Abstract
Solanum lycopersicum L. has long been used to promote diuresis in Ethiopia. Although strong ethnobotanical evidence is available, its diuretic activity has not been scientifically proven. The aim of this study was to provide scientific evidence supporting the traditional use of S. lycopersicum L. leaves as a diuretic. The leaves of S. lycopersicum L. were extracted using a maceration technique with 80% methanol. The 80% hydro-methanol extract and solvent fractions (ethyl acetate, n-hexane, and aqueous) were administered orally to the mice at doses of 100, 200, and 400 mg/kg. The effects of these extracts and solvent fractions were compared over 5 h’s period with a negative control and a standard medication (furosemide, 10 mg/kg). The extract at a dosage of 2000 mg/kg was shown to be safe in the acute oral toxicity test. The 400 mg/kg hydro-methanol extract of S. lycopersicum L. leaves showed a significant (p < 0.001) increase in both urine volume and sodium excretion compared to the negative control. The 400 mg/kg aqueous solvent fraction and the standard drug (furosemide 10 mg/kg) showed significant (p < 0.001) increases in both urine volume and sodium excretion compared to the negative control. The 400 mg hydro-methanol extract and 400 mg aqueous solvent fraction of S. lycopersicum L. leaves showed urine and salt excretion profiles comparable to those of the standard drug. The results of this study reveal that 80% hydro-methanol extract and the solvent fraction of the leaves of S. lycopersicum L. demonstrate a diuretic effect and validate traditional assertions.
Similar content being viewed by others
Introduction
Diuretics are medications that help the body to remove excess water and salt by increasing their excretion through urine1. They are among the most commonly prescribed drugs and are used to manage conditions such as high blood pressure, heart failure, ascites, kidney failure, diabetic insipidus, etc.2. They raise osmolality and cause a negative fluid balance by preventing electrolyte reabsorption from the nephron lumen. Diuretics are classified into five main categories: loop diuretics, thiazides, potassium-sparing diuretics, osmotic diuretics, and carbonic anhydrase inhibitors3. Furosemide and other loop diuretics work by inhibiting the Na+/K+/2Cl− co-transporter in the thick ascending part of the loop of Henle, while thiazide diuretics act on the Na+/Cl− co-transporter located in the distal convoluted tubule. Spironolactone and other potassium-sparing diuretics work by counteracting the effects of aldosterone or by blocking sodium channels in the epithelial cells of the collecting ducts. Various classes of diuretics have a unique impact on fluid homeostasis and electrolyte balance, which influences the choice of treatment depending on the clinical needs of the patient4.
However, diuretic use has been associated with several side effects and resistance in some patients. For example, electrolyte imbalance, hypotension, syncope, dizziness, headache, and urinary frequency are some of the most frequent side effects5. While more recent medications such as vaptans and Vasopressin receptor antagonists have shown promise in large-scale clinical trials, they do not significantly impact long-term morbidity and death6. A global cohort study found that approximately 21% of heart failure patients experience resistance to diuretic treatment7. The prevalence of diuretic resistance has become a subject of increasing concern within the medical community because of its potential impact on patient outcomes, healthcare costs, and the overall management of cardiovascular and renal diseases. Thus, it is necessary to search for new diuretics with a novel mechanism of action, improved effectiveness, ease of accessibility and cost, and manageable side effect profiles.
It has long been a customary practice to treat human illnesses using medicinal plants. In developing countries, most people depend on traditional medicine. Herbal medicines are primarily used to manage various medical conditions8. Because several plants have diuretic effects, they have become an area of application for botanical studies. Several published reports support the level of clinical evidence in favor of using folklore medicines as diuretics9,10.
The plant Solanum lycopersicum L. (tomato) was confirmed using the World Flora Online database (www.worldfloraonline.org). This plant is a Solanaceae family, the genus includes more than 3000 species of plants grown worldwide under outdoor and indoor conditions11. In Ethiopia, the plant is commonly known as “Timatim” in Amharic. The plant has 4–24 inches of leaves with soft and hairy stems and can grow up to 9 feet tall. The flowers are yellow, small, and have five pointed lobes in the corolla12.
Based on insights gathered from Ethiopian communities and backed by an ethnobotanical report, Solanum lycopersicum L. leaves were used to promote diuresis. The leaves were crushed, filtered, and orally administered to manage urinary retention, body swelling, and urinary problems13. In addition, another study revealed Solanum lycopersicum L. has diuretic14, antihypertensive15,16, malaria17, excessive bleeding after birth, anthrax18, ulcers, and eye disease19 effects. Previous reports have indicated that Solanum lycopersicum L. exhibits in vitro antibacterial20, antioxidant21, and antiproliferative22 activity. Studies in animals have reported that Solanum lycopersicum L. has antihypertensive15, Antiplatelet23, and antiviral24 activity. Although there is strong ethnobotanical evidence, little research is available on the ability of Solanum lycopersicum L. to diuresis. Therefore, the purpose of this study was to provide scientific evidence to support the traditional use of this plant as a diuretic.
