Abstract
The “dolomite problem” has long been a significant challenge in sedimentology, particularly regarding the determination of dolomite formation temperatures, a subject that remains highly debated. Magnesium (Mg) isotopes, due to their stability in dolomite during diagenesis and the strong relationship between isotopic equilibrium fractionation and temperature, present a promising tool for estimating the formation temperatures of dolomite. In this study, we analyzed the Mg isotope composition (δ26Mg) of various dolomite samples from the Ediacaran to Ordovician in the Tarim Basin, China, to assess the potential of Mg isotopes as a thermometer for dolomite formation. The δ26Mg values of micro-fine crystalline dolomites (D1) ranged from − 1.98 to − 1.69‰, fine-medium crystalline dolomites (D2) from − 1.68 to − 1.33‰, and medium-coarse crystalline dolomites (D3) from − 2.35 to − 1.99‰. Based on the Mg isotope temperature equilibrium fractionation equations, Δ26Mgdol−fluid = − 0.1554(± 0.0096) × 106/T2 or Δ26Mgdol−cal=0.1453(± 0.0106)×106/T2, the calculated formation temperatures for D1 ranged from 45.0 to 65.4 °C, for D2 from 53.1 to 73.9 °C, and for D3 from 142.4 to 173.9 °C or 156.4 to 189.0 °C. These temperature estimates align closely with those derived from fluid inclusions and clumped isotopes in previous literature studies of the same formations, supporting the reliability of Mg isotopes as a method for determining dolomite formation temperatures. This study introduces an innovative approach for assessing dolomite formation temperatures using Mg isotopes, offering crucial insights into the resolution of the “dolomite problem.”
Similar content being viewed by others
Introduction
Research on the synthesis of dolomite has revealed that hydration effects hinder the incorporation of Mg2+ ions into carbonate minerals, making the formation of dolomite under surface temperature and pressure conditions challenging1,2. This finding aligns with geological observations indicating the scarcity of dolomite in contemporary sediments3. In contrast, massive dolostone strata have been identified in the rock record from the Precambrian to Paleozoic eras, with depositional thicknesses exceeding several hundred meters and covering thousands of square kilometers4,5. This distinctive geological phenomenon, coupled with unsuccessful synthesis attempts, has given rise to the “dolomite problem,” which has perplexed geologists for centuries6.
Following extensive exploration, three primary hypotheses have been proposed regarding the mechanisms of dolomite genesis7. (I) Near-Surface Low-Temperature Genesis: Disordered dolomite forms initially in the pores of oceanic or surface sediments under low-temperature conditions (≤ 60 °C), and later transforms into lattice-ordered dolomite through early diagenesis. (II) Burial Genesis: Calcium carbonate mineral precursors, such as calcite, undergo transformation into dolomite during long-term burial, facilitated by high-temperature underground- or pore-space-fluids, typically exceeding 60 °C8,9,10. (III) Hydrothermal Genesis: Mg-rich hydrothermal fluids, primarily controlled by tectonic-hydrothermal activity and usually exceeding 100 °C, facilitate dolomitization when their temperatures are significantly higher than those of the surrounding rocks11,12. These hypotheses emphasize the importance of formation temperature in explaining dolostone genesis. Accurate determination of the temperatures during dolomite formation is essential, as it can reveal the physicochemical conditions of the dolostone formation environment and offer valuable insights for addressing the “dolomite problem”13.
Thermodynamic theory predicts that the extent of isotopic fractionation diminishes as temperature increases, suggesting that isotopes could serve as geological thermometers14. For instance, the oxygen isotopes have been widely used to determine the formation temperature of dolomite in the past4,15,16,17. However, oxygen isotopes are relatively susceptible to diagenetic alteration and can be affected by subsequent fluid mixing, leading to changes in original oxygen isotope signals and belittling the reliability of oxygen isotope thermometry18,19. Recent experimental simulations have demonstrated that the equilibrium fractionation of Mg isotopes in dolomite is also dependent on temperature, with the degree of fractionation of Mg isotopes changing by approximately 0.011‰/°C20. Furthermore, it is believed that the Mg isotopes of dolomite are more resistant to late diagenetic alteration or hydrothermal activity, and can preserve the original geochemical signals21,22. Given the strong correlation between the equilibrium fractionation of dolomite Mg isotopes and temperature, as well as their stability during diagenetic alteration, it is possible to suggest that Mg isotopes have the potential to provide insights into dolomite formation temperatures.
The dolostone reservoirs in the Ediacaran to Ordovician formations in the Tarim Basin of China are important for oil and gas exploration, and a large number of measurements of fluid inclusions and clumped isotopes have been carried out in these formations, which provide adequate data for determining the forming temperature of dolomite23,24,25,26,27,28. In this study, we systematically measured the Mg isotopic composition (δ26Mg) of dolomite formed under different temperature conditions in the Ediacaran to Ordovician strata in the Tarim Basin to scrutinize the effectiveness of high-precision Mg isotopes as thermometers for dolomite formation. The results show that the dolomite formation temperatures calculated from Mg isotopes are close to those of fluid inclusions and clumped isotopes, and are consistent with the corresponding dolomite genesis mechanism, confirming that Mg isotopes can be used to constrain the formation temperature of dolomite effectively. It should be noted that determining the δ26Mg of the dolomitizing fluid is a challenging task due to the variability of fluid sources and the complexity of diagenetic processes. The temperature dependency of δ26Mg values allows for the potential reconstruction of fluid characteristics and dolomitization pathway at the time of dolomitization, thus enhancing our understanding of the mechanisms driving this process.
Geological background
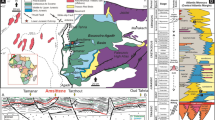
The Tarim Basin, a vast hydrocarbon-rich basin in northwestern China, spans approximately 530,000 km[2 29,30. The basement of the basin dates back to the Proterozoic, with its geological evolution significantly influenced by the assembly and break-up of the Columbia-Rodinia supercontinent31. The craton rift basin initialized during the Paleozoic and Mesozoic periods due to the closing of the Paleotethys and the Paleo-Asian Oceans32. The collision between the Indian Plate and the Eurasian Plate during the Cenozoic led to the formation of an intracontinental foreland basin along the periphery of Tarim Basin, resulting in a superimposed basin system consisting of the Cenozoic foreland basin and the Paleozoic-Mesozoic cratonic basin33,34 (Fig. 1).
The tectonic delineation of the Tarim Basin, with the sampling and drilling locations35.
