Abstract
Nutraceuticals exert a series of health benefits, including protection against cardiovascular diseases. In this study, naringin, naringenin, and quercetin were tested for their safety and efficacy in ameliorating angiotensin (Ang) II-induced cardiac hypertrophy through carbonic anhydrase II (CA-II) inhibition. In silico molecular docking and MD simulations exhibited that naringin strongly binds CA-II with a docking score of -9.55 kcal/mol and hydrogen bonding energy of -6.07 kcal/mol. Naringin formed stable hydrogen bond interactions with Asn62, Trp5, and N-acetyl His4 via catalytic water molecule, and a continuous interaction via major water bridge with N-acetyl His4, His4, and Trp5. Moreover, naringin effectively inhibited CA-II activity with an IC50 value of 82.99 ± 4.92 nM, followed by naringenin and quercetin. Of note, all the tested nutraceuticals were found to be safe as evident from the cell viability assays. Further, naringin effectively attenuated cardiac hypertrophy, as indicated by the reductions in the Ang II-induced increases in cell surface area of H9c2 cardio myoblasts (165.6 ± 1.26% Ang II vs. 109.8 ± 1.88% Ang II + naringin), followed by naringenin and quercetin. Furthermore, naringin significantly inhibited CA-II activity (191.77 ± 7.69% Ang II vs. 120.16 ± 5.52% Ang II + naringin) and suppressed Ang II-induced CA-II and Na+/H+ exchanger 1 (NHE1) protein expression. Besides, naringin suppressed Ang II-induced CA-II, NHE1, Na+/Ca2+ exchanger 1 (NCX1), and angiotensin-converting enzyme (ACE1) mRNA expression. Collectively, naringin when compared to naringenin and quercetin effectively attenuated Ang II-induced cardio myoblast hypertrophy, CA-II activity, CA-II, and NHE1 expression. The naringin-mediated attenuation of cardiac hypertrophy might be through the inhibition of CA-II enzyme activity, and the suppression of NHE1, and NCX1.
Similar content being viewed by others
Introduction
Cardiovascular diseases account for the increased morbidity and mortality in India and worldwide. Globally, the three biggest causes of increased mortality are myocardial infarction, stroke, and heart failure1. Hypertension-induced cardiac hypertrophy is one of the main risk factors for heart failure. Hypertension is caused by many factors, including increases in angiotensin (Ang) II levels, acid-base imbalance, sodium and calcium levels, and hyperactivity of membrane transporters, including NHE1, NCX1, and Sodium/Bicarbonate co-transporter (NBC). CA-II plays a significant role in cardiopulmonary diseases by regulating NHE1. CA-II influences NHE1 transport efficiency, with direct interaction at the C-terminal region, and regulates the NHE1 activity via phosphorylation2. It has been shown that the induced expression of NHE1 augments cardiac hypertrophy and myocardial pathology3. Notably, CA-II provides substrates for the membrane transporters such as NHE1, NBC, and Anion Exchanger-3 (AE3)4. NHE1, involved in the uptake of intracellular Na+, causes an overload of Ca2 + ions and involved in the uptake of intracellular Na+, causes an overload of Ca2 + ions and, thus, myocardial injury. Together, the elevated cardiac NHE1 and increased CA-II activity result in heart failure2,3,4,5. However, NHE1 inhibition is also a sensible strategy to attenuate cardiac remodelling. However, a clinical trial reported a higher incidence of mortality and cerebrovascular side effects with direct NHE1 inhibition6. Moreover, CA-II is a key enzyme that regulates NHE1 activity and provides the substrate for a transporter. Of note is that the inhibition of CA-II activity might indirectly suppress NHE1 activity and contribute to the reduced risk of heart diseases. The CA-II inhibition decreases inflammation and cardiac hypertrophy4. However, considering the adverse effects of the existing CA-II inhibitory drugs7,8. It is imperative to develop effective and safer nutraceutical-based treatment strategies for cardiovascular diseases. Multiple studies have shown that nutraceuticals, including quercetin, naringenin, and its glycoside component naringin, offer substantial protective effects against various cardiovascular diseases, including myocardial ischemia and cardiac hypertrophy9,10. Martinez et al. reported that nutraceuticals such as quercetin, naringenin and genistein showed direct cardiovascular actions. Testai et al. (2013) demonstrated that naringenin provides direct cardioprotective effects against damage caused by severe ischemia/reperfusion in Langendorff-perfused rat hearts11. Hence, in this study, we aim to test the nutraceuticals naringin, naringenin, and quercetin for their safety and efficacy as CA-II inhibitors, followed by evaluating their efficiency in attenuating angiotensin II-induced cardio myoblast hypertrophy using H9c2 cells.
Results
Molecular Docking
Naringin, naringenin, and quercetin exhibited similar interactions with CA-II compared to acetazolamide, a standard CA-II inhibitor. Among the docked nutraceuticals, naringin exhibited the highest docking score of -9.55 kcal/mol with a greater hydrogen bonding energy of -6.07 kcal/mol (Table 1). Besides, naringin binds well with NHE1, while naringenin and quercetin bind loosely to CA-II. These data suggest that naringin strongly interacts with the CA-II and NHE1 compared to naringenin and quercetin. However, in case of NHE1, naringin binds comparatively more efficiently with a docking score of -7.93 kcal/mol (Table 1). Of note, naringin exhibited a similar extent of binding as compared to acetazolamide (Table 1). Further, MM/GBSA was performed for all the docked complexes to validate the molecular docking findings.
MM/GBSA
The MMGBSA data of ΔG (binding energy), ΔG(Coulomb), ΔG (Covalent energy), ΔG(H−bond), ΔG(Lipo), and ΔG(vdW) are given in Table 1. Naringin showed an acceptable Gtotal and hydrogen bond score. However, the total ΔG (total binding energy) was found to be superior (-29.83 kcal/mol) against CA-II among the tested nutraceuticals (Table 1). Naringenin and quercetin showed the least binding with CA-II. It is worth noting that similar observations were reflected in the case of NHE1 with less efficiency, as represented in Table 1. The more coulombic forces and Van der Waals forces are observed to be governed by naringin during interaction with CA-II. However, in case of NHE1, Van der Waals forces contributed more to binding with naringin. Hence, CA-II complexed with naringin, naringenin, quercetin, and acetazolamide were analysed to understand the naringin-CA-II complex stability and interaction strength by molecular dynamics.
*Standard CA-II inhibitor drug.
Molecular dynamics simulations
RMSD is one of the fundamental factors which is used to estimate protein stability compared to the initial structure. RMSF analysis was performed to evaluate the average fluctuations of residues during the simulation. In this study, naringin formed stable interactions with CA-II throughout 200 ns, and protein RMSD and ligand RMSD fluctuated within the limit ranging from 1-2.2Å. In the first phase of the interaction, protein RMSD was found to be stable throughout 200 ns, and ligand RMSD fluctuated from 10 ns to 130 ns (Fig. 1a). Herein, the seventh oxygen atom of naringin showed bonding with Asn62 and Asn61 of CA-II (Fig. 1b and e). Based on these observations, it is confirmed that the naringin and CA-II form stable complex throughout molecular dynamic simulations. The two-dimensional pictorial representation exhibits hydrogen bonds between naringin and amino acid residues Asn62, Trp5, and N-acetyl His4 (ACE3) of CA-II via a catalytic water molecule (Fig. 1b and d). The L-RMSF plot of naringin with CA-II shows that oxygen atoms 7, 9, and 14 form active interactions with strengths of 32%, 59%, and 30%, respectively (Fig. 1b and c) and with Glu69 with bond strength of 31%. Naringin forms a major water bridge with His4, Trp5, and Asn62, which is a continuous interaction (Fig. 1e), and the number of contact points is represented in color-coded form (Fig. 1e). Naringin forms hydrogen bonds with Asn67 and Glu69. Hence, the catalytical water molecules play a vital role in holding naringin in the putative binding site of CA-II.