Method
Drugs, chemicals, and instrument
The following medications and chemicals equipment were used in this study: furosemide 40 mg tablet (Salutas Pharma, Germany), distilled water (University of Gondar Medical Laboratories, Ethiopia), ethyl acetate (Blulux Laboratories, India), n-hexane (Blulux Laboratories, India), and tween 80 (Uni-chem, India). Every chemical that was used was analytical grade.
Equipment and supplies used in this study include: Metabolic cages, urine collectors, digital weighing balance, oral gavage, lyophilizer, deep freezer, digital pH meter (HANNA Instruments, Italy), rotary evaporator (Yamato, Japan), vacuum pump (AB-288-I, India), separatory funnel, beaker, water bath (Yamato, Japan), Whatman filter paper (No.1) (Maid Stone), examination gloves (Malaysia), and gauze were used.
Plant material
Fresh leaves of Solanum lycopersicum L. were collected during the rainy spring season of May 2023 from Kebele 18, St. George Church in Gondar Town, Amhara Regional State, Ethiopia. Mr. Getenet Chekole, Assistant Professor of Botanical Science at the Department of Biology, College of Natural and Computational Sciences, University of Gondar, recognized and verified the plant material. The specimens were placed in the University of Gondar Herbarium and given voucher number 02/AT/2023.
Extraction and fractionation of Solanum lycopersicum L. leaves
The leaves of Solanum lycopersicum L. were thoroughly washed with tap water to remove dirt and debris. Afterward, the leaves were manually cut into small pieces and dried in the shade. Once dried, the leaves were ground into a fine powder using a mortar and pestle. The powdered material was then stored in airtight containers to maintain its quality.
The extraction and fractionation procedures followed in this study were based on the methodology outlined in our previous work (Beyna AT, 2024)25. Eight hundred grams powdered of Solanum lycopersicum L. leaves were macerated in an 80% V/V methanol solution at room temperature under a round-bottom flask and periodically agitated for 72 continuous hours to exhaustively extract bioactive compounds. Then it was filtered out first through gauze to clear the bigger particles and then through Whatman No. 1 filter paper to get an even clear solution of the extract. The residue was further subjected to two more macerations using the same procedure and merging the filtrates to ensure maximum yield. The combined filtrates of all filtration steps were evaporated in a rotary evaporator at 40 °C to concentrate the extract. The concentrates obtained from the extraction were frozen overnight in a deep freezer to assist the succeeding processes. The solution was dried in a freeze dryer to a fine powder, which was weighed and packed in an airtight container till further use.
For the fractionation process, a 60-g extract was suspended in distilled water at a ratio of 1:6 using a separator funnel. To this aqueous suspension, an equal volume of n-hexane was added. The mixture was thoroughly mixed and allowed to separate into two distinct layers.
The n-hexane layer was carefully separated and collected into a separate flask. This process was repeated twice, each time with fresh n-hexane. After collecting the n-hexane fractions, equal volumes of ethyl acetate were added to the remaining aqueous fraction. The mixture was allowed to separate into distinct layers once again. The ethyl acetate fraction was collected, and the remaining aqueous layer was set aside for further processing. This procedure was repeated twice. Finally, the aqueous fraction was concentrated in an oven at 40 °C before being lyophilized, while the n-hexane and ethyl acetate fractions were evaporated using a rotary evaporator. All the resulting fractions were stored in a refrigerator for further analysis.
Experimental animals
Aged 6–8 weeks and weighing 25–30 g, healthy Swiss albino mice of both sexes were acquired from the animal house facilities of the University of Gondar’s Department of Pharmacology. These mice were employed in main investigations as well as acute toxicity testing. The animals were kept in normal housing polypropylene cages and given a week’s acclimatization before the studies began. The National Institutes of Health’s Guidelines for the Care and Use of Laboratory Animals were followed in all aspects of handling and caring for the animals during the study26.
Grouping and dosing of animal
The diuretic activity of the 80% methanol extract of Solanum lycopersicum L. leaves was assessed by using a total of thirty mice which were organized into five distinct groups, each group consisting of six mice. Group I was allocated as the negative control group, while groups II, III, and IV were assigned as treatment groups which receiving doses of 100, 200, and 400 mg/kg, respectively. Group V was allocated as the positive control group which received doses of 10 mg/kg furosemide.
To evaluate the efficacy of the various solvent fractions, an additional experiment was conducted involving eleven groups, each group containing six mice. Allocate as, Group I assigned as the negative control group. Groups II, III, and IV were treated with the aqueous fraction which received doses of 100, 200, and 400 mg/kg aqueous fraction extract, respectively. While groups V, VI, and VII received the ethyl acetate fraction which received doses of 100, 200, and 400 mg/kg ethyl acetate fraction extract, respectively. Groups VIII, IX, and X were assigned to the n-hexane fraction treatment which received doses of 100, 200, and 400 mg/kg n-hexane fraction extract, respectively. Finally, Group XI was assigned as the positive control group which received10 mg/kg furosemide.