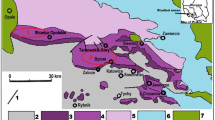
The Tarim Basin exhibited a paleogeographic pattern that a basin to the east and a carbonate platform to the west from the Late Ediacaran to the Ordovician. During the late Ediacaran, the central and western parts of the Tarim Basin was characterized by carbonate ramp deposits, leading to the formation of considerable dolostone strata within the Qigebulake Formation under arid and hot climates24. The Keping Movement at the end of the Late Ediacaran resulted in an angular unconformity between the top of the Ediacaran and the bottom of the Cambrian36,37. With global transgression during the early Cambrian, the basin transitioned into an epicontinental sea sedimentary environment, forming the black shale of the Yuertusi Formation38,39. From the Early to Late Cambrian, the sedimentary environment shifted to carbonate platform deposits at passive continental margin. Notably, the Lower Cambrian experienced the dolostone accumulation of the Xiaoerbulake Formation on a homoclinal ramp. In the Middle-Late Cambrian, a large rimmed platform emerged due to the rise of sea level, leading to the development of considerable dolomite-gypsum interbedded strata within the platform40,41. The range of Ordovician carbonate platform deposits expanded notably, with the central and western parts of the basin characterized by extensive carbonate platform deposits and the eastern part featuring slope and basin deposits42. The Penglaiba Formation of Lower Ordovician was set in a restricted platform, where a substantial dolostone was formed43. The Yingshan Formation of Middle-Lower Ordovician, influenced by rising sea levels, exhibites gentle slope open platform facies and developed dolostone, limestone dolostone, and limestone successively44. In the Late Ordovician, the carbonate platform contracted quickly due to a rapid rise of sea level and increased terrigenous depositions, leading to the limestone strata in the Yijianfang and Lianglitage Formation of the Upper Ordovician45 (Fig. 2).
The tectonic activity and geological composition within the Tarim Basin from the Ediacaran to Ordovician periods46.
Sampling and experimental methods
Sampling
A systematic sampling was conducted on the dolostones from the Ediacaran to Ordovician periods in the Tarim Basin. Micro-fine crystalline dolostone (D1) from the Upper Ediacaran Qigebulake Formation and fine-medium crystalline dolostone (D2) from the Lower Cambrian Xiaoerbulake Formation were collected from the Xiaoxigou profile at the northwestern margin of the Tarim Basin. Additionally, medium-coarse crystalline dolostone (D3) from the Lower Ordovician Penglaiba Formation and the Middle-Lower Ordovician Yingshan Formation were sampled from Well LS2 in the Tabei Uplift and Well GC17 in the Gucheng Low Uplift, respectively (Figs. 1 and 2). While there was some variation in dolomite fabrics within each formation, the D1, D2 and D3 samples were chosen to represent the most dominant dolomite types within their respective sampled strata.
We used one half of the samples to create thin sections, while the other half was used for micro-area sampling. Adequate micro-area powder samples were obtained for element, carbon isotope, oxygen isotope, and Mg isotope analyses. During the sampling process, specially cleaned drill bits and sampling tools were used, and careful cleaning and disinfection were performed before and after each sample collection to prevent any rock debris or external contaminants from entering the samples. Additionally, the drill bits were regularly replaced and cleaned to ensure they remained contamination-free throughout the sampling procedure. To minimize the risk of environmental contamination, all operations were carried out in a clean environment, and strict quality control and inspection of all tools and equipment were implemented. These measures ensured the accuracy and representativeness of the samples.
Experimental methods
The thin sections were observed using the Nikon Eclipse LV100 N POL optical microscope at the School of Earth and Space Sciences of Peking University, Beijing, China. Mg and Ca elements were analyzed using an Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES) at the ICP spectral analysis laboratory of the same institution, with an instrument error of less than 5%. Carbon and oxygen isotope analyses were performed using the Thermo Fisher 253plus Gas Bench in the ultra-clean laboratory of Beijing Kehui Testing Technology Co., Ltd., employing the McCrea orthophosphate method. Both carbon and oxygen isotopes were calibrated to the Vienna Pee Dee Belemnite (VPDB) standard, with δ18O and δ13C measurement errors of less than 0.1‰.
Mg isotopes were purified via cation-exchange chromatography in the ultra-clean laboratory of the School of Earth and Space Sciences, Peking University. After purification, the ratios of Ca/Mg, Al/Mg, Na/Mg, K/Mg, and Fe/Mg were all kept below 5%, with sample recovery rates exceeding 95%47. Mg isotope measurements were conducted using the Neptune Plus Multi-Collector Inductively Coupled Plasma Mass Spectrometer (MC-ICP-MS) in the ultra-clean laboratory of Beijing Aloe Testing Technology Co. To correct for mass fractionation effects in the mass spectrometer, a standard-sample bracketing (SSB) method was applied during the Mg isotope analysis. The laboratory standard used was a pure Mg solution, IGGMg1. Prior to the analysis, the Mg isotope composition of IGGMg1 was measured using MC-ICP-MS and compared with its long-term Mg isotope value (− 0.0852‰ ± 0.046‰) to assess instrument stability. All data were calibrated to the International Mg Isotope Standard (DSM-3) values, with δ26Mg of dolostone reported using the traditional δ-notation (Eqs. 1 and 2)48. To check for mass-independent fractionation in the measured δ26Mg, all data were evaluated on an isotopic plot (δ26Mg vs. δ25Mg). The root means square error (R2) for δ26Mg and δ25Mg in the samples was 0.9943 (Fig. 3).
Results
Petrology
Considering both the crystal sizes and the morphology of the dolomite, three distinct categories of dolostone (D1, D2, and D3) have been identified in the collected samples from the Ediacaran to Ordovician periods in the Tarim Basin (Table 1).
The hand specimens of D1 appear grayish black and have a compact texture with no apparent pores (Fig. 4A). The hand specimens of D1 are grayish-black and exhibit a compact texture with no apparent pores (Fig. 4A). The crystal size of D1 is less than 50 μm, and their shapes are either planar anhedral crystals or planar subhedral crystals (Fig. 4B). Foamy-layered or algal-clotted sedimentary fabrics are visible in D1, and these fabrics are primarily composed of dolomite crystals with diameters smaller than 20 μm (Fig. 4C). The hand specimen of D2 is dark gray (Fig. 4D). The crystal size of D2 range from 50 μm to 250 μm, displaying planar euhedral crystals or planar subhedral crystals (Fig. 4E). Some D2 samples retain the residual granule structure, with the original grains likely being sand-sized debris or ooids (Fig. 4F). The hand specimens of D3 are light gray, and their thin sections reveal a coarse crystalline structure that is visible to the naked eye (Fig. 4G). The crystal size of D3 ranges from 250 μm to 500 μm, primarily exhibiting non-planar subhedral or non-planar anhedral crystals (Fig. 4H). Identifying the original sedimentary fabrics of D3 is challenging, but dolomite in these samples displays distinctive features, including a “foggy core and bright edge” as well as undulatory extinction (Fig. 4I).