Molecular dynamics simulations of naringin with CA-II. (a) RMSD of naringin and CA-II complex, giving insight into its structural conformation throughout the simulation, showing fluctuation within the acceptable limit. (b) 2D geometrical interactive plot showing interactions of amino acids with naringin. (c) L-RMSF depicting the interaction of naringin atoms (given in percentage) with CA-II throughout the MDSs. (d) Summary of the interactions of CA-II-naringin complex in a normalized stacked bar chart. (e) The contact strengths between the putative binding site amino acids of CA-II with naringin throughout the MDS’s up to 200ns.
Naringenin also formed stable interactions and hydrogen bonds with Asn62, Trp5, and ACE3 of CA-II via a catalytic water molecule (Figure S1a and S1b). The L-RMSF plot of naringenin with CA-II showed that the oxygen atoms 1, 2, 3, and 5 form active interactions with strengths of 42%, 33%, 32%, and 42%, respectively (Figure S1c). Of note, naringenin formed a major hydrophobic interaction with Tyr7 and Ala65 and hydrogen bonding with Asn62, His64, and Glu92, which is a continuous interaction (Figure S1d), and the contact points are represented in color-coded form (Figure S1e). The RMSD of quercetin and CA-II exhibited a stable complex (Figure S2a), having hydrogen bonds with ACE3 and Gln36 (Figure S2b). The L-RMSF plot of quercetin with CA-II showed that oxygen atoms 4 and 6 form active interactions with strengths of 33% and 36%, respectively (Figure S2c). Quercetin formed hydrophobic interactions with Tyr7 and Ala65, hydrogen bond, and water bridge with ACE3 and Gln92, which is a continuous interaction (Figure S2d), and the contact points are represented in color-coded form (Figure S2e).
Notably, RMSD of acetazolamide and CA-II remained stable with a strong interaction between the nitrogen of acetazolamide and zinc atom of CA-II active site and hydrogen bonding with Asn62, Tyr7, and Thr200 via catalytic water (Figure S3a and S3b). L-RMSF plot of acetazolamide with CA-II showed that oxygen atoms 3, 4, and 6 form active interactions with strengths of 83%, 94%, and 63%, respectively (Figure S3c). Acetazolamide formed hydrophobic interactions with Tyr7, His94, Val143, and Trp209, hydrogen bond and water bridge with Thr199 and Thr200, which is a continuous interaction (Figure S3d), and the contact points are represented in color-coded form (Figure S3e).
Collectively, in silico data demonstrated a strong binding affinity between naringin and CA-II with multiple interactions, including hydrogen bonding, ionic bonding, hydrophobic interactions, and water bridges. Moreover, the binding orientation of naringin was similar to that of acetazolamide, suggesting the potential of naringin as an effective CA-II inhibitor.
Carbonic anhydrase-II inhibitory activity
A series of concentration-dependent inhibition assays were performed to determine the effective enzyme inhibitory concentrations of individual molecules. Of the tested molecules, naringin and acetazolamide significantly inhibited CA-II enzyme activity at nanomolar concentrations while naringenin and quercetin exhibited enzyme inhibitory activity at micromolar concentrations (Figure S4). Importantly, the concentrations of the tested molecules ranged from 1 nM to 1 mM, and henceforth, to ensure precise and accurate inhibitions, the varied concentrations of individual were considered. Among the tested nutraceuticals, naringin efficiently inhibited CA-II activity with an IC50 value of 82.99 ± 4.92 nM (Fig. 2a). The inhibitory efficiency of naringin is comparable, yet higher than acetazolamide, a standard CA-II inhibitor (IC50 value 26.23 ± 0.376 nM; Fig. 2b), followed by naringenin and quercetin (IC50 values of 68.95 ± 0.56 µM and 70.44 ± 2.50 µM respectively) (Fig. 2c and d). Further, these nutraceuticals were tested for their toxicity and effects on Ang II-induced cell hypertrophy.
Concentration-dependent inhibition of CA-II by nutraceuticals (a) naringin, (b) acetazolamide, (c) naringenin and (d) quercetin. The decreased concentration of P-nitrophenol in the test reaction compared to control and enzyme activity reaction was expressed as percentage of CA-II inhibition. Data represents mean ± SEM (n = 3).
Cell viability
Cell viability analysis of naringin, naringenin, and quercetin did not show cytotoxicity in H9c2 cells (Fig. 3a-c), which is similar to that of acetazolamide and losartan (Figure S5a-b). The cells treated with angiotensin II alone (Figure S5c) showed 87% viability at its concentration of 200nM. Of note, naringin, naringenin, quercetin (80µM), acetazolamide, and losartan (60µM) were found to be protective, and thus, these doses were considered for the evaluation; thus, these doses were considered to evaluate hypertrophy parameters.
Cardio myoblast hypertrophy
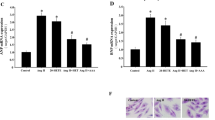
Naringin, naringenin, quercetin, and standard drugs were tested for their efficacy in attenuating Ang II-induced cardio myoblast hypertrophy. Of note, Ang II-induced cell hypertrophy, as indicated by the increased cell surface area (165.6 ± 1.26% vs. 100% control). Besides, naringin effectively attenuated Ang II-induced hypertrophy (109.8 ± 1.88% vs. 165.6 ± 1.26%), followed by naringenin (115.15 ± 1.46%), which is similar to that of losartan (106.87 ± 1.69%). However, acetazolamide and quercetin exhibited moderate effects on Ang II-induced cell hypertrophy (126.36 ± 1.8% and 132.37 ± 2.53% respectively) when compared to naringin and naringenin (Fig. 4).
Carbonic anhydrase-II activity
The effect of nutraceuticals on CA-II activity in Ang II-induced cardiac hypertrophy were analysed. Ang II-induced cell hypertrophy, as indicated by the increased CA-II activity (191.77 ± 7.69% vs. 100% control). Besides, naringin effectively attenuated Ang II-induced hypertrophy (120.16 ± 5.52% vs. 191.77 ± 7.69%), followed by naringenin (115.15 ± 1.46%), which is similar to that of losartan (106.87 ± 1.69%), which is almost similar to that of standard CA-II inhibitor acetazolamide (114.79 ± 2.51%) and antihypertensive drug losartan (111.23 ± 4.18%) followed by naringenin and quercetin exhibited moderate inhibitory activity of CA-II (133.63 ± 11.8% and 133.64 ± 8.03% respectively) when compared to controls. Naringin, acetazolamide and losartan significantly reduced Ang II-induced CA-II activity compared to other tested molecules (Fig. 5).