Before the experiment started, every mouse was kept in a metabolic cage for three days straight, for a total of 3 h every day. The purpose of this acclimatization phase was to minimize stress and variability in the experiment outcomes by ensuring that the mice were comfortable and acclimated to their surroundings27.
Acute oral toxicity study
This study followed the limit test outlined in the Organization for Economic Cooperation and Development Guideline No. 42528. Five non-pregnant female mice, weighing 25–30 g and aged 6–8 weeks, were used for acute oral toxicity study. All of the mice were allowed full access to water and were fasted for 4 h before and 2 h following the administration of the extract. The dosage was calculated based on the body weight of the fasted mice. At first, one mouse received a single dosage of the extract (2000 mg/kg). Because the mice survived after a day, the single dose (2000 mg/kg) was repeated for four more mice. After extract administration, mice were extensively examined for short-term toxicity effects for up to 24 h and mortality profiles for up to 14 days.
Diuretic activities
The assessment of diuretic action procedures followed in this study was based on previous published work of Hailu, W. and E. Engidawork29. All the procedure of the study is followed by the ARRIVE guideline. To evaluate the diuretic activity of 80% hydro-methanol extract and solvent fraction Solanum lycopersicum L., each mouse was individually housed in a metabolic cage for 24 h to allow for adaptation (Fig. 1). The mice were pretreated orally with physiological saline (0.9% NaCl2) at a dose of 0.15 ml per 10 g of body weight to maintain a constant water and salt load, and they were given unrestricted access to water during their overnight fast30. Subsequently, based on the specified grouping and dosage, treatment was administered orally to each animal via gavage.
The mice were immediately placed into individual metabolic cages designed to separate urine from feces. Urine was collected and measured at the first, second, third, fourth, and fifth hours following the dose and was stored at − 20 °C29. During the urine collection process, the animals were deprived of both water and food. For each mouse, the total urine volume, concentrations of Na+, K+, and Cl−, and urine pH were measured. The parameters of urine excretion, diuretic action, and diuretic activity were evaluated to compare the effects of the extracts with those of the vehicle and standard treatments. The percentage of urine excretion (1), diuretic action (2), and diuretic activity (3) were calculated independently of the animal’s weight.
Urine electrolyte analysis
The concentrations of Na+, K+, and C− in urine were determined for all groups using an ion-selective electrode analyzer (Beckman Coulter DxC 700 AU, USA). The electrolyte concentration in the urine was quantified in mmol/L. To evaluate the natriuretic index and carbonic anhydrase inhibition, Na+/K+ and C−/(K+ + Na+) ratios were calculated based on the electrolyte levels. The pH of urine samples was measured using a digital pH meter. To eliminate any potential influence on urinary electrolyte concentration, the salt levels of both the hydro-methanol extract and fractions were measured using the same instrumentation.
Post-experiment euthanasia method
After the completion of the experimental procedures all animals used in this experiment were humanely euthanized in accordance with established ethical guidelines. To euthanize mice humanly we used a high dose of anesthesia to ensure a painless method. We were administered ketamine at a dose of 200 mg/kg via intraperitoneal injection, which effectively induced mice in a deep-state anesthesia. After we confirmed the absence of pain reflexes, the cervical dislocation method was performed as a final step to ensure euthanasia was conducted humanely and effectively. This procedure adhered strictly to the guidelines set forth by institutional and ethical standards, which are designed to promote the humane treatment of laboratory animals throughout all stages of research31.
Phytochemical screening test
The presence of secondary metabolites, such as alkaloids, phenols, anthraquinones, glycosides, saponins, phytosterols, tannins, triterpenoids, and flavonoids, in the hydro-methanol extract and solvent fraction of Solanum lycopersicum L. leaves was qualitatively evaluated following the method of Shaikh and Patil32.
Data analysis
Data analyses were conducted using SPSS version 24. Statistical significance for each dataset was assessed using a one-way analysis of variance, followed by Tukey’s multiple comparison tests. The study findings are described using the mean ± standard error of the mean. A significance level of p < 0.05 was applied to ascertain whether mean differences were statistically significant.
Ethical clearance
The experimental animals were handled and cared for according to the internationally accepted laboratory animal use, care, and welfare guidelines. Ethical approval was obtained from the Bioethics Committee of the Department of Pharmacology, University of Gondar (reference number Sop7/08/09/2015).
Results
From the total of eight hundred g of coarse leaf powder of Solanum lycopersicum L., 128 g (16%) hydro-methanol extract was obtained. From the total of 60 g hydro-methanol extract, 33.6 g (56%), 13.2 g (22%), and 10.8 g (18%) distilled water, n-hexane, and ethyl acetate fractions were produced, respectively.