The petrological characteristics of dolostone from the Ediacaran to Ordovician periods in the Tarim Basin. (A) Hand specimens of micro-fine crystalline dolostone (D1), grayish black, XX-15; (B) Microphotograph of D1 showing the dolomite crystal diameter is less than 50 μm, XX-15, imaged under plane-polarized light; (C) Foamy-layered or algal-clotted sedimentary fabric of D1, XX-27, imaged under plane-polarized light; (D) Hand specimen of fine-medium crystalline dolostone (D2), dark gray, XX-68; (E) Microphotograph of D2, XX-41, imaged under plane-polarized light; (F) Microphotograph of D2 has obvious residual granule structure, XX-68, imaged under plane-polarized light; (G) Hand specimen of medium-coarse crystalline dolostone (D3), light gray, GC17-23; (H) Microphotograph of D3, LS2-7, imaged under plane-polarized light; (I) Dolomite with “foggy core and bright edge” and undulatory extinction, GC17-1, imaged under crossed polars.
Geochemistry
Table 2 presents the Mg/Ca(mol/mol) ratios and δ13C-δ18O-δ26Mg values for dolostone samples from the Ediacaran to Ordovician periods in the Tarim Basin. All dolostone samples show Mg/Ca(mol/mol) ratios exceeding 0.7. For D1, the δ26Mg values range from − 1.98 to − 1.69%, with corresponding δ13C values varying from 5.30 to 7.96‰ and δ18O values ranging from − 5.04 to 1.45‰. For D2, the δ26Mg values range from − 1.68 to − 1.33‰, with δ13C values ranging from 0.29 to 2.58‰, and δ18O values varying from − 8.72 to − 4.94‰. Lastly, D3 exhibits δ26Mg values from − 2.35 to − 1.99‰, δ13C values from − 2.08 to − 0.85‰, and δ18O values from − 7.29 to − 3.54‰ (Fig. 5). There is no significant correlation between the Mg/Ca (mol/mol) ratios and δ26Mg values for D1 and D2, while a notable correlation exists between the Mg/Ca (mol/mol) ratio and δ26Mg values for D3 (Fig. 5A). The δ13C and δ18O values for D1, D2, and D3 show distinct distribution patterns (Fig. 5B).
Discussion
Origin of different dolostones
Micro-fine crystalline dolostone (D1)
Dolomite crystal grains are usually small in sizes and capable of preserving structural characters when they are forming in near-surface settings, where rapid crystallization occurs49. The crystal sizes of D1 are less than 50 μm (Fig. 4B), suggesting that D1 are likely formed in a near-surface environment. The average order degree of D1 is only 0.68 50, which is typically interpreted as a consequence of rapid dolomite crystallization at low temperatures. Related studies suggest that microbial degradation of organic matter consumes sulfate and increases alkalinity, which promotes dolomite precipitation51. D1 exhibits foamy-layered or algal-clotted structures (Fig. 4C), and these microbial-constructed features indicate that the formation of D1 may be associated with microbial activity52.
Seafloor sediments can be divided into two zones from top to bottom: the oxygen-rich zone and the oxygen-poor zone. The oxygen-poor zone can be further subdivided into the methane oxidation zone and the methane generation zone. In the methane generation zone, methane produced is relatively depleted in 13 C, and as a result, 13C becomes enriched in HCO3−53. This13C-enriched HCO3− serves as an important carbon source for dolomite formation under the influence of methane-generating bacteria. As a result, dolomites formed in the methane generation zone often exhibit a significant positive carbon isotope anomaly, with δ13C values ranging from 0 to + 26‰54. The positive δ13C values observed in D1 (5.30–7.96‰, Fig. 5B) are similar to those of dolomites formed in the methane generation zone in drilling cores from the west coast of Africa (4–7‰)55, suggesting that D1 may have been induced by methane-generating bacteria in an oxygen-poor seawater environment. The extremely small dolomite size and significantly positive δ13C values together imply that D1 was likely co-precipitationally formed in a low-temperature, oxygen-poor seawater environment influenced by anaerobic microorganisms (Fig. 6A).
Fine-medium crystalline dolostone (D2)
Dolomite formed at temperatures below the “critical coarsening temperature (50 °C)” typically exhibits a small crystal size (< 50 μm) and a flat crystal morphology56. However, the crystal size of D2 ranges between 50 μm and 250 μm, with most crystals being planar euhedral or subhedral (Fig. 4E). Additionally, the residual granule structures are preserved in D2 (Fig. 3F), which could be attributed to the alteration of the original rock structure during diagenetic process57. The δ13C value of D2 closely resembles the carbon isotope composition of Early Cambrian seawater, suggesting a connection between the dolomitized fluid and the Early Cambrian seawater58 (Fig. 5B). In contrast, the δ18O value of D2 is lower than that of the Early Cambrian seawater, potentially attributed to the dolomitized fluid being a permeable reflux brine in a shallow burial environment, with its δ18O value being reset at a higher temperature59. Based on all aforementioned evidence, it can be deduced that D2 was formed by seepage reflux dolomitization in shallow burial environment (Fig. 6B).
Medium-coarse crystal dolomite (D3)
The crystal sizes of D3 are the largest, and its crystal morphology is more curved (Fig. 4H). These coarse and curved dolomite crystals are the result of rapid crystallization at high temperatures typically56. The D3 is primarily formed through metasomatism of calcite in a high-temperature environment during deep burial periods, leading to the destruction of the original rock fabric and the manifestation of dolomite crystals with a “fog core and bright edge” and undulatory extinction60,61,62 (Fig. 4I).
Generally, dolomitization fluid in the medium-deep burial environment is primarily sourced from the fluid in the pore space or fault hydrothermal fluid11. The δ13C value of D3 ranges from − 2.08 to − 0.85‰, slightly lower than the carbon isotope composition of Ordovician seawater, suggesting that the dolomitization fluid may have inherited characteristics from high-temperature residual modified seawater63. In summary, D3 is formed in a medium-deep burial environment, with the fluid being derived from residual seawater in the pores and significantly influenced by deep hydrothermal fluids (Fig. 6C).
Using fluid inclusions, clumped isotopes and U-Pb age to calibrate dolomite formation temperature
Currently, the predominant techniques for determining the temperature at which dolomite forms include the analysis of fluid inclusions64,65,66,67, clumped isotopes thermometer (TΔ47)68,69,70,71, and U-Pb age72,73,74. In the Tarim Basin, the Ediacaran to Ordovician dolostone formations serve as a significant reservoir for oil and gas. Extensive testing of fluid inclusions, clumped isotopes, and U-Pb age has been conducted by researchers, providing abundant data for calibrating the formation temperature of dolomite23,24,25,26,27,28 (Table 3).