Immunoblotting
The expression levels of CA-II and NHE1 and the effect of nutraceuticals on CA-II and NHE1 levels were analysed using immunoblot technique. Ang II-induced group showed the increased expression levels of CA-II and NHE1 proteins when compared to control. Of note, naringin, naringenin and losartan significantly suppressed Ang II-induced CA-II expression compared to acetazolamide and quercetin (Fig. 6). Besides all the tested compounds profoundly suppressed Ang II-induced NHE1 protein expression (Fig. 6). However, naringin and naringenin significantly supressed CA-II and NHE1 expression, similar to that of antihypertensive drug losartan, followed by acetazolamide and quercetin.
Effect of nutraceuticals on CA-II and NHE1 protein expression in H9c2 cells. The β-actin served as internal control. Data represents mean ± SEM (n = 3) (# vs. control group; * vs. Ang II group; * P, 0.05, ## and **P, 0.01, ### and ***P, 0.001). (Original blots/gels are presented in Supplementary Figure S6-S8)
Gene expression
Ang II significantly enhanced the mRNA levels of CA-II, NHE1, NCX1, and ACE1 in H9c2 cells compared to controls. Of note, all the tested nutraceuticals significantly attenuated Ang II-induced mRNA levels. However, naringin seems more effective in suppressing Ang II-induced gene expression than naringenin and quercetin. Besides, the effect of naringin on Ang II-induced gene expression was similar to that of losartan and acetazolamide (Fig. 7). comparison attenuated Ang II-induced cardio myoblast hypertrophy, CA-II activity mRNA, and protein expression levels of CA-II and NHE1 compared with naringenin and quercetin.
Effect of nutraceuticals on (a) CA-II, (b) NHE1, (c) NCX1, and (d) ACE1 gene expression in H9c2 cells. The β-actin served as internal control. Data represents mean ± SEM (n = 3). Data represents mean ± SEM (n = 3) (# vs. control group; * vs. Ang II group; * P, 0.05, ## and **P, 0.01, ### and ***P, 0.001).
Discussion
Despite available treatment for cardiac hypertrophy and heart failure, many patients still experience disease progression, highlighting the need for additional measures12. Importantly, nutraceuticals and dietary as well as lifestyle measures are advised due to their safety, affordability, and potential as preventive medicine13. So far, various nutraceuticals have been reported to alleviate oxidative and endoplasmic reticulum stress, support nitric oxide and hydrogen sulphide bioactivity, prevent cardiomyocyte pH reduction, and positively regulate cardioprotective hormones14. Moreover, nutraceuticals offer a promising approach into managing cardiac hypertrophy by targeting various pathways involved in inflammation, oxidative stress, and metabolic regulation13.
Of note, the flavonoids have shown potential in attenuating cardiac hypertrophy and are known for anti-inflammatory and antioxidant activities15. Importantly, the flavonoids could protect endothelial function, which is vital for maintaining cardiovascular health16. They prevent the oxidation of low-density lipoprotein and offer vasodilatory effects, helping to reduce the workload on the heart17. Quercetin has been shown to possess cardioprotective effects, including ACE inhibition, antioxidant, and anti-inflammatory properties18. Quercitrin could reduce the creation of ROS and promote the regeneration of endogenous antioxidant defences, which have been linked to the suppression of oxidative stress19. The ability of quercetin to inhibit the renin-angiotensin system (RAS) is crucial in the regulation of blood pressure and fluid balance since the overactivation of RAS leads to cardiac hypertrophy18.
Naringin and naringenin are also flavonoids with proven efficiency in cardioprotection. Notably, naringenin attenuates cardiac hypertrophy induced by various factors20,21,. For instance, naringenin can reduce oxidative stress and inhibit the AMPK/NOX2/MAPK signalling pathway, which is involved in of developing cardiac hypertrophy20. Additionally, naringenin has been shown to inhibit the PI3K/Akt, ERK, and JNK signalling pathways, further contributing to its cardioprotective effects20. Similarly, naringin exhibits cardioprotective properties and has been shown to control the serum levels of creatinine, blood urea nitrogen, and other markers of cardiac health. Also, naringin can ameliorate cardiac toxicity and hypertrophy, displaying its importance as a preventive medicine for cardiovascular diseases22.
The literature reveals that the NHE1, NCX1, and CA-II play interconnected roles in maintaining cardiac function, and their dysregulation might contribute to an imbalance in cardiac function, including hypertrophy23,24,25. CA-II activates the NHE1 efflux through the interaction with the C-terminal of NHE1 and donates H+ ion as a substrate for NHE126,27. NHE1 activity affects intracellular pH, which in turn influences NCX1 function23. The changes in intracellular pH can modulate NCX1 activity, affecting Ca2+ homeostasis28,29. Thus, the intricate relationship between NHE1, NCX1, and CA-II might impact cardiac contractility, arrhythmogenesis, and overall cellular homeostasis.
In this study, quercetin, naringenin, and naringin showed a higher affinity towards CA-II. MD simulation results suggested the similarity between naringin and acetazolamide in their binding pattern, binding regions, and bonding interactions. The MM/GBSA score is important in validating in silico molecular docking data, and the bioactivity data set is typically linked with MM/GBSA scores13. Of note, Gscore was calculated to validate molecular docking results and predict the protein-ligand complex’s behavior. Naringin showed a sufficient Gscore and hydrogen bond score and higher binding energy than other compounds. Naringenin and quercetin showed the least binding with CA-II. It is worth noting that similar observations were reflected in the case of NHE1 with less efficiency, as represented.
MDSs were employed to study the molecular dynamics of a docked complex of ligands against the targeted macromolecule. The molecular docking analyses revealed that the naringin formed stable interactions with CA-II via four ionic bonds, nine hydrogen bonds, and six hydrophobic interactions. In contrast, acetazolamide formed four ionic bonds, four hydrogen bonds, and four hydrophobic interactions. Moreover, the binding mode of naringin was similar to that of acetazolamide. On the other hand, naringenin and quercetin formed four and seven hydrogen bonds respectively, with CA-II, and did not form ionic bonds. However, naringenin and quercetin had three and two hydrophobic interactions with CA-II, respectively. Importantly, naringin and acetazolamide were similar in making ionic and hydrogen bonding and showed similar binding modes. Overall, the in-silico data confirmed a strong binding affinity of naringin for CA-II and the similarity in binding orientations of naringin and acetazolamide with CA-II.
In this study, we tested naringin, naringenin, quercetin along with acetazolamide for their CA-II inhibitory efficacy in vitro and demonstrated their efficacy in attenuating cardiac hypertrophy in comparison with losartan in H9c2 cells. Acetazolamide, a standard CA-II inhibitor is widely used as diuretic drug in humans30,31 and losartan, an angiotensin II receptor blocker exert antihypertensive effects through distinct mechanisms32. The antihypertensive mechanism of acetazolamide involves the reduction of sodium bicarbonate reabsorption in the kidney33. This leads to loss of bicarbonate in the urine, pulling water along with it, reducing plasma volume and extracellular fluid volume, thus lowering blood pressure33,34. On the other hand, losartan exerts antihypertensive effect by blocking angiotensin II binding to the AT1 receptor, thus suppressing angiotensin II-mediated effects including vasoconstriction, systemic vascular resistance and aldosterone secretion. Together, losartan reduces hypertension by decreasing sodium and water retention, blood volume and preload32. Remarkably, naringin exhibited higher inhibitory potential with an IC50 value at a nanomolar concentration, comparable with the standard CA-II inhibitor acetazolamide. In contrast, quercetin and naringenin were effective at micromolar concentrations. Of note, previously, the studies have also reported similar CA-II inhibitory effects of naringin and quercetin35,36.
