. H., Camps, K., Zachee, P. & Wu, K. L. Massilia timonae infection presenting as generalized lymphadenopathy in a man returning to Belgium from Nigeria. J. Clin. Microbiol. 49, 2763–2765. https://doi.org/10.1128/JCM.00160-11 (2011).
Kandi, V. et al. Emerging bacterial infection: Identification and clinical significance of Kocuria species. Cureus 8, e731. https://doi.org/10.7759/cureus.731 (2016).
Simon, J. H., Glick, D. H. & Frank, A. L. The relationship of endodontic-periodontic lesions. J. Endod. 39, e41–46. https://doi.org/10.1016/j.joen.2013.02.006 (2013).
Jansson, L. E. & Ehnevid, H. The influence of endodontic infection on periodontal status in mandibular molars. J. Periodontol. 69, 1392–1396. https://doi.org/10.1902/jop.1998.69.12.1392 (1998).
Claassen, S. et al. A comparison of the efficiency of five different commercial DNA extraction kits for extraction of DNA from faecal samples. J. Microbiol. Methods 94, 103–110. https://doi.org/10.1016/j.mimet.2013.05.008 (2013).
Ferdous, T. et al. The rise to power of the microbiome: power and sample size calculation for microbiome studies. Mucosal Immunol. 15, 1060–1070. https://doi.org/10.1038/s41385-022-00548-1 (2022).
Glassing, A., Dowd, S. E., Galandiuk, S., Davis, B. & Chiodini, R. J. Inherent bacterial DNA contamination of extraction and sequencing reagents may affect interpretation of microbiota in low bacterial biomass samples. Gut Pathog. 8, 24. https://doi.org/10.1186/s13099-016-0103-7 (2016).
Alves, F. R. et al. Bacterial community profiling of cryogenically ground samples from the apical and coronal root segments of teeth with apical periodontitis. J. Endod. 35, 486–492. https://doi.org/10.1016/j.joen.2008.12.022 (2009).
Abraham, S. B. et al. Molecular analyses indicate profuse bacterial diversity in primary and post- treatment endodontic infections within a cohort from the united Arab Emirates—A preliminary study. PLoS One 19, e0305537. https://doi.org/10.1371/journal.pone.0305537 (2024).
Manohar, S. et al. Simplified method of detection of Dialister invisus and Olsenella Uli in oral cavity samples by polymerase chain reaction. J. Adv. Oral Res. 8, 47–52 (2017).
Chidambar, C. K., Shankar, S. M., Raghu, P., Gururaj, S. B. & Bushan, K. S. Detection of Enterococcus faecalis in subgingival biofilms of healthy, gingivitis, and chronic periodontitis subjects. J. Indian Soc. Periodontol. 23, 416–418. https://doi.org/10.4103/jisp.jisp_44_19 (2019).
Duggan, J. M. & Sedgley, C. M. Biofilm formation of oral and endodontic Enterococcus faecalis. J. Endod. 33, 815–818. https://doi.org/10.1016/j.joen.2007.02.016 (2007).
Pinto, K. P., Barbosa, A. F. A., Silva, E., Santos, A. P. P. & Sassone, L. M. What is the microbial profile in persistent endodontic infections? A scoping review. J. Endod. 49, 786–798 (2023). https://doi.org/10.1016/j.joen.2023.05.010
Spratt, D. A., Weightman, A. J. & Wade, W. G. Diversity of oral asaccharolytic Eubacterium species in periodontitis-identification of novel phylotypes representing uncultivated taxa. Oral Microbiol. Immunol. 14, 56–59. https://doi.org/10.1034/j.1399-302x.1999.140107.x (1999).
Sogodogo, E. et al. First characterization of methanogens in oral cavity in Malian patients with oral cavity pathologies. BMC Oral Health 19, 232. https://doi.org/10.1186/s12903-019-0929-8 (2019).
Shen, C., Simpson, J., Clawson, J. B., Lam, S. & Kingsley, K. Prevalence of oral pathogen Slackia Exigua among clinical orthodontic and non-orthodontic saliva samples. Microorganisms 11 https://doi.org/10.3390/microorganisms11040867 (2023).
You, M., Mo, S., Leung, W. K. & Watt, R. M. Comparative analysis of oral Treponemes associated with periodontal health and disease. BMC Infect. Dis. 13, 174. https://doi.org/10.1186/1471-2334-13-174 (2013).
Shahravan, A. & Haghdoost, A. A. Endodontic epidemiology. Iran. Endod. J. 9, 98–108. https://doi.org/10.22037/iej.v9i2.5001 (2014).
Aloutaibi, Y. A., Alkarim, A. S., Qumri, E. M., Almansour, L. A. & Alghamdi, F. T. Chronic endodontic infections and cardiovascular diseases: Does the evidence support an independent association? Cureus 13, e19864. https://doi.org/10.7759/cureus.19864 (2021).
Roy, R., Tiwari, M., Donelli, G. & Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 9, 522–554. https://doi.org/10.1080/21505594.2017.1313372 (2018).
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Research Foundation of Korea, which is funded by the Korean government (NRF-2021R1A2C2006952, NRF-2022M3A9F3082330, RS-2022-00164722, NRF-2022M3A9F3082331, NRF-2023R1A2C1002595, and RS-2025-00518511).
Author information
Authors and Affiliations
Contributions
D.H. Park, E.J. Tak, and O.-J. Park contributed to design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. H. Perinpanayagam, Y.-J. Yoo, and H.-J. Lee contributed to data conception and design, critically revised the manuscript. Y.-S. Jeong, J.-Y. Lee, and H.S. Kim contributed to data analysis and acquisition. J.-W. Bae, K.-Y. Kum, and S.H. Han, contributed to conception, design, data analysis and interpretation, drafted and critically revised the manuscript. All authors gave their final approval and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Park, D.H., Tak, E.J., Park, OJ. et al. Association between root canals and gingival sulci microbiota in secondary and persistent endodontic infections. Sci Rep 15, 11253 (2025). https://doi.org/10.1038/s41598-025-95522-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-95522-9