Phytochemical screening
The hydro-methanol extract and solvent fractions of Solanum lycopersicum L. were analyzed for medicinally active compounds and tested positive for alkaloids, phenols, saponins, triterpenoids, flavonoids, glycosides, and anthraquinones in the hydro-methanol extract (Table 1).
Acute oral toxicity
Female mice underwent acute oral toxicity assessment according to the guidelines outlined in Organization for Economic Cooperation and Development Guideline No. 425. The study revealed that administering a maximum dose of 2000 mg/kg of Solanum lycopersicum L. hydro-methanol extract did not result in any fatalities within the initial 24-h period nor the subsequent 14 days. Additionally, thorough physical and behavioral assessments of the experimental subjects showed no discernible signs of acute toxicity. Consequently, the lethal dose 50 value of the extract exceeded 2000 mg/kg.
Urinary output
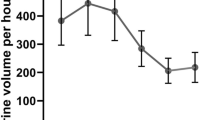
The effects of the methanol extract of Solanum lycopersicum L. leaves on urinary output are shown in Table 2. The methanol extracts elicited diuretic effects that seemed to correlate with dosage and duration. The lower dose (100 hydro-methanol extract) did not produce any significant difference in urine volume in the first hour, but it produced a significant increase in urine volume after the second hour (p < 0.01) compared with the negative control. The medial (200 hydro-methanol extract) and larger (400 hydro-methanol extract) doses of the methanol extract of Solanum lycopersicum L. leaves showed a significant (p < 0.001) increase in urine volume from the first hour of treatment to the end of the fifth hour of urine collection period compared to the negative control.
The effect of the solvent fraction of Solanum lycopersicum L. leaves on the urinary output is shown in Table 3. Ethyl acetate 100 mg/kg and n-hexane 100 mg/kg fractions did not show any significant difference in urine volume within the first 3 h of dosing, except n-hexane 100 mg/kg fraction, which produced a significant urine volume difference in the second hour (p < 0.05) compared to the negative control. Ethyl acetate 400 mg/kg, n-hexane 400 mg/kg, and aqueous fraction 400 mg/kg showed significant differences in urine volume (p < 0.001) from the third hour of treatment to the end of the fifth hour urine collection period compared to the negative control. In contrast, ethyl acetate 100 mg/kg, n-hexane 100 mg/kg, and aqueous fraction 100 mg/kg did not show any significant difference in urine volume during the first hour of treatment compared with the negative control. Intergroup analysis revealed that the ethyl acetate 400 mg/kg fraction showed a significant difference in urine volume (p < 0.001) in the fourth and fifth hours of treatment compared to the ethyl acetate 100 mg/kg fraction. The aqueous fraction of 400 mg/kg and the standard drug (furosemide 10 mg/kg) showed significant differences in urine volume (p < 0.001) from the first hour of treatment to the end of the fifth-hour urine collection period compared to all doses of ethyl acetate, n-hexane fraction, and negative control.
Urinary electrolyte excretion
The electrolyte contents (Na+, K+, and Cl−) of urine samples collected over 5 h were examined and are shown in Table 4. Maximum significant sodium excretion was observed in mice treated with the 400 mg/kg hydro-methanol extract and furosemide 10 mg/kg. The increase sodium excretion produced by 400 mg/kg hydro-methanol extract and furosemide 10 mg/kg was 47.3% and 54.8%, respectively (p < 0.001), compared to negative control. Increased potassium excretion was also observed at all doses of 100, 200, and 400 mg/kg hydro-methanol extract (23.1%, 50.3%, and 89.2%, p < 0.001), respectively, compared to negative control. Similarly, chloride excretion was also increased at all doses of 100 (11.4%), 200 (29.1%), and 400 (40.1%) mg/kg hydro-methanol extract as compared to negative control (Table 4).
The n-hexane 200 (p < 0.01) and 400 mg/kg (p < 0.001) fractions significantly increased sodium excretion compared with the negative control. In addition, n-hexane 400 mg/kg fraction significantly increased sodium excretion compared with n-hexane 100 mg/kg (p < 0.001) and n-hexane 200 mg/kg (p < 0.05). The increase in sodium excretion produced by ethyl acetate 200 and 400 mg/kg was 21.8% and 31.4%, respectively, p < 0.001), compared to negative control. Both 200 and 400 mg/kg aqueous fraction significantly increase sodium excretion by 29.1% and 49.8%, respectively (p < 0.001), as compared with negative control. All of the highest doses of n-hexane, ethyl acetate, and aqueous fractions significantly (p < 0.001) increased potassium excretion by 41.2%, 52.4%, and 79.9%, respectively, as compared with negative control. All treatment groups and the standard medication significantly (p < 0.001) increased chloride excretion (p < 0.001) compared to the negative control, except for n-hexane 100 mg/kg (Table 5).