Previously, naringin exhibited the cardioprotective effects against anoxia/reoxygenation injury at a concentration of 40 µg/mL (equivalent to 68.90 µM) in H9c2 cells37, while 80 µM of naringenin protected against hypoxia/reoxygenation injury in cardiomyocytes38. In another study, 30 µM and 100 µM quercetin demonstrated cardioprotection against oxidative stress-induced cell death, with no cardiotoxic effects observed even at higher concentrations39. Considering the safety, efficacy, and preliminary standardization, the nutraceutical concentrations of 80 µM were tested in H9c2 cells. Importantly, 80 µM naringin, naringenin, and quercetin did not display toxicity against H9c2 cells, which is also evident from other studies37,38,40. Further, Ang II-induced hypertrophy model using H9c2 cardio myoblasts41 was considered for testing the effects of these nutraceuticals and standard drugs. Notably, naringin attenuated Ang II-induced cell hypertrophy, and the effect was similar to that of losartan, while superior to acetazolamide, naringenin, and quercetin. Moreover, the drug treatment alone did not show increases in cell hypertrophy or cell death, suggesting the safety and efficacy of naringin in the attenuation of cardiac hypertrophy.
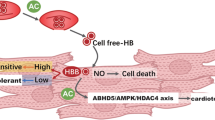
It has been shown that the pro-hypertrophic agonist Ang II acts on G-protein-coupled receptors42, which in turn activates protein kinase C and indirectly stimulates NHE1 via mitogen-activated protein kinase43. Besides, the catalytic activity of CA-II generates and provides H+ ions to NHE1, thus stimulating the efflux action of NHE1. Of note, NHE1 activation eventually led to the rise in cytosolic Na+ ions44, which compromises or reverses the ability of Na+ / Ca2+ exchanger1 (NCX1). Physiologically, NCX1 is involved in the efflux of Ca2+ ions, driven by the transmembrane Na+ ion gradient. However, the dysregulated NCX1 increases intracellular Ca2+ levels and activates calcineurin, eventually contributing to the development of cardiac hypertrophy and heart failure (Fig. 8)45,46,47.
To decipher the mode of action of nutraceuticals, the cell lysates were evaluated for both the protein and mRNA expression levels of CA-II and their counterparts. Importantly, the cell lysates of Ang II-induced hypertrophic cells exhibited significant increases in CA-II enzyme activity. However, Ang II-induced increases in CA-II enzyme activity were effectively inhibited by naringin. Likewise, naringenin and quercetin moderately inhibited CA-II enzyme activity. Of note, the observed enzyme inhibitory efficiency of naringin was similar to acetazolamide and losartan. It is critical to understand that the CA-II regulates NHE1 and NCX1, thus contributing to cardiac hypertrophy2. Hence, the Ang II-induced expression levels of CA-II and NHE1 proteins and the effect of nutraceuticals on Ang II-induced CA-II and NHE1 protein levels were analysed. Importantly, Ang II increased CA-II and NHE1 protein levels, while naringin and losartan significantly suppressed Ang II-induced CA-II protein expression. On the other hand, naringenin efficiently suppressed Ang II-induced NHE1 protein expression, followed by naringin, losartan, quercetin, and acetazolamide.
Lastly, the mRNA expression analyses were undertaken to comprehend the nutraceutical-mediated molecular mechanisms. It is known that CA-II, NHE1, and NCX1 interplay, as well as ACE1 expression, play a key role in cardiac hypertrophy48. Principally, CA-II, NHE1, NCX1, and ACE1 mRNA levels were significantly amplified in Ang II-infused hypertrophic cells. Though, all the tested nutraceuticals attenuated Ang II-induced mRNA levels, naringin alone was found to be effective and similar to that of losartan and acetazolamide. Collectively, naringin might have inhibited CA-II enzyme activity either through direct interaction with CA-II protein or regulation of mRNA and protein expression levels of CA-II. Furthermore, naringin-mediated regulation of CA-II expression and enzyme activity along with the suppression of NHE1, NCX1, and ACE1 might have collectively contributed to the attenuation of Ang II-induced cardiac hypertrophy (Fig. 8).
Conclusions
Naringin, naringenin, and quercetin were found to be safe and effective at 80 µM concentrations in ameliorating Ang II-induced cardio myoblast hypertrophy. Of note, nutraceutical-mediated attenuation of cardio myoblast hypertrophy corresponds to the inhibition of CA-II enzyme activity and suppression of CA-II and NHE1 expression. Perhaps, nutraceutical-mediated effects might be through the suppression of CA-II-mediated signal transduction pathways involved in the development of Ang II-induced cardiac hypertrophy, via negative regulation of CA-II, NHE1, NCX1 and ACE1.
Materials and methods
Materials
Carbonic anhydrase II (Cat# C2522-5MG), Quercetin (Cat# 1592409), naringin (Cat# N1376-25G), naringenin (Cat# N5893-5G), acetazolamide (Cat# A6011-10G), losartan (Cat# SML3269-10MG), angiotensin II (Cat# A9525), 4-nitrophenyl acetate (Cat. No: N8130-5G), and crystal violet (Cat# 61135) were obtained from Sigma (St. Louis, MO, USA). Nitrocellulose membrane (Cat# 1620115) and ECL substrate (Cat. No: 1705060) were obtained from BioRad Laboratories (USA). MTT assay kit (Cat# CCK003-1000 LOT# 0000578578) was from HiMedia Laboratories Pvt Ltd (Bengaluru, India), DMEM medium (Cat# 12100046), FBS (Cat# A3160801), penicillin, and streptomycin (Cat# 10378016) from Gibco (Grand Island, NY, USA). Internal control β-actin (Cat#4967, LOT #ZC385732), Primary antibodies for CA-II (Cat# MA5-49145 LOT#ZA4177591) and Nhe-1 (Cat# PA5-116471, LOT #ZA4173385B), secondary antibodies, goat anti-mouse IgG2b (Cat# A-21242, LOT #2465093), and goat anti-rabbit IgG (Cat# 32460 LOT #YK383320), and BCA assay kit (Cat#23235) were from Thermo Fisher Scientific (Waltham, MA, USA). Prime Script RT Reagent (Cat# RR037A LOT#AN51001N) and TB Green® Premix Ex Taq II (Tli RNaseHPlus) (Cat# RR820A LOT #AN41007N) obtained from TaKaRa (Shiga, Japan). All primers were from Integrated DNA Technologies (Singapore).