Although this study did not directly obtain fluid inclusion, clumped isotope, or U-Pb age data, existing literature demonstrates a high degree of petrological compatibility with the D1, D2, and D3 samples in this study. This allows us to use the available fluid inclusion, clumped isotope, and U-Pb data to infer the formation temperatures of the D1, D2, and D3 samples. Shen23 measured the TΔ47 and U-Pb ages of dolostones from the same profile (Xiaoxigou profile) and the same stratigraphic unit (Qigebulake Formation) as in our current study. These dolomites exhibited a micro-crystalline structure consistent with D1 (Fig. 3B,C). The results showed that the TΔ47 of the dolostone ranged from 30 to 32 °C, with U-Pb ages of 576 ± 16.0 Ma and 560 ± 26.0 Ma. Additionally, foamy-layered dolostones from the Qigebulake Formation of the northern Tarim Basin, well Qitan-1, also exhibited similar lithological characteristics to D1, with a TΔ47 of 51 °C24. These relatively low TΔ47 values (30–51 °C) and the U-Pb ages consistent with late Ediacaran strata (576 ± 16.0 Ma or 560 ± 26.0 Ma) indicate that D1 is a near-surface, low-temperature dolomite (< 60 °C).
Fine crystalline dolostone from the Xiaorubulake Formation in the Yutixi profile of the Tarim Basin has crystal diameters ranging from 50 μm to 200 μm and retains residual grain structures25. This is consistent with the lithological features of D2 from the Xiaoxigou profile (Fig. 3E,F). Previous measurements of the homogenization temperatures of fluid inclusions in fine crystalline dolostone from the Xiaoerbulake Formation in the Yutixi profile is between 70 and 110 °C25. We speculate that the formation temperature and depth of D2 from the Xiaoxigou profile are higher than those of D1. Hu26 used laser U-Pb isotope dating techniques to determine the ages of D2 from the Xiaoerbulak Formation, yielding ages of 491.6 ± 9.2 Ma and 488 ± 24 Ma. These ages correspond to the Middle-Late Cambrian period. Based on the latest burial-thermal evolution history of the early Cambrian in the Tarim Basin46 and the U-Pb age of D2, we calculated that the depth of formation of D2 is approximately ~ 500 m, which falls within the shallow burial depth range (Fig. 7). The homogenization temperature of fluid inclusions and the burial-thermal evolution history confirm that D2 is a medium-low temperature dolomite formed in a shallow burial environment (60–100 °C).
Burial-thermal evolution history of Tarim Basin (Revised after46).
Previous studies measured the U-Pb ages of the core and edge of coarse crystalline dolostone from the Penglaiba Formation in the Yong’anba profile of the Tarim Basin, yielding ages of 433 ± 22 Ma and 382 ± 29 Ma, respectively75. Considering that the lithological features of the coarse crystalline dolostone from the Penglaiba Formation in the Yong’anba profile are similar to those of D3 from the LS2 well, and combining the U-Pb ages with the burial-thermal evolution history of the Early Ordovician Penglaiba Formation in the Tarim Basin, we infer that the formation depth of D3 is approximately 2600 to 4200 m, with corresponding formation temperatures of approximately 90–115 °C (Fig. 7). This is consistent with the lowest homogenization temperature of fluid inclusions in D3, which was measured at 119.8 °C27. Previous studies also collected medium-coarse crystalline dolostone with crystal sizes ranging from 250 to 500 μm from the Yingshan Formation in the Tarim Basin’s Tazhong area, at the ZG9 and ZG512 wells, and measured ∆T47 temperatures ranging from 94 to 111 °C28. The Gucheng area in the Tarim Basin formed several faults during the early Caledonian movement, and then the faults further expanded under the continuous activity in the middle Caledonian. The fault-rich system provides favorable migration channels for the hydrothermal fluids77,78. The previously determined U-Pb age of D3 in Yingshan Formation is 464 ± 12 Ma, which coincides with the Middle and Late Ordovician hydrothermal fluid action period and confirms that D3 of the Yingshan Formation is the product of high temperature hydrothermal activity76. In summary, we believe that D3 belongs to medium-to-deep burial or high-temperature hydrothermal dolomite (> 100 °C).
Using Mg isotopes to constrain the formation temperature of dolomite
Constraining the forming temperature of D1
The equilibrium fractionation of Mg isotopes in dolomite is controlled by temperature. Li20 summarized an equation for the temperature-dependent Mg isotope fractionation coefficient of dolomite, with temperature (T) serves as a variable in the equation (Eq. 3).
Notes: ∆26Mgdol−fluid: the Mg isotope fractionation coefficient between dolomite and fluid; T: the temperature in Kelvin.
The D1 was formed in a near-surface environment, with dolomitization fluids originating from seawater (Fig. 6A). Although Eq. (3) was calibrated through experiments conducted in the 130–220 °C range, it is consistent with the measurement deviations between low-temperature dolostone and pore water in natural environments79. Therefore, the application of Eq. (3) is reasonable at lower temperatures. However, a crucial factor in this calculation is to know the δ26Mg value of seawater. Zhang80 suggested the δ26Mg value at − 0.54‰ for Late Ediacaran seawater based on fibrous dolomite from the Upper Ediacaran Dengying Formation in the Sichuan Basin, China. Given the long retention time of Mg isotopes in seawater, the δ26Mg value of global seawater is expected to remain stable over short time scales81. Since the Qigebulake Formation belongs to the same Late Ediacaran period as the Dengying Formation, it is reasonable to assume that the δ26Mg value of the seawater involved in the formation of D1 in the Qigebulake Formation is −0.54‰. Additionally, the Mg isotope could be fractionating during the formation of dolomite, with the24Mg in the fluid entering the dolomite lattice preferentially, leading to the gradual enrichment of the heavier26Mg in the fluid, and increasing the δ26Mg value in later formed dolomite5. In one set of dolostone strata, the lowest δ26Mg value is considered to be the equilibrium value between dolomite and seawater82. Thus, − 1.98‰ was chosen as the Mg isotope equilibrium value between D1 and seawater. By combining δ26Mg value of seawater (− 0.54‰) with the δ26Mg value of D1 (− 1.98‰) into Eq. (3), the temperatures at which D1 formed were determined to be within the range of 45.0–65.4 °C, a finding that aligns closely with the ∆T47 (30–51.1 °C) (Fig. 8; Table 4).