Urinary pH
Urine pH analysis showed that the various treatments had distinct effects. Both the 80% hydro-methanol extract and the solvent fractions produced relatively alkaline urine. The pH of urine treated with the extract had shown an increase from 100 mg hydro-methanol extract (7.49) to 400 mg hydro-methanol extract (7.83). The negative control group had the lowest pH (7.32), and the positive control group had an intermediate pH (7.71) between the negative control and extract-treated groups (Fig. 2). The pH of urine treated with n-hexane fraction had shown an increase from 100 mg/kg n-hexane fraction (7.21) to 400 mg/kg n-hexane fraction (7.5). The pH of urine treated with ethyl acetate fraction had shown an increase from 100 mg/kg ethyl acetate fraction (7.3) to 400 mg/kg ethyl acetate fraction (7.53). The highest urine pH was produced by a higher dose of aqueous solvent fraction (7.83) (Fig. 3).
Urinary pH of mice treated with solvent fraction of the leaves of Solanum lycopersicum L. Notes: NHF100, n-hexane fraction 100 mg/kg; NHF200, n-hexane fraction 200 mg/kg; NHF400, n-hexane fraction 400 mg/kg; EAF100, ethyl acetate fraction 100 mg/kg; EAF200, ethyl acetate fraction 200 mg/kg,EAF400, ethyl acetate fraction 400 mg/kg; AQF 100 mg, Aqueous fraction100mg/kg; AQF 200 mg, aqueous fraction 200 mg/kg; AQF 400 mg, aqueous fraction 400 mg/kg; F10: Furosemide 10 mg/kg; 2%TW80: 2% Tween 80.
Discussion
According to a previous ethnobotanical survey conducted in Ethiopia, the leaves of S Solanum lycopersicum L. are used to manage body swelling, urinary retention, and urinary problems (as a diuretic agent). However, there is no scientific evidence to support its diuretic activity. Taking these facts into consideration, the current study describes the diuretic and natriuretic characteristics of the Solanum lycopersicum L. leaf extract and solvent fractions. In this study, mice were used to assess the diuretic effect of Solanum lycopersicum L. leaves by measuring urine volume, electrolytes, and pH.
The mice were treated along with three test doses of 100 mg/kg, 200 mg/kg, and 400 mg/kg of 80% hydro-methanol extract. Especially at the maximum test doses, there was a notable increase in urine output, starting from the 1st hr and continuing till the 5th hr compared to the negative control. This might be due to the presence of a higher percentage of the active component diuretics in the Solanum lycopersicum L. 80% hydro-methanol extract. Among the solvent fractions, higher doses of the aqueous fraction Solanum lycopersicum L. revealed a notable increase in urine output compared to the negative, n-hexane, and ethyl acetate fractions. The results of this study could be attributed to the fact that the majority of bioactive elements in plants that have diuretic action are water-soluble. To extract more polar chemicals, including alkaloids, flavonoids, and glycosides, polar solvents such as water are favorable33. In contrast, there was no appreciable difference in urine output between the groups treated with the ethyl acetate and n-hexane fractions. This could be because the aqueous fraction contained the majority of the bioactive substances with diuretic potential.
The diuretic potential was classified as "nil," "mild," "moderate," and “good” based on the estimated diuretic index if the computed values were < 0.72, 0.72–0.99, 1.00–1.5, and > 1.5, respectively34. 200 hydro-methanol extract (0.76) and 400 hydro-methanol extract (0.99) showed mild diuretic activities. By contrast, 100 hydro-methanol extract (0.69) showed no diuretic activity. Thus, the diuretic effect of Solanum lycopersicum L. appears to be dose-dependent. This is in accordance with a previous study on ajuga remota benth, which showed dose-dependent diuretic activity29. Among the solvent fractions, only 200 mg/kg aqueous fraction (0.79) and 400 mg/kg aqueous fraction (0.95) showed mild diuretic effects. None of the other solvent fractions showed a diuretic effect at any dose. This suggested that the polar extract may contain a higher concentration of active ingredients that cause diuretic activity. This is in line with earlier research highlighting the role of highly polar components in diuretic activity, as observed in Withania aristata Ait35, Moringa stenopetala 36, Withania somnifera L37. In contrast, other studies on plants such as Avicennia officinalis.L.38, Clerodendrum myricoides Hochst39, and Rumex abyssinicus Jacq40, emphasized that less polar substances are responsible for diuretic activity.
Medicinal plants have been shown to reduce volume overload caused by electrolytes and water retention10. Hence, it is imperative to demonstrate the effectiveness of plant extracts in the presence of water and electrolytes41.
The electrolyte content and ion excretion patterns of the solvent fractions of the Solanum lycopersicum L. leaf extract were slightly different. In contrast to the negative control, ethyl acetate, and n-hexane fractions, a higher dose of the aqueous solvent fraction markedly increased the output of urine electrolytes. This might be the reason for the better diuretic activity of the aqueous solvent fraction compared to the ethyl acetate and n-hexane fractions.
