Abstract
Techniques to analyze proteins often involves complex workflows and/or sophisticated equipment with modest limits-of-detection. While fluorescence spectroscopy can interrogate single molecules, it often requires fluorescence labeling with lasers and microscopes. We report herein a label-free approach for analyzing intact proteins using resistive pulse sensing (RPS). RPS data were secured using a unique RPS device, which we call a dual in-plane nanopore sensor, fabricated in a thermoplastic. The nanopore sensor was produced via nano-injection molding with critical structures of 30 nm, enabling the detection of individual protein molecules and providing an approach toward their identification. Following nano-injection molding, the pore size could be reduced to ∼ 10 nm using thermal fusion bonding of a cover plate to the molded substrate. The device architecture contained two in-plane nanopores flanking a nanochannel (50 × 50 nm width × depth and 5 µm length) that facilitated the measurement of the apparent electrophoretic mobilities of protein molecules in a label free manner via their molecular-dependent time-of-flight (ToF; time-difference between two consecutive RPS events—peak pair). We investigated four model proteins and collected multiple characteristics including RPS peak amplitude and dwell time, as well as an RPS-independent value, which was the ToF. Furthermore, we analyzed the temporal profiles of RPS events revealing distinct peak shapes for spherical and non-spherical proteins that were influenced by their rotational motion when resident within the nanopore.
Similar content being viewed by others
Introduction
Advancements in measurement science have led to the ability to detect individual molecules1. Single-molecule and single-cell measurements are valuable because they offer more detailed information without the confounding issues associated with ensemble averaging when making bulk measurements. Therefore, single molecule detection is capable of unveiling the diversity within biological systems2. Studying proteins at the single-molecule level is highly intriguing for several reasons: (1) offers insights into complex molecular mechanisms; (2) provides a dynamic perspective on the probabilistic aspects of chemical processes; (3) reveals the diversity within a group of molecules of the same kind; and (4) opens doors for the creation of novel protein-based molecular diagnostics3. In this context, resistive pulse sensing (RPS) is a method available to characterize and quantify unlabeled proteins in their native state at the single-molecule level4.
RPS of single-molecules typically utilizes two electrolyte-filled cavities separated by a nanoscale pore poised within an insulating barrier membrane. When the pore is electrically biased and connected to a high-gain current amplifier, individual molecules can translocate through the pore causing a temporary reduction in the ionic current. This current eventually returns to its original level after the molecule has passed through the pore5,6. This altered current is known as a resistive pulse or translocation event, and by analyzing parameters such as the current amplitude (ΔI), dwell time (tD), and capture rate (CR), valuable information about the molecule can be obtained7.
Nanopores can be either biological (e.g., α-hemolysin8, MspA9 and ClyA) or solid-state10. While biological nanopores with well-defined structures exhibit single-molecule sensitivity, they are susceptible to pore failure due to buffer composition and other external conditions such as mechanical or chemical instability of the lipid bilayer11. In contrast, nanopores created from inorganic or organic materials (e.g., silicon nitride (SiNx), SiO2, graphene, and plastics) can be customized in terms of their size, structure, and surface characteristics to provide optimal readout of single molecules using RPS. Solid-state nanopores, with their adjustable pore size and durability, can expand the range of biological targets that can be analyzed. In addition, they offer flexibility in device designs, materials used for the RPS measurements, and are well-suited for integration to other processing steps besides RPS readout12. Solid-state nanopores can be further classified into two categories based on the pore orientation relative to the substrate; out-of-plane (pore orifice perpendicular to the substrate surface) and in-plane (pore orifice parallel to the substrate surface).
Since Li et al.13 detected DNA using solid-state nanopores in a thin silicon nitride membrane, nanopore-based research has evolved to encompass the detection of various biomolecules, including proteins using out-of-plane solid-state nanopores. In 2006, Han et al.14 reported the first instance of single bovine serum albumin (BSA) sensing using solid-state nanopores. In recent years, numerous studies have focused on characterizing proteins using solid-state nanopores, particularly emphasizing the utilization of out-of-plane nanopores15,16,17. However, several challenges are associated with out-of-plane nanopores such as the requirement of complex fabrication techniques for example, focused ion beam (FIB) milling or transmission electron microscopy (TEM) drilling to make each device with well-defined geometries18. In addition, out-of-plane pores typically have poor sampling efficiency, which results in modest concentration limits-of-detection19. Also, out-of-plane nanopores pose challenges for device integration with other fluidic components19.
In this study, we demonstrate the use of a plastic-based in-plane nanopore sensor to address the challenges associated with the out-of-plane architecture. Our experiment utilized two in-plane nanopores arranged in series that flanked either end of a single nanochannel. This configuration enabled the measurement of the time-of-flight (ToF) of single protein molecules in a label-free manner, providing crucial information about their molecular-dependent electrophoretic mobility under a constant electric field20. Additionally, these planar devices facilitated improved fluid management, integration to various detection techniques (such as electrical and optical), and enhanced mass transfer of the analyte providing favorable concentration LODs21,22.
Only a few publications have focused on single-molecule sensing using in-plane nanopores19. Zhang et al. fabricated glass in-plane nanopores ranging in diameter from 40 to 90 nm for sensing virus capsids. Similarly, Haywood et al.23 employed silicon to create in-plane nanopores and channels to assess the electrophoretic mobility of translocating species. Unfortunately, the authors utilized FIB milling to craft nanostructures and photolithography to create microstructures for each device, which is costly and time-consuming.
In this work, we utilized thermoplastics such as cyclic olefin polymer (COP) and cyclic olefin copolymer (COC), along with nano-injection molding for producing the dual in-plane nanopore sensor. The combination of thermoplastics and injection molding facilitated the production of low-cost, disposable nanofluidic devices24. Thermoplastics offer several benefits including the ability to customize the size of the in-plane nanopore not only during the injection molding process, but also during thermal fusion bonding, which places a cover plate over the molded substrate25. Additionally