Molecular Docking
The in-silico docking methods were performed as described previously by Jiang et al.49. CA-II (PDB ID: 1A42)50 and NHE1 (PDB ID: 7DSX)51 Were retrieved from the Protein Data Bank (PDB). Each protein was constructed using automated default parameters, ensuring accurate bond configurations and charge assignments. The protein preparation involved the retention of catalytic water molecules and the application of an OPLS3 force field for the minimization52. A root-mean-square deviation (RMSD) of 0.30Å was set for heavy atom convergence before docking in Maestro’s Prime module, and a receptor grid of 30Å was generated. Structures of naringin, naringenin, quercetin and acetazolamide were imported from the PubChem database. Energy minimization was performed with OPLS3 for each ligand, followed by extra-precision docking to evaluate docking scores53.
MM/GBSA calculation
Molecular mechanics with generalized Born and surface area solvation (MM/GBSA) is a widely used technique to estimate the free energy associated with binding small ligands to biological macromolecules. It lies between rigorous alchemical perturbation approaches and empirical scoring functions regarding accuracy and computational complexity. MM/GBSA was employed to predict binding free energies (ΔGbind) of quercetin, naringin, naringenin, and acetazolamide to CA-II and NHE154. The docked complex structures were visualized using the pose viewer, and the results were analyzed using the Prime MM/GBSA module in Maestro, force field OPLS3 and VSGB 2.0 solvation model was used in Maestro to predict ΔGbind55The binding free energy was calculated using the minimized energies of the receptor, ligand, and receptor-ligand complex, using the equation below to calculate ΔGbind.
\(\Delta G\left( {bind} \right)~=~Ecomplex~\left( {\hbox{min} imized} \right)~--~\left[ {Eligand~\left( {\hbox{min} imized} \right)+~Rreceptor~\left( {\hbox{min} imized} \right)} \right]\)
Molecular dynamic simulations (MDSs)
The MDSs were performed on a 64-bit Ubuntu 20.04 system using the Maëstro software. The docking scores for CA-II with ligands, validated through MM/GBSA, were utilized in the MDSs. The protein-ligand complex was solubilized using the TIP3P water model by employing the system-builder option within an orthorhombic periodic boundary box. To achieve charge neutrality, Hg²⁺ ions (2.403 mM), Cl⁻ ions (52.875 mM), and Na⁺ ions (50.472 mM) were added. The default relaxation method from the Desmond program was applied for MDSs, maintaining several atoms, pressure, and temperature under NPT ensemble conditions at 310 K and 1 atmosphere. The analysis focused on root mean square deviation (RMSD), root mean square fluctuation (RMSF), and total energy of the complexes, utilizing event analysis and simulation-interaction diagrams over a simulation period of 200 nanoseconds56,57.
CA-II inhibitory assay
P-nitrophenyl acetate, a synthetic substrate, is hydrolyzed by CA-II to create p-nitrophenol, which exhibits an absorption peak at 405 nm. The generation of p-nitrophenol enables colourimetric detection of the product and subsequent estimation of CA-II activity57. In summary, a 96-well plate was filled with triplicate solutions of 1.26 U/180 µl of CA-II in the presence or absence of inhibitors and 3 mM of p-nitrophenol acetate. The reaction buffer contained 50 mM TrisSO4, pH 7.6, and a final volume of 180 µl. The changes in the absorbance at 405 nm following a 30-minute incubation period at 25 °C were detected and recorded using a MultiskanTM FC Microplate photometer. The maximal activity obtained without an inhibitor was considered 100% activity and used to define the % inhibition. IC50 values were computed from a graph of per cent inhibition vs. inhibitor concentration using GraphPad Prism 8.0 software.
Cell culture
H9c2 embryonic rat heart-derived cells were obtained from NCCS, Pune. The cells cultured in DMEM medium were enriched with 10% FBS, 3.7 g/L NaHCO₃, and antibiotics (streptomycin at 100 µg/ml, penicillin at 100 U/ml). Cells were maintained in a humidified incubator with an atmosphere of 95% oxygen and 5% carbon dioxide. For various assays, the cells were seeded at different densities: 0.5 × 104 cells per well in 96-well plates for cell viability tests, 3 × 104 cells in 24-well plates for crystal violet staining and surface measurement, and 1 × 106 cells in 6-well plates for extracting proteins and mRNA.
Cell viability assay
The MTT assay was performed to analyze the effects of the test compounds. For this, a density of 0.5 × 104 cells per well was seeded in 96-well plates, allowing the culture plate to get 70–85% confluence. Once cells are confluent, they were exposed to different concentrations of quercetin, naringenin, and naringin and standard CA-II inhibitor acetazolamide, antihypertensive drug losartan (20, 40, 60, 80, and 100µM), and Ang II at (50, 100,150, 200, and 250 nM). Acetazolamide and nutraceuticals were dissolved in a final DMSO concentration of less than 0·1% (v/v). Control cells received the same dosage of DMSO as a vehicle. The control and treated cells were incubated for 48 h. Then, MTT solution (1 mg/mL) was added to each well at a volume of 70 µL and incubated for 3 to 4 h. Afterwards, the media was discarded, and 200 µL of DMSO and ethanol (50:50) was added to each well to dissolve the formazan crystals. The plates were then gently shaken at room temperature for 5 to 10 min and the absorbance was measured at 570 nm by a multimode plate reader ((Multiskan Sky, Thermo Fisher Scientific). The percentage of cell viability was calculated as the relative absorbance of the treated cells vs. untreated control cells58.
Cell surface area measurement
Crystal violet staining of H9c2 cardio myoblasts was employed as previously reported, with slight modifications41. Briefly, treated cardiomyoblasts were rinsed 2–3 times with PBS, incubated with 3% crystal violet dissolved in methanol for 1 h at room temperature, and washed the wells with PBS until the stain was removed. Then, the cardiomyoblasts were observed under a light microscope. The cross-sectional area of at least 100 representative cells from each experimental group was quantified using microscopic images captured from randomly selected fields. The measurements were performed using ImageJ software.
Protein extraction
Cells were seeded into a 10 cm dish at a density of 2.5 × 105. Dishes were grouped into 12 groups: control, Ang II (200nM) alone; nutraceutical (quercetin, naringenin, and naringin; 80 µM) alone; nutraceutical with Ang II, acetazolamide (60 µM) alone, acetazolamide with Ang II, losartan (60 µM) alone, and losartan with Ang II. The cells were then harvested in cell lysis buffer [4% sodium dodecyl sulfate in 50 mM triethylammonium bicarbonate, with 1 mM sodium orthovanadate, 2.5 mM sodium pyrophosphatase, and 1 mM beta-glycerophosphate] after 48 h and cells were lysed by sonication on ice with discontinuous disruption at 30% amplification. The cells were centrifuged at 12,000 g for 30 min at 4ºC, supernatants were transferred to sterile tubes and stored at -20 ºC for further experiments59.
Protein expression studies
Protein concentration was estimated using a BCA protein estimation kit. The protein (20 µg) samples were loaded onto SDS- PAGE (NHE1- 10% gel and CA II 12% gel, respectively) for the western blot analyses. The proteins were blotted using a nitrocellulose membrane and blocked with 5% BSA in 1X PBST. Next, the nitrocellulose membrane was independently incubated with primary antibodies (NHE1 and CA II − 1:500 dilutions) at 4 ºC temperature overnight and then with respective HRP-conjugated secondary antibodies (1:3000 dilution) for 90 min at room temperature. The protein bands were observed using ECL substrate (Bio-Rad), and densitometry analyses were performed using ImageJ software59. The β-actin was used as a reference control.