The Na+/K+ ratio was used to evaluate natriuretic activity. Ratios > 1 suggest a satisfactory natriuretic effect, values > 2 indicate a favorable natriuretic effect and values > 10 signify potassium-sparing action42,43. A higher Na+: K+ ratio indicates greater excretion of Na+ than K+, which is considered an excellent profile for diuretic medicines. Satisfactory natriuretic activity was observed at all the extract doses. However, none of these compounds exhibited potassium-sparing activity. These results align with those of earlier studies on the diuretic action of thymus serrulatus44, and Erica arborea L45, indicating an acceptable natriuretic index and no K+ sparing activity.
The carbonic anhydrase inhibitor effect of diuretics can be predicted using the Cl−/(Na+ + K+) ratio of the test substance44. The carbonic anhydrase inhibitor impact is excluded if the Cl−/(Na+ + K+) ratio is between 0.8 and 1.00; if it is less than 0.8, there is thought to be significant carbonic anhydrase inhibitor activity46. In our study, the carbonic anhydrase inhibitor index of the extracts decreased with higher dosages. This suggests that the primary mechanism of action for the extracts is likely the inhibition of carbonic anhydrase inhibitor enzymes. The significant increase in urine pH compared to the controls supports this hypothesis, indicating that carbonic anhydrase inhibition is a key mechanism of the plant’s diuretic effect. This finding aligns with previous research on the diuretic effects of Moringa stenopetala47.
K+, Na+, and Cl− excretion patterns in urine and various bioactive components found in the extracts. The plant seems to function in a way that is comparable to certain herbal remedies that function through a variety of diuretic modes of action48. Therefore, osmotic effects and an increased glomerular filtration rate might also play a role in the diuretic effect of the plant, alongside the proposed carbonic anhydrase inhibition activity.
Phytochemical analysis revealed the presence of triterpenoids, alkaloids, anthraquinones, glycosides, phenols, flavonoids, and saponins. According to certain claims, triterpenoids interfere with the Na+-K+-2Cl− co-transport carrier in the luminal membrane of the thick ascending loop of Henle, which explains their diuretic properties49. Phenols, flavonoids, and alkaloids have been shown to exhibit carbonic anhydrase inhibitor activity50. This suggests that these compounds might contribute to the simultaneous increase in urine pH and the observed effects related to carbonic anhydrase inhibitor. Additionally, saponins are known to inhibit the furosemide-sensitive Na+-ATPase in the basolateral layer of the proximal convoluted tubule. Consequently, the saponins present in the extract could be responsible for its diuretic effects.
Conclusion
Our study found that the 80% hydro-methanol extract of Solanum lycopersicum L. leaves, along with its different solvent fractions, showed notable diuretic effects. Among these, the aqueous fraction was the most effective, suggesting it has the strongest potential for diuretic activity compared to the other fractions. These results support the traditional use of Solanum lycopersicum L. leaves as a natural remedy for urinary retention. Overall, this study helps us better understand the plant’s therapeutic potential and paves the way for its future use in treating urinary disorders.
Data availability
All relevant data are within the manuscript and its Supporting Information files.
References
Kehrenberg, M. C. & Bachmann, H. S. Diuretics: A contemporary pharmacological classification?. Naunyn Schmiedebergs Arch. Pharmacol. 395(6), 619–627 (2022).
Pandey, M. et al. Clinical pharmacology & therapeutic uses of diuretic agents: A review. J. Res. Appl. Sci. Biotechnol. 1(3), 11–20 (2022).
Friedman-Jakubovics, M. & Fazylov, R. Diuretics. In Side Effects of Drugs Annual 227–236 (Elsevier, 2019).
Chakraverty, R. et al. Mechanism of action of diuretic and anti-diuretic drugs. In How Synthetic Drugs Work 369–390 (Elsevier, 2023).
Zinkovsky, D. Side effects, ADRs & ADEs of diuretics. In Side Effects of Drugs Annual 259–265 (Elsevier, 2021).
Masella, C. et al. Diuretic resistance in cardio-nephrology: role of pharmacokinetics, hypochloremia, and kidney remodeling. Kidney Blood Press. Res. 44(5), 915–927 (2019).
Trullàs, J.-C. et al. Prevalence and outcome of diuretic resistance in heart failure. Intern. Emerg. Med. 14, 529–537 (2019).
Ouoba, K. et al. Prevalence of traditional medicines use and adverse events: A population-based cross-sectional survey in Burkina Faso. Eur. J. Integr. Med. 51, 102129 (2022).
Wright, C. et al. Herbal medicines as diuretics: a review of the scientific evidence. J. Ethnopharmacol. 114(1), 1–31 (2007).
Dutta, K. N. et al. Herbal plants used as diuretics: a comprehensive review. J Pharm Chem Biol Sci 2(1), 27–32 (2014).
Kalloo, G. Genetic Improvement of Tomato Vol. 14 (Springer, Berlin, 2012).