Constrain the temperature of D2
The D2 was formed in a shallow burial setting with a seepage reflux brine as the fluid (Fig. 6B). While D2 was not directly formed in a seawater environment, there is often a fluid cycle maintained between the infiltration reflux brine and the basin seawater83. Dolostones formed in such shallow burial environments typically belong to a ‘’fluid-buffered’’ system, where their Mg isotopes undergo extensive exchange with infiltrating recirculated seawater, reaching equilibrium84. Meanwhile, several studies have confirmed that the Mg isotopes in early dolostones are resistant to subsequent diagenetic alteration22,85,86,87. The δ26Mg values of D2 range from − 1.68 to − 1.33‰, and the lowest δ26Mg value (− 1.68‰) is chosen as the equilibrium value between dolomite and seawater for calculating the dolomite formation temperature. Based on the latest constraint on the δ26Mg value (ranging from − 0.31 to + 0.15‰) of Early Cambrian seawater from Li et al.21, this study selects the lower bound of δ26Mg for Early Cambrian seawater (− 0.31‰) as the fluid δ26Mg value for calculating the temperature of D2 formation. This is because the temperature derived from this lower bound corresponds to the upper limit of the formation temperature of D2 dolostone. When the fluid δ26Mg value is − 0.31‰, the ∆26Mgdol−fluid of D2 is − 1.37‰. Using Eq. (3), the calculated formation temperature of D2 ranges from 53.1 to 73.9 °C (Fig. 8; Table 4).
Constrain the temperature of D3
The dolomitization fluid of D3 is believed to originate from either residual pore water buried at medium to deep depths or from hydrothermal fluid that has risen along a fault line (Fig. 6C). The hydrochemical properties of this fluid differ significantly from those of seawater, making it impossible to determine the fractionation value of dolomite and fluid using the δ26Mg value of seawater89. Previous research has indicated that the equilibrium fractionation of Mg isotopes in carbonate minerals is influenced by the internal coordination number of the minerals. Minerals with lower coordination numbers have shorter Mg–O chemical bond lengths, resulting in heavier δ26Mg values90. Dolomite and calcite have Mg-O bond lengths of 2.08 and 2.12, respectively. This suggests that the δ26Mg value of dolomite is higher than that of calcite91,92. Empirical data confirms that the δ26Mg values of dolomite and calcite are significantly different, with the δ26Mg values of calcite being approximately 2‰ lower than those of dolomite21,93. Furthermore, the Mg isotope composition of calcite and dolomite will readjust at higher temperatures during later burial diagenesis94. Based on the studies of Li20, Saenger and Wang95, and Mavromatis96, the Mg isotope temperature fractionation calibration formula for dolomite and calcite is as follows:
Notes: ∆26Mgdol−cal: the Mg isotope fractionation coefficient between dolomite and calcite; T: the temperature in Kelvin.
The Mg isotope measurements of dolomite and calcite in the carbonate rocks of the Middle Cambrian Xuzhuang Formation indicate that during the relatively low-temperature mineral transformation process in the penecontemporaneous stage, the δ26Mg difference between dolomite and calcite is approximately 1.6‰ 97. The δ26Mg difference of 0.72‰ between dolomite and calcite in the Triassic Geshan profile of eastern China. By applying the Mg isotope fractionation coefficient derived from experimental methods, they calculated the corresponding equilibrium temperatures to be 158 °C and 191 °C, which is consistent with the burial-thermal evolution history of the studied strata82. The distinct Mg isotope differences between dolomite and calcite in the Geshan area reflect the reequilibration of Mg isotopes between the minerals during the maximum burial period, supporting the use of the dolomite-calcite Mg isotope geothermometer.
The previous studies on the Penglai Ba Formation and the Yingshan Formation documented that the calcite coexisting with D3 dolomite exhibits undulatory extinction and recrystallized structure75,76, indicating that both underwent recrystallization after experiencing the same thermal burial event. The δ26Mg values of calcite in the Penglaiba and Yingshan Formations of the Tarim Basin are − 2.82‰ and − 3.13‰, respectively (data form75,76). We selected the minimum δ26Mg values of -2.09‰ from D3 of the Penglaiba Formation and − 2.35‰ from D3 of the Yingshan Formation, resulting in δ26Mgdol−cal values of 0.73‰ for D3 in the Penglaiba Formation and 0.78‰ for D3 in the Yingshan Formation. Isotope fractionation theory suggests that the degree of isotope fractionation decreases with increasing temperature14. For D3, the δ26Mg values show a positive correlation with Mg/Ca (mol/mol) (Fig. 5A), which aligns with the behavior of many mineral systems at elevated temperatures. The Mg isotope variation between D3 and calcite is much smaller than the δ26Mgdol−cal values observed during the penecontemporaneous dolomitization process97, indicating that during the later burial diagenesis, the Mg isotope composition of both calcite and D3 underwent re-equilibration under higher temperature conditions. Using Eq. (4), we calculated the formation temperatures of D3 in the Penglaiba Formation and the Yingshan Formation to be 156.4 °C to 189.0 °C and 142.4 °C to 173.9 °C, respectively (Fig. 9; Table 4).
Conclusion
Based on the sizes, shapes of dolomite crystals, together with the rock fabric, three types of dolostones have been identified in the Tarim Basin from the Ediacaran to Ordovician periods, which could be listed as micro-fine crystalline dolostone (D1), fine-medium crystalline dolostone (D2), and medium-coarse crystalline dolostone (D3). The formation environments and fluid sources for these different dolomites exhibit significant variations. D1 was formed in a near-surface environment, with fluid derived from contemporaneous seawater. D2 was formed in a shallow burial environment, with fluid sourced from seepage reflux brine. D3, on the other hand, was formed in a medium-deep burial environment, with fluid derived from residual seawater or deep hydrothermal fluid in the pores. The formation temperatures were estimated using Mg isotopes, with temperatures of D1 ranging from 45.0 °C to 65.4 °C, D2 from 53.1 °C to 73.9 °C, and D3 from 142.4 °C to 173.9 °C or 156.4 °C to 189.0 °C. The calculated temperatures of dolomite using Mg isotopes were consistent with the temperature obtained by previous researchers from fluid inclusions and clumped isotopes, thus confirming the potential of Mg isotopes in determining the temperature at which dolomite forms. We emphasize that while the use of magnesium isotope temperature proxies provides valuable temperature estimates, it is more prudent to validate these estimates by combining them with other independent temperature proxies (such as bulk isotopes), given the assumptions of δ26Mg for dolomitizing fluids/calcite and potential fractionation effects. Therefore, we recommend that future studies integrate multiple temperature proxies to further investigate the relationship between dolomite and dolomitizing fluids, in order to enhance the reliability of the research findings.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Land, L. S. Failure to precipitate dolomite at 25 °C from dilute solution despite 1000-foldoversaturation after 32 years. Aquat. Geochem. 4, 361–368 (1998).
Gregg, J. M., Bish, D. L., Kaczmarek, S. E. & Machel, H. G. Mineralogy, nucleation and growth of dolomite in the laboratory and sedimentary environment: A review. Sedimentology 62, 1749–1769 (2015).
Warren, J. Dolomite: Occurrence, evolution and economically important associations. Earth Sci. Rev. 52, 1–81 (2000).
Land, L. S. The origin of massive dolomite. J. Geol. Educ. 33, 112–125 (1985).
Ning, M. et al. Towards Understanding the origin of massive dolostones. Earth Planet. Sci. Lett. 545, 116403 (2020).