Gene expression studies
Cells were seeded into a 10 cm dish at a density of 2.5 × 105. Dishes were grouped into 12 groups: control, Ang II (200nM) alone; nutraceutical (quercetin, naringenin, and naringin; 80 µM) alone; nutraceutical with Ang II, acetazolamide (60µM) alone, acetazolamide with Ang II, losartan (60 µM) alone, and losartan with Ang II. Total RNA was extracted from cell lysates using Tri-reagent and then reverse-transcribed into cDNA using Prime Script RT Reagent (TaKaRa) cDNA synthesis kit for CA-II, NHE1, NCX1, and ACE1 (Table 2). Reverse transcription PCR (RT-PCR) was performed using TB Green® Premix Ex Taq II (Tli RNaseHPlus) SYBR Green PCR Master Mix. The β-actin serves as the reference control60.
Statistical analysis
All data were expressed as mean ± standard error (n = 3). Statistical analyses were conducted using GraphPad Prism 8.0 software. One-way analysis of variance (ANOVA) was used to evaluate significant difference among multiple groups followed by Dunnett’s test61,62,63. A value of p < 0.05 was considered statistically significant. *p < 0.05; **p < 0.01; ***p < 0.001.
Data availability
The data could be obtained upon request to the corresponding author.
Change history
28 May 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-03168-4
References
Kalra, A. et al. The burgeoning cardiovascular disease epidemic in Indians – perspectives on contextual factors and potential solutions, May 01, Elsevier Ltd. (2023). https://doi.org/10.1016/j.lansea.2023.100156
Li, X., Alvarez, B., Casey, J. R., Reithmeier, R. A. F. & Fliegel, L. Carbonic anhydrase II binds to and enhances activity of the Na+/H + exchanger, Journal of Biological Chemistry, vol. 277, no. 39, pp. 36085–36091, Sep. (2002). https://doi.org/10.1074/jbc.M111952200
Mraiche, F., Oka, T., Gan, X. T., Karmazyn, M. & Fliegel, L. Activated NHE1 is required to induce early cardiac hypertrophy in mice, Basic Res Cardiol, vol. 106, no. 4, pp. 603–616, Jul. (2011). https://doi.org/10.1007/s00395-011-0161-4
Alvarez, B. V. et al. Feb., Carbonic anhydrase inhibition prevents and reverts cardiomyocyte hypertrophy, Journal of Physiology, vol. 579, no. 1, pp. 127–145, (2007). https://doi.org/10.1113/jphysiol.2006.123638
Nakamura, T. Y., Iwata, Y., Arai, Y., Komamura, K. & Wakabayashi, S. Activation of Na+/H + exchanger 1 is sufficient to generate Ca2 + signals that induce cardiac hypertrophy and heart failure, Circ Res, vol. 103, no. 8, pp. 891–899, Oct. (2008). https://doi.org/10.1161/CIRCRESAHA.108.175141
Mentzer, R. M. et al. Apr., Sodium-Hydrogen Exchange Inhibition by Cariporide to Reduce the Risk of Ischemic Cardiac Events in Patients Undergoing Coronary Artery Bypass Grafting: Results of the EXPEDITION Study, Annals of Thoracic Surgery, vol. 85, no. 4, pp. 1261–1270, (2008). https://doi.org/10.1016/j.athoracsur.2007.10.054
Sanah Aslam and & Gupta, V. Carbonic Anhydrase Inhibitors (StatPearls Publishing, 2023).
Supuran, C. T., Scozzafava, A. & Casini, A. Carbonic anhydrase inhibitors. Mar https://doi.org/10.1002/med.10025 (2003).
Yaseen, H. S. et al. Naringin: cardioprotective properties and safety profile in diabetes treatment. Fitoterapia 176, 106011. https://doi.org/10.1016/j.fitote.2024.106011 (2024).
Galán-Martínez, L., Herrera-Estrada, I. & Fleites-Vázquez, A. Direct actions of the flavonoids naringenin, quercetin and genistein on rat cardiac and vascular muscles [Acciones directas de los flavonoides naringenina, quercetina y genisteína sobre los músculos cardiaco y vascular de rata], J Pharm Pharmacogn Res, vol. 6, no. 3, pp. 158–166, [Online]. (2018). Available: http://jppres.com/jppres
Testai, L., Martelli, A., Cristofaro, M., Breschi, M. C. & Calderone, V. Cardioprotective effects of different flavonoids against myocardial ischaemia/reperfusion injury in Langendorff-perfused rat hearts. J. Pharm. Pharmacol. 65 (5), 750–756. https://doi.org/10.1111/jphp.12032 (May 2013).
McCarty, M. F. Nutraceutical, dietary, and lifestyle options for prevention and treatment of ventricular hypertrophy and heart failure. Apr 01 2021 MDPI AG https://doi.org/10.3390/ijms22073321
Dama, A. et al. Targeting Metabolic Diseases: The Role of Nutraceuticals in Modulating Oxidative Stress and Inflammation, Feb. 01, Multidisciplinary Digital Publishing Institute (MDPI). (2024). https://doi.org/10.3390/nu16040507
Mazza, A. et al. Nutraceuticals for serum lipid and blood pressure control in hypertensive and hypercholesterolemic subjects at low cardiovascular risk. Adv. Ther. 32 (7), 680–690. https://doi.org/10.1007/s12325-015-0229-x (Jul. 2015).
Gupta, L., Chauhan, M., Kumar, A., Chauhan, D. & Saini, P. Flavonoids and cardiovascular diseases, in Advances in Flavonoids for Human Health and Prevention of Diseases, Apple Academic, 73–94. doi: https://doi.org/10.3109/13880200490893483. (2024).
Choy, K. W. et al. Flavonoids as natural anti-inflammatory agents targeting nuclear factor-kappa B (NFκB) signaling in cardiovascular diseases: A mini review, Front Pharmacol, vol. 10, no. OCT, (2019). https://doi.org/10.3389/fphar.2019.01295
Kozłowska, A. & Szostak-Węgierek, D. Targeting Cardiovascular Diseases by Flavonols: An Update, Apr. 01, MDPI. (2022). https://doi.org/10.3390/nu14071439
Dmello, J. R., Chandekar, L. P., Bavadekar, T. A., Buttar, H. S. & Kaur, G. Quercetin: A promising flavonoid for the therapy of cardiac hypertrophy and heart failure mediated by the Renin angiotensin system, in The Renin Angiotensin System in Cardiovascular Disease, (eds Dhalla, N. S., Bhullar, S. K. & Shah, A. K.) Cham: Springer International Publishing, 377–405. doi: https://doi.org/10.1007/978-3-031-14952-8_23. (2023).
Charachit, N., Sukhamwang, A., Dejkriengkraikul, P. & Yodkeeree, S. Hyperoside and Quercitrin in houttuynia cordata extract attenuate UVB-Induced human keratinocyte cell damage and oxidative stress via modulation of MAPKs and Akt signaling pathway. Antioxidants 11 (2). https://doi.org/10.3390/antiox11020221 (Feb. 2022).
Li, Y. et al. Naringenin attenuates Isoprenaline-Induced cardiac hypertrophy by suppressing oxidative stress through the AMPK/NOX2/MAPK signaling pathway. Nutrients 15 (6). https://doi.org/10.3390/nu15061340 (Mar. 2023).