Knapp, S. & Peralta, I. E. The tomato (Solanum lycopersicum L., Solanaceae) and its botanical relatives. In The Tomato Genome, 7–21 (2016).
Birhan, Y. S. et al. Ethnobotanical study of medicinal plants used to treat human diseases in Enarj Enawga district, East Gojjam zone, Amhara region, Ethiopia. SM J. Med. Plant. Stud. 1(1), 1–9 (2017).
Rouhi-Boroujeni, H., et al. Hypolipidemic herbals with diuretic effects: A systematic review. Biol. Sci. (2017).
Fadlilah, S., Sucipto, A. & Judha, M. Cucumber (Cucumis sativus) and tomato (Solanum lycopersicum) juice effective to reduce blood pressure. GSC Biol. Pharmaceut. Sci. 10(1), 001–008 (2020).
Marcolongo, P. et al. Chemical characterisation and antihypertensive effects of locular gel and serum of Lycopersicum esculentum L. var “Camone” tomato in spontaneously hypertensive rats. Molecules 25(16), 3758 (2020).
Chekole, G. Ethnobotanical study of medicinal plants used against human ailments in Gubalafto District, Northern Ethiopia. J. Ethnobiol. Ethnomed. 13(1), 1–29 (2017).
Araya, S., Abera, B. & Giday, M. Study of plants traditionally used in public and animal health management in Seharti Samre District, Southern Tigray, Ethiopia. J. Ethnobiol. Ethnomed. 11, 1–25 (2015).
Yeshiwas, Y., Tadele, E. & Workie, M. Utilization, cultivation practice and economic role of medicinal plants in Debre Markos town, East Gojjam zone, amhara region, Ethiopia. J. Med. Plants Res. 13(1), 18–30 (2019).
sajet AL-Oqaili, R.M. & Salman, B.B.M.M.A. In vitro antibacterial activity of Solanum lycopersicum extract against some pathogenic bacteria. In Vitro 27 (2014).
Sbartai, H. et al. Antioxidative response in tomato plants Lycopersicon esculentum L. roots and leaves to zinc. Am. Eurasian J. Toxicol. Sci. 3(1), 41–46 (2011).
Saturnino, C. et al. Antiproliferative activity of “Lycopersicon esculentum” leaves (Var Paul Robenson): Preliminary study. Food Nutr. Sci. 04(06), 632–635 (2013).
Fuentes, E. et al. Effect of tomato industrial processing on phenolic profile and antiplatelet activity. Molecules 18(9), 11526–11536 (2013).
Spinu, K. et al. Antiviral Activity of Tomatoside from Lycopersicon esculentum Mill. In Saponins Used in Traditional and Modern Medicine 505–509 (Springer, 1996).
Beyna, A. T. et al. Evaluation of wound healing and anti-inflammatory activity of hydro-alcoholic extract and solvent fractions of the leaves of Clerodendrum myricoides (Lamiaceae) in mice. PLoS ONE 19(7), e0306766 (2024).
National Research Council. Guide for the care and use of laboratory animals. 8th ed. Washington (DC): National Academies Press; 2011.
Asif, M. et al. Diuretic activity of aqueous extract of Nigella sativa in albino rats. Acta Pol Pharm 72(1), 129–135 (2015).
Co-operation, O.f.E. and Development, Test No. 425: acute oral toxicity: up-and-down procedure (OECD Publishing, 2008).
Hailu, W. & Engidawork, E. Evaluation of the diuretic activity of the aqueous and 80% methanol extracts of Ajuga remota Benth (Lamiaceae) leaves in mice. BMC Complement. Altern. Med. 14(1), 1–8 (2014).
Prathibhakumari, P. A comparative study on the diuretic activity of neolamarckia cadamba. Int. Res. J. Pharmacy 8(12), 55–61 (2018).
Reilly JS, editor. Euthanasia of animals used for scientific purposes. 2nd ed. Glen Osmond (SA): Australian and New Zealand Council for the Care of Animals in Research and Teaching; 2001.
Shaikh, J. R. & Patil, M. Qualitative tests for preliminary phytochemical screening: An overview. Int. J. Chem. Stud. 8(2), 603–608 (2020).
Chen, H. et al. Study on the polar extracts of Dendrobium nobile, D. officinale, D. loddigesii, and Flickingeria fimbriata: Metabolite identification, content evaluation, and bioactivity assay. Molecules 23(5), 1185 (2018).
Ergena, A., Rajeshwar, Y. & Solomon, G. Synthesis and diuretic activity of substituted 1, 3, 4-thiadiazoles. Scientifica 2022, 3011531 (2022).
Martın-Herreraa, D. et al. Diuretic activity of some Withania aristata Ait. fractions. J. Ethnopharmacol. 117, 496–499 (2008).
Fekadu, N. et al. Diuretic activity of the aqueous crude extract and hot tea infusion of Moringa stenopetala (Baker f.) Cufod leaves in rats. J. Exp. Pharmacol. 9, 73–80 (2017).