Chang, B. et al. Massive formation of early diagenetic dolomite in the Ediacaran Ocean: Constraints on the dolomite problem. Proc. Natl. Acad. Sci. 117, 14005–14014 (2020).
Zhao, W. et al. Genetic types and distinguished characteristics of dolomite and the origin of dolomite reservoirs. Pet. Explor. Dev. 45, 983–997 (2018).
Müller, D. W., McKenzie, J. A. & Mueller, P. A. Abu Dhabi Sabkha, Persian Gulf, revisited: Application of strontium isotopes to test an early dolomitization model. Geology 18, 618–621 (1990).
Roberts, J. A. et al. Surface chemistry allows for abiotic precipitation of dolomite at low temperature. Proc. Natl. Acad. Sci. 110, 14540–14545 (2013).
Petrash, D. A. et al. Microbially catalyzed dolomite formation: From near-surface to burial. Earth Sci. Rev. 171, 558–582 (2017).
Davies, G. R. & Smith, L. B. Structurally controlled hydrothermal dolomite reservoir facies: An overview. AAPG Bull. 90, 1641–1690 (2006).
Koeshidayatullah, A. et al. Origin and evolution of fault-controlled hydrothermal dolomitization fronts: A new insight. Earth Planet. Sci. Lett. 541, 116291 (2020).
Hu, Z. et al. Temperatures of dolomitizing fluids in the Feixianguan formation from the Northeastern Sichuan basin. Sci. China Earth Sci. 55, 1627–1640 (2012).
Urey, H. C. The thermodynamic properties of isotopic substances. J. Chem. Soc. 1947, 562–581 (1947).
Vasconcelos, C., McKenzie, J. A., Warthmann, R. & Bernasconi, S. M. Calibration of the δ18O paleothermometer for dolomite precipitated in microbial cultures and natural environments. Geology 33, 317–320 (2005).
Kırmacı, M. Z. & Akdağ, K. Origin of dolomite in the late Cretaceous–Paleocene limestone turbidites, Eastern Pontides, Turkey. Sed. Geol. 181, 39–57 (2005).
Horita, J. Oxygen and carbon isotope fractionation in the system dolomite–water–CO2 to elevated temperatures. Geochim. Cosmochim. Acta. 129, 111–124 (2014).
Nelson, B. K., DeNiro, M. J., Schoeninger, M. J., De Paolo, D. J. & Hare, P. E. Effects of diagenesis on Strontium, carbon, nitrogen and oxygen concentration and isotopic composition of bone. Geochim. Cosmochim. Acta. 50, 1941–1949 (1986).
Dickson, J. & Coleman, M. Changes in carbon and oxygen isotope composition during limestone diagenesis. Carbonate Diagenesis 27, 107–118 (1980).
Li, W., Beard, B. L., Li, C., Xu, H. & Johnson, C. M. Experimental calibration of Mg isotope fractionation between dolomite and aqueous solution and its geological implications. Geochim. Cosmochim. Acta. 157, 164–181 (2015).
Li, X. et al. Conservative behavior of Mg isotopes in dolomite during diagenesis and hydrothermal alteration: A case study in the lower cambrian qiulitage formation, Gucheng area, Tarim basin. Appl. Geochem. 148, 105540 (2023).
Hu, Z. et al. Conservative behavior of Mg isotopes in massive dolostones: From diagenesis to hydrothermal reworking. Sed. Geol. 381, 65–75. https://doi.org/10.1016/j.sedgeo.2018.12.007 (2019).
Shen, A., Hu, A., Zheng, J., Liang, F. & wang, Y. Reconstruction of tectonic-burial evolution based on the constraints of laser in situ U–Pb date and clumped isotopic temperature: A case study from Sinian Qigebulak formation in Akesu area, Tarim basin. Mar. Origin Pet. Geol. 26, 200–210 (2021).
Chen, X. et al. Dolomite reservoir formation and diagenesis evolution of the upper Ediacaran qigebrak formation in the Tabei area, Tarim basin. Sci. China Earth Sci. 66, 2311–2331 (2023).
Bai, Y. et al. Characteristics and multiple dolomitization mode of the lower cambrian dolomite reservoir, Northwestern Tarim basin. Acta Petrolei Sinica. 42, 1174–1191 (2021).
Hu, A. et al. Application of laser in-situ U-Pb dating to reconstruct the reservoir porosity evolution in the cambrian Xiaoerbulake formation. Tarim Basin Oil Gas Geol. 41, 37–49 (2020).
Ye, N. et al. Dolomitization and its impact on porosity development and preservation in the deeply burial lower ordovician carbonate rocks of Tarim basin, NW China. J. Petrol. Sci. Eng. 182, 106303 (2019).
Liu, J. et al. Diagenetic fluid evolution of dolomite from the lower ordovician in Tazhong area, Tarim basin: Clumped isotopic evidence. Oil Gas Geol. 41, 68–82 (2020b).
Jia, C. & Wei, G. Structural characteristics and petroliferous features of Tarim basin. Chin. Sci. Bull. 47, 1–11 (2002).
Deng, S., Li, H., Zhang, Z., Zhang, J. & Yang, X. Structural characterization of intracratonic strike-slip faults in the central Tarim basin. AAPG Bull. 103, 109–137 (2019).
Zhang, C. L., Zou, H. B., Li, H. K. & Wang, H. Y. Tectonic framework and evolution of the Tarim block in NW China. Gondwana Res. 23, 1306–1315 (2013).
He, B. et al. The paleotectonic and paleogeography reconstructions of the Tarim basin and its adjacent areas (NW China) during the late early and middle paleozoic. Gondwana Res. 30, 191–206 (2016).
Jin, Z. & Wang, Q. Recent developments in study of the typical superimposed basins and petroleum accumulation in China: Exemplified by the Tarim basin. Sci. China Ser. D-Earth Sci. 47, 1–15 (2004).
Yin, A. Cenozoic tectonic evolution of Asia: A preliminary synthesis. Tectonophysics 488, 293–325 (2010).
Zhu, D., Meng, Q., Jin, Z., Liu, Q. & Hu, W. Formation mechanism of deep cambrian dolomite reservoirs in the Tarim basin, Northwestern China. Mar. Pet. Geol. 59, 232–244 (2015).
Carroll, A. R., Graham, S. A., Chang, E. Z. & McKnight, C. Sinian through permian tectonostratigraphic evolution of the Northwestern Tarim basin, China. Geol. Soc. Am. Mem. 194, 47–69 (2001).
Chen, L. et al. Characteristics and formation mechanisms of the unconformity-related paleokarst reservoirs in the upper Sinian, Northwestern Tarim basin, China. Mar. Pet. Geol. 120, 104559 (2020b).