Zhang, N. et al. Dec., Naringenin attenuates pressure overload-induced cardiac hypertrophy, Exp Ther Med, vol. 10, no. 6, pp. 2206–2212, (2015). https://doi.org/10.3892/etm.2015.2816
Shackebaei, D. et al. Cardioprotective effect of naringin against the ischemia/reperfusion injury of aged rats. Naunyn Schmiedebergs Arch. Pharmacol. 397 (2), 1209–1218. https://doi.org/10.1007/s00210-023-02692-2 (2024).
Tóth, A. & Varró, A. Role of NCX1 and NHE1 in ventricular arrhythmia, in Heart Rate and Rhythm: Molecular Basis, Pharmacological Modulation and Clinical Implications, (eds Tripathi, O. N., Quinn, T. A. & Ravens, U.) Cham: Springer International Publishing, 293–307. doi: https://doi.org/10.1007/978-3-031-33588-4_13. (2023).
Watanabe, Y. Cardiac Na+/Ca2 + exchange stimulators among cardioprotective drugs, Nov. 01, Springer. (2019). https://doi.org/10.1007/s12576-019-00721-5
Herrmann, S. et al. Sep., The cardiac sodium-calcium exchanger NCX1 is a key player in the initiation and maintenance of a stable heart rhythm, Cardiovasc Res, vol. 99, no. 4, pp. 780–788, (2013). https://doi.org/10.1093/cvr/cvt154
Sjøgaard-Frich, L. M. et al. Mar., Dynamic Na+/H + exchanger 1 (NHE1) - calmodulin complexes of varying stoichiometry and structure regulate Ca2+-dependent NHE1 activation, Elife, vol. 10, (2021). https://doi.org/10.7554/eLife.60889
Vallés, P. G., Bocanegra, V., Gil Lorenzo, A. & Costantino, V. V. Physiological Functions and Regulation of the Na+/H + Exchanger [NHE1] in Renal Tubule Epithelial Cells, Aug. 22, S. Karger AG. (2015). https://doi.org/10.1159/000368521
Magi, S. et al. Intracellular calcium and ischemic damage: dual role of the Na+/Ca2 + Exchanger, in The First Outstanding 50 Years of Università Politecnica Delle Marche: Research Achievements in Life Sciences, (eds Longhi, S., Monteriù, A., Freddi, A., Aquilanti, L., Ceravolo, M. G., Carnevali, O., Giordano, M. & Moroncini, G.) Cham: Springer International Publishing, 361–372. doi: https://doi.org/10.1007/978-3-030-33832-9_24. (2020).
Chou, A. C., Ten Ju, Y. & Pan, C. Y. Calmodulin interacts with the sodium/calcium exchanger NCX1 to regulate activity. PLoS One. 10 (9). https://doi.org/10.1371/journal.pone.0138856 (Sep. 2015).
Claude, A. Diuretics, in Pharmacology in Veterinary Anesthesia and Analgesia, pp. 315–323. (2024). https://doi.org/10.1002/9781118975169.ch24
Sabina, M., Barakat, Z., Feliciano, A., Lamb, A. & Alsamman, M. M. Unlocking the Potential of Acetazolamide: A Literature Review of an Adjunctive Approach in Heart Failure Management, Jan. 01, Multidisciplinary Digital Publishing Institute (MDPI). (2024). https://doi.org/10.3390/jcm13010288
Li, D. et al. Binding of Losartan to Angiotensin AT1 Receptors Increases Dopamine D1 Receptor Activation, Journal of the American Society of Nephrology, vol. 23, no. 3, [Online]. (2012). Available: https://journals.lww.com/jasn/fulltext/2012/03000/binding_of_losartan_to_angiotensin_at1_receptors.11.aspx
Koeppen, B. M. The kidney and acid-base regulation. Adv. Physiol. Educ. 33, 275–281. https://doi.org/10.1152/advan.00054.2009.-Since (2009).
Eskandari, D., Zou, D., Grote, L., Hoff, E. & Hedner, J. Acetazolamide reduces blood pressure and sleep-disordered breathing in patients with hypertension and obstructive sleep apnea: A randomized controlled trial, Journal of Clinical Sleep Medicine, vol. 14, no. 3, pp. 309–317, Mar. (2018). https://doi.org/10.5664/jcsm.6968
Taslimi, P., Caglayan, C. & Gulcin, İ. The impact of some natural phenolic compounds on carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase, and α-glycosidase enzymes: an antidiabetic, anticholinergic, and antiepileptic study. J. Biochem. Mol. Toxicol. 31 (12). https://doi.org/10.1002/jbt.21995 (Dec. 2017).
Ekinci, D. et al. Carbonic anhydrase inhibitors: In vitro inhibition of α isoforms (hCA I, hCA IV) by flavonoids, J Enzyme Inhib Med Chem, vol. 28, no. 2, pp. 283–288, (2013). https://doi.org/10.3109/14756366.2011.643303
Chen, R. C., Sun, G. B., Wang, J., Zhang, H. J. & Sun, X. B. Naringin protects against anoxia/reoxygenation-induced apoptosis in H9c2 cells via the Nrf2 signaling pathway, Food Funct, vol. 6, no. 4, pp. 1331–1344, Apr. (2015). https://doi.org/10.1039/c4fo01164c
Tang, J. Y. et al. Naringenin ameliorates hypoxia/reoxygenation-induced Endoplasmic reticulum stress-mediated apoptosis in H9c2 myocardial cells: involvement in ATF6, IRE1α and PERK signaling activation. Mol. Cell. Biochem. 424, 1–2. https://doi.org/10.1007/s11010-016-2848-1 (Jan. 2017).
Daubney, J., Bonner, P. L., Hargreaves, A. J. & Dickenson, J. M. Cardioprotective and cardiotoxic effects of quercetin and two of its in vivo metabolites on differentiated H9c2 cardiomyocytes, Basic Clin Pharmacol Toxicol, vol. 116, no. 2, pp. 96–109, Feb. (2015). https://doi.org/10.1111/bcpt.12319
Angeloni, C., Spencer, J. P. E., Leoncini, E., Biagi, P. L. & Hrelia, S. Role of Quercetin and its in vivo metabolites in protecting H9c2 cells against oxidative stress. Biochimie 89 (1), 73–82. https://doi.org/10.1016/j.biochi.2006.09.006 (Jan. 2007).
Abdulrahman, N. et al. Empagliflozin inhibits angiotensin II-induced hypertrophy in H9c2 cardiomyoblasts through Inhibition of NHE1 expression. Mol. Cell. Biochem. 477 (6), 1865–1872. https://doi.org/10.1007/s11010-022-04411-6 (Jun. 2022).
Zhang, X., Zhang, S., Wang, M., Chen, H. & Liu, H. Advances in the allostery of angiotensin II type 1 receptor. Dec. 01 2023 BioMed. Cent. Ltd. https://doi.org/10.1186/s13578-023-01063-x
Verma, K., Pant, M., Paliwal, S., Dwivedi, J. & Sharma, S. An Insight on Multicentric Signaling of Angiotensin II in Cardiovascular system: A Recent Update, Aug. 20, Frontiers Media S.A. (2021). https://doi.org/10.3389/fphar.2021.734917
Fliegel, L. Role of Genetic Mutations of the Na+/H + Exchanger Isoform 1, in Human Disease and Protein Targeting and Activity, Feb. 01, Springer. (2021). https://doi.org/10.1007/s11010-020-03984-4
Bers, D. M., Barry, W. H. & Despa, S. Intracellular Na + regulation in cardiac myocytes. Apr 01. https://doi.org/10.1016/S0008-6363(02)00656-9 (2003).