Dayib, K., Debella, A. & Abay, S. Diuretic activities of hydro-alcoholic extract and solvent fractions of the roots of Withania somnifera L. (Solanacaea) in rats. Int. J. Complement Alt. Med. 14(3), 131–136 (2021).
Islam, M. N. et al. Identification of potential diuretic and laxative drug candidates from Avicennia officinalis L. bark through in vivo mice model studies and in vitro gas chromatography-mass spectrometry and molecular docking analysis. Evid.-Based Complement. Altern. Med. 2022, 4409250 (2022).
Welu, G. G. et al. In vivo diuretic activity of hydromethanolic extract and solvent fractions of the root bark of clerodendrum myricoides hochst (Lamiaceae). Evid.-Based Complement. Altern. Med. 2020, 1–9 (2020).
Mekonnen, T., Urga, K. & Engidawork, E. Evaluation of the diuretic and analgesic activities of the rhizomes of Rumex abyssinicus Jacq in mice. J. Ethnopharmacol. 127(2), 433–439 (2010).
Nedi, T., Mekonnen, N. & Urga, K. Diuretic effect of the crude extracts of Carissa edulis in rats. J. Ethnopharmacol. 95(1), 57–61 (2004).
Alexander, W. D. et al. The urinary sodium: Potassium ratio and response to diuretics in resistant oedema. Postgrad. Med. J. 53(617), 117–121 (1977).
Shanmuganathan, P. & Kumarappan, M. Evaluation of diuretic, saluretic and natriuretic activity of hydrochlorothiazide in combination with misoprostol in Wistar rats. Natl. J. Physiol. Pharmacy Pharmacol. 8(8), 1226–1229 (2018).
Melka, A. E. et al. Diuretic activity of the aqueous crude extract and solvent fractions of the leaves of Thymus serrulatus in mice. J. Exp. Pharmacol. 8, 61–67 (2016).
Wondimu, N. L., Mengistie, M. G. & Yesuf, J. S. Evaluation of diuretic activity of aqueous and hydro methanolic crude extracts and solvent fraction of the hydromethanolic flower extract of erica Arborea L(Ericaceae) in Swiss Albino Mice. J. Exp. Pharmacol. 16, 175–187 (2024).
Lacorte, L. H. et al. Diuretic activity of kalumpang (Stercula foetida L) methanolic leaf extract in male albino Sprague Dawley rats. Asian J. Biol. Life Sci. 7(2), 33–39 (2018).
Geleta, B. et al. Evaluation of diuretic activity of hydro-ethanolic extract of Moringa stenopetala leaves in Swiss albino mice. Clin. Exp. Pharmacol. 5(190), 2161 (2015).
Jayakody, J. et al. Diuretic activity of leaves extract of hot water infusion of Ruta graveolens L. in rats. J. Pharmacol. Toxicol. 6(5), 525–532 (2011).
Zhang, X. et al. Diuretic activity of compatible triterpene components of Alismatis rhizoma. Molecules 22(9), 1459 (2017).
Imran, M. et al. Carbonic anhydrase and cholinesterase inhibitory activities of isolated flavonoids from Oxalis corniculata L. and their first-principles investigations. Ind. Crops Prod. 148, 112285 (2020).
Acknowledgements
We would like to thank University of Gondar
Funding
The project was not funded.
Author information
Authors and Affiliations
Contributions
Conceptualization: A.T.B, W.S, D. E, H.S.A, W.A, G.S.C, M.L.M. Y. Y.G, A. K. M. Data Curation: A. M.S, G.K.B, L. W. L, A. K.M. Formal analysis: M.M, W.S, G.K.B, A.T.B. Investigation: M.M, W.S, Y.Y.G, A.T. B. Methodology: Y. Y. G, G.K.B, D.E, M.L.M, H.S.A. Project administration: A.K.M, L.W.L. Resources: M.M, W.A, A.T.B. Software: Y.Y.G, G.S.C, M.L. M. Supervision: G.K.B, H.S.A, W.S, W.A. Validation: A.M.S, A.T. B, H.S.A, D.E, A.K.M. Visualization: Y.Y.G, L.W. L. Writing—review & editing: A.T.B, M.M, W. S, G. K. B, Y. Y G, L. W. L, W.A, G.S.C, M.L.M, D. E, A. K. M, H.S.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All experiments were conducted in accordance with the internationally accepted laboratory animal use, care, and guidelines, and the study was approved by the School of Pharmacy Ethics Committee (protocol number Sop7/08/09/2015).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Beyna, A.T., Limenh, L.W., Simegn, W. et al. Diuretic activity of hydro-alcoholic extract and solvent fractions of Solanum lycopersicum L. leaves in mice. Sci Rep 15, 17917 (2025). https://doi.org/10.1038/s41598-025-95628-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-95628-0