Zhu, G. et al. Discovery of the lower cambrian high-quality source rocks and deep oil and gas exploration potential in the Tarim basin, China. AAPG Bull. 102, 2123–2151 (2018).
Wu, L., Zhu, G., Yan, L., Feng, X. & Zhang, Z. Late Ediacaran to early cambrian tectonic–sedimentary controls on lower cambrian black shales in the Tarim basin, Northwest China. Glob. Planet Change. 205, 103612 (2021).
Shen, A., Zheng, J., Chen, Y., Ni, X. & Huang, L. Characteristics, origin and distribution of dolomite reservoirs in Lower-Middle cambrian, Tarim basin, NW China. Pet. Explor. Dev. 43, 375–385 (2016).
Huang, Y., Fan, T. & Berra, F. Architecture and paleogeography of the early paleozoic carbonate systems in the east-central Tarim basin (China): Constraints from seismic and well data. Mar. Pet. Geol. 113, 104147 (2020).
Lin, C. et al. Sequence architecture and depositional evolution of the ordovician carbonate platform margins in the Tarim basin and its response to tectonism and sea-level change. Basin Res. 24, 559–582 (2012).
Guo, C. et al. Depositional environments and Cyclicity of the early ordovician carbonate ramp in the Western Tarim basin (NW China). J. Asian Earth Sci. 158, 29–48 (2018).
Shi, P. et al. Carbonate diagenesis in fourth-order sequences: A case study of Yingshan formation (Lower Ordovician) from the Yubei area-Tarim basin, NW China. J. Petrol. Sci. Eng. 195, 107756 (2020).
He, D., Zhou, X., Zhang, C. & Yang, X. Tectonic types and evolution of ordovician proto-type basins in the Tarim region. Chin. Sci. Bull. 52, 164–177 (2007).
Jia, C. & Zhang, S. The formation of marine ultra-deep petroleum in China. Acta Geol. Sinica. 97, 2775–2801. https://doi.org/10.19762/j.cnki.dizhixuebao.2023201 (2023).
Shen, B., Jacobsen, B., Lee, C. T. A., Yin, Q. Z. & Morton, D. M. The Mg isotopic systematics of granitoids in continental arcs and implications for the role of chemical weathering in crust formation. Proc. Natl. Acad. Sci. 106, 20652–20657 (2009).
Galy, A. et al. Magnesium isotope heterogeneity of the isotopic standard SRM980 and new reference materials for magnesium-isotope-ratio measurements. J. Anal. At. Spectrom. 18, 1352–1356 (2003).
Zhao, W. et al. The porosity origin of dolostone reservoirs in the Tarim, Sichuan and Ordos basins and its implication to reservoir prediction. Sci. China Earth Sci. 57, 2498–2511 (2014).
Zheng, J., Liu, Y., Zhu, Y. & Liang, F. Geochemical features and its geological significances of the upper Sinian qigeblak formation in Wush area, Tarim basin. J. Palaeogeogr. 23, 983–998 (2021).
Baker, P. A. & Kastner, M. Constraints on the formation of sedimentary dolomite. Science 213, 214–216 (1981).
Yuan, Y., Shi, X., Tang, D., Shi, Q. & Li, Y. Microfabrics and organominerals as indicator of microbial dolomite in deep time: An example from the mesoproterozoic of North China. Precambrian Res. 382, 106881 (2022).
Whiticar, M. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 161, 291–314 (1999).
Roberts, J. A., Bennett, P. C., González, L. A., Macpherson, G. & Milliken, K. L. Microbial precipitation of dolomite in methanogenic groundwater. Geology 32, 277–280 (2004).
Moore, T. S., Murray, R., Kurtz, A. & Schrag, D. Anaerobic methane oxidation and the formation of dolomite. Earth Planet. Sci. Lett. 229, 141–154 (2004).
Gregg, J. M. & Sibley, D. F. Epigenetic dolomitization and the origin of xenotopic dolomite texture. J. Sediment. Res. 54, 908–931 (1984).
Shen, A., Luo, X., Hu, A., Qiao, Z. & Zhang, J. Dolomitization evolution and its effects on hydrocarbon reservoir formation from penecontemporaneous to deep burial environment. Pet. Explor. Dev. 49, 731–743 (2022).
Veizer, J. et al. Sr/86Sr, δ13C and δ18O evolution of phanerozoic seawater. Chem. Geol. 161. 87, 59–88 (1999).
Qing, H. et al. δ26Mg-δ13C-δ18O systems as geochemical tracers for dolomite recrystallization: A case study of lower ordovician dolomite from Tarim basin. Chem. Geol. 619, 121302 (2023).
Zenger, D. H. Burial dolomitization in the lost Burro formation (Devonian), east-central California, and the significance of late diagenetic dolomitization. Geology 11, 519–522 (1983).
Lee, Y. I. & Friedman, G. M. Deep-burial dolomitization in the ordovician ellenburger group carbonates, West Texas and southeastern new Mexico. J. Sediment. Res. 57, 544–557 (1987).
Jiang, L. et al. Multiphase dolomitization of deeply buried cambrian petroleum reservoirs, Tarim basin, north-west China. Sedimentology 63, 2130–2157 (2016).
Zhu, D., Jin, Z. & Hu, W. Hydrothermal recrystallization of the lower ordovician dolomite and its significance to reservoir in Northern Tarim basin. Sci. China Earth Sci. 53, 368–381 (2010).
Prezbindowski, D. R. & Larese, R. E. Experimental stretching of fluid inclusions in calcite—implications for diagenetic studies. Geology 15, 333–336 (1987).
Shelton, K. L., Bauer, R. M. & Gregg, J. M. Fluid-inclusion studies of regionally extensive epigenetic dolomites, Bonneterre dolomite (Cambrian), Southeast Missouri: Evidence of multiple fluids during dolomitization and lead-zinc mineralization. Geol. Soc. Am. Bull. 104, 675–683 (1992).
Goldstein, R. H. Fluid inclusions in sedimentary and diagenetic systems. Lithos 55, 159–193 (2001).
Bi, D. J. et al. Constraints of fluid inclusions and C, O isotopic compositions on the origin of the dolomites in the Xisha Islands, South China sea. Chem. Geol. 493, 504–517 (2018).
Dennis, K. J. & Schrag, D. P. Clumped isotope thermometry of carbonatites as an indicator of diagenetic alteration. Geochim. Cosmochim. Acta. 74, 4110–4122 (2010).
Ferry, J. M., Passey, B. H., Vasconcelos, C. & Eiler, J. M. Formation of dolomite at 40–80 C in the latemar carbonate buildup, dolomites, Italy, from clumped isotope thermometry. Geology 39, 571–574 (2011).
MacDonald, J., John, C. & Girard, J. P. Dolomitization processes in hydrocarbon reservoirs: Insight from geothermometry using clumped isotopes. Proced. Earth Planet. Sci. 13, 265–268 (2015).