Molkentin, J. D. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Aug 15 https://doi.org/10.1016/j.cardiores.2004.01.021 (2004).
Williams, R. S. Calcineurin signaling in human cardiac hypertrophy. May 14. https://doi.org/10.1161/01.CIR.0000017141.01845.FA (2002).
Xia, H. et al. Na+/H + Exchanger 1, a Potential Therapeutic Drug Target for Cardiac Hypertrophy and Heart Failure, Jul. 01, MDPI. (2022). https://doi.org/10.3390/ph15070875
Jiang, Y. et al. Radical scavenging and anti-inflammatory activities of (hetero)arylethenesulfonyl fluorides: synthesis and structure-activity relationship (SAR) and QSAR studies. Bioorg. Chem. 89, 103015. https://doi.org/10.1016/j.bioorg.2019.103015 (2019).
Stams, T. et al. Structures of murine carbonic anhydrase IV and human carbonic anhydrase II complexed with Brinzolamide: molecular basis of isozyme-drug discrimination. Protein Sci. 7 (3), 556–563. https://doi.org/10.1002/pro.5560070303 (1998).
Dong, Y. et al. Structure and mechanism of the human NHE1-CHP1 complex. Nat. Commun. 12 (1). https://doi.org/10.1038/s41467-021-23496-z (Dec. 2021).
Shivakumar, D. et al. Prediction of absolute solvation free energies using molecular dynamics free energy perturbation and the OPLS force field. J. Chem. Theory Comput. 6 (5), 1509–1519. https://doi.org/10.1021/ct900587b (May 2010).
Jyothi, M., Khamees, H. A., Patil, S. M., Ramu, R. & Khanum, S. A. Microwave-Assisted synthesis, characterization, Docking studies and molecular dynamic of some novel phenyl thiazole analogs as Xanthine oxidase inhibitor. J. Iran. Chem. Soc. 19 (9), 3919–3933. https://doi.org/10.1007/s13738-022-02574-z (2022).
Greenidge, P. A., Kramer, C., Mozziconacci, J. C. & Wolf, R. M. Successes, Failures, and Directions for Further Improvement. J. Chem. Inf. Model. 53 (1), 201–209. https://doi.org/10.1021/ci300425v (Jan. 2013). MM/GBSA Binding Energy Prediction on the PDBbind Data Set:.
Li, J. et al. The VSGB 2.0 model: A next generation energy model for high resolution protein structure modeling. Proteins Struct. Funct. Bioinform. 79 (10), 2794–2812. https://doi.org/10.1002/prot.23106 (Oct. 2011).
Suryakoppa, K. S., Kameshwar, V. H., Appadurai, R., Eranna, S. & Khan, M. H. M. Enantiomeric Separation of Indole-3-Propanamide Derivatives by Using Supercritical Fluid Chromatography on a Polysaccharide-Based Chiral Stationary Phase, J Chromatogr Sci, vol. 60, no. 7, pp. 692–704, Sep. (2022). https://doi.org/10.1093/chromsci/bmab102
Ho, Y. T. et al. Jun., Inhibition of carbonic anhydrase II by steroidal and non-steroidal sulphamates, Biochem Biophys Res Commun, vol. 305, no. 4, pp. 909–914, (2003). https://doi.org/10.1016/S0006-291X(03)00865-9
DH, H. S., Sultana, R., Prabhu, A., Mohanto, P. S. R. S. & Subramaniyan, V. Biomedicine and pharmacotherapeutic effectiveness of combinatorial Atorvastatin and Quercetin on diabetic nephropathy: an in vitro study. Biomed. Pharmacotherapy. 174 https://doi.org/10.1016/j.biopha.2024.116533 (May 2024).
Pavan, S. R. & Prabhu, A. Novel pH responsive hesperidin nanoformulation exerts anticancer activity on lung adenocarcinoma cells by targeting Akt/mTOR and MEK/ERK pathways. J. Mater. Res. 39 (8), 1217–1231. https://doi.org/10.1557/s43578-024-01304-w (2024).
Meduri, B. et al. New LPA1 receptor modulators: design, synthesis, in-silico, and anticancer studies of Triazole and oxadiazole analogs. J. Mol. Struct. 1295, 136672. https://doi.org/10.1016/j.molstruc.2023.136672 (2024).
Li, R. et al. MIR-7a/b attenuates post-myocardial infarction remodeling and protects H9c2 cardiomyoblast against hypoxia-induced apoptosis involving Sp1 and PARP-1. Sci. Rep. 6 https://doi.org/10.1038/srep29082 (Jul. 2016).
huai Hu, Y. et al. Sep., sFRP1 protects H9c2 cardiac myoblasts from doxorubicin-induced apoptosis by inhibiting the Wnt/PCP-JNK pathway, Acta Pharmacol Sin, vol. 41, no. 9, pp. 1150–1157, (2020). https://doi.org/10.1038/s41401-020-0364-z
Zweifach, A. Samples in many cell-based experiments are matched/paired but taking this into account does not always increase power of statistical tests for differences in means. Mol. Biol. Cell. 35 (1), br1. https://doi.org/10.1091/mbc.E23-05-0159 (Jan. 2024).
Acknowledgements
The authors thank Department of Science and Technology, Ministry of Science and Technology, India, and JSS Academy of Higher Education and Research for the financial support, JSS Academy of Higher Education and Research, and Yenepoya Research Centre Yenepoya (Deemed to be University) for providing laboratory facilities.
Funding
Research supported by Department of Science and Technology (DST-SERB), Ministry of Science and Technology, Government of India, New Delhi (SRG/2021/001016), JSS Academy of Higher Education and Research, Mysuru (JSSAHER/REG/RES/URG/54/2023-24 and JSSAHER/REG/RES/URG/54/2011-12/7951), and King Saud University, Riyadh, Saudi Arabia, Project Number (RSPD2025R709).
Author information
Authors and Affiliations
Contributions
J.M.S. and M.M., developed the research idea; M.M., S.G.P., P.S.R., and N.S.D., performed all laboratory experiments, and M.M., C.S., V.H.K., performed and analyzed computational experiments; J.M.S., M.M., S.D., V.H.K., T.S.K., C.S., and S.K.P., contributed to the design of the study, and evaluation of the results, and J.M.S., M.M., S.D., V.H.K., T.S.K., C.S., S.K.P., S.F.A., and S.M.A., contributed in writing and revising the manuscript. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: Shaik F. Ahmad was incorrectly affiliated, and the Funding information contained an incorrect project number. Full information regarding the correction made can be found in the correction for this Article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mahadevaswamy, M., Suchitha, G.P., Pavan, S.R. et al. Naringin attenuates angiotensin II induced cardiac hypertrophy by inhibiting carbonic anhydrase II. Sci Rep 15, 11789 (2025). https://doi.org/10.1038/s41598-025-95537-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-95537-2