Lloyd, M. K., Ryb, U. & Eiler, J. M. Experimental calibration of clumped isotope reordering in dolomite. Geochim. Cosmochim. Acta. 242, 1–20 (2018).
Elisha, B., Nuriel, P., Kylander-Clark, A. & Weinberger, R. Towards in situ U–Pb dating of dolomite. Geochronology 3, 337–349 (2021).
Su, A. et al. In situ U-Pb dating and geochemical characterization of multi-stage dolomite cementation in the Ediacaran Dengying formation, central Sichuan basin, China: Constraints on diagenetic, hydrothermal and paleo-oil filling events. Precambrian Res. 368, 106481 (2022).
Jiang, L. et al. U–Pb geochronology and clumped isotope thermometry study of neoproterozoic dolomites from China. Sedimentology 69, 2925–2945 (2022).
Qiao, Z. et al. Laser ablated U-Pb dating-based determination of burial dolomitization process: A case study of lower ordovician Penglaiba formation of Yonganba outcrop in Tarim basin. Acta Petrol. Sinica. 36, 3493–3509 (2020).
Liu, H. et al. Application of U-Pb dating technique in the study of hydrothermal activities of dolomite reservoir: A case study on 3rd member of Yingshan formation in Gucheng area, Tarim basin, NW China. Acta Petrologica Sinica. 38, 765–776 (2022).
Cao, Y. et al. Petroleum geological conditions and exploration potential of lower paleozoic carbonate rocks in Gucheng area, Tarim basin, China. Pet. Explor. Dev. 46, 1099–1114 (2019).
Guo, R. et al. Hydrothermal dolomite reservoirs in a fault system and the factors controlling reservoir formation-A case study of lower paleozoic carbonate reservoirs in the Gucheng area, Tarim basin. Mar. Pet. Geol. 120, 104506 (2020).
Shalev, N., Bontognali, T. R. & Vance, D. Sabkha dolomite as an archive for the magnesium isotope composition of seawater. Geology 49, 253–257 (2021).
Zhang, P., Huang, K. J., Luo, M., Cai, Y. & Bao, Z. Constraining the terminal Ediacaran seawater chemistry by Mg isotopes in dolostones from the Yangtze platform, South China. Precambrian Res. 377, 106700 (2022).
Tipper, E. et al. The magnesium isotope budget of the modern Ocean: Constraints from riverine magnesium isotope ratios. Earth Planet. Sci. Lett. 250, 241–253 (2006).
Hu, Z. et al. Resetting of Mg isotopes between calcite and dolomite during burial metamorphism: Outlook of Mg isotopes as geothermometer and seawater proxy. Geochim. Cosmochim. Acta. 208, 24–40 (2017).
Manche, C. J. & Kaczmarek, S. E. Evaluating reflux dolomitization using a novel high-resolution record of dolomite stoichiometry: A case study from the cretaceous of central Texas, USA. Geology 47, 586–590 (2019).
Higgins, J. A. et al. Mineralogy, early marine diagenesis, and the chemistry of shallow-water carbonate sediments. Geochim. Cosmochim. Acta. 220, 512–534 (2018).
Jacobson, A. D., Zhang, Z., Lundstrom, C. & Huang, F. Behavior of Mg isotopes during dedolomitization in the Madison aquifer, South Dakota. Earth Planet. Sci. Lett. 297, 446–452 (2010).
Geske, A. et al. Impact of diagenesis and low grade metamorphosis on isotope (δ26Mg, δ13C, δ18O and 87Sr/86Sr) and elemental (Ca, Mg, Mn, Fe and Sr) signatures of triassic Sabkha dolomites. Chem. Geol. 332, 45–64 (2012).
Li, W. et al. Effects of early diagenesis on Mg isotopes in dolomite: The roles of Mn(IV)-reduction and recrystallization. Geochim. Cosmochim. Acta. 250, 1–17. https://doi.org/10.1016/j.gca.2019.01.029 (2019).
Qiao, Z. et al. Magnesium isotope-based forming process of large sized burial dolomite: A case study of the Penglaiba formation in Tarin basin. Acta Geol. Sinica. 97, 2293–2310. https://doi.org/10.19762/j.cnki.dizhixuebao.2023239 (2023).
Biehl, B. C., Reuning, L., Schoenherr, J., Lüders, V. & Kukla, P. A. Impacts of hydrothermal dolomitization and thermochemical sulfate reduction on secondary porosity creation in deeply buried carbonates: A case study from the lower Saxony basin, Northwest Germany. AAPG Bull. 100, 597–621 (2016).
Guo, B. et al. Mg isotopic systematics and geochemical applications: A critical review. J. Asian Earth Sci. 176, 368–385 (2019).
Finch, A. & Allison, N. Coordination of Sr and Mg in calcite and Aragonite. Mineral. Mag. 71, 539–552 (2007).
Wang, Z. et al. Experimental calibration of Mg isotope fractionation between Aragonite and seawater. Geochim. Cosmochim. Acta. 102, 113–123 (2013).
Teng, F. Z. Magnesium isotope geochemistry. Rev. Mineral. Geochem. 82, 219–287 (2017).
Liu, J., Tian, S. & Wang, L. Application of magnesium stable isotopes for studying important geological processes—A review. Earth Sci. Front. 30, 399–424 (2023).
Saenger, C. & Wang, Z. Magnesium isotope fractionation in biogenic and abiogenic carbonates: Implications for paleoenvironmental proxies. Q. Sci. Rev. 90, 1–21 (2014).
Mavromatis, V., Gautier, Q., Bosc, O. & Schott, J. Kinetics of Mg partition and Mg stable isotope fractionation during its incorporation in calcite. Geochim. Cosmochim. Acta. 114, 188–203 (2013).
Peng, Y. et al. Constraining dolomitization by Mg isotopes: A case study from partially dolomitized limestones of the middle Cambrian Xuzhuang Formation, North China. Geochem. Geophys. Geosyst. 17, 1109–1129 (2016).
Acknowledgements
This project is funded by the Scientific Research and Technology Development Project of PetroChina Company Limited (Grant No.:2021DJ05). We acknowledge this generous funding from the China National Petroleum Corporation. This work benefited from valuable core samples provided by the Tarim Basin Exploration and Development Research Institute.
Author information
Authors and Affiliations
Contributions
Xi Li and Guangyou Zhu designed the research ideas experimental methods, discussed the results and implications, and commented on the manuscript at all stages. Yifei Ai, Zhiyao Zhang and Weiyan Chen performed the experiments. Tingting Li, Yan Zhang, and Pengzhen Duan compiled geological maps.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, X., Zhu, G., Ai, Y. et al. Using Mg isotopes to constrain the formation temperature of dolomite. Sci Rep 15, 10910 (2025). https://doi.org/10.1038/s41598-025-95540-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-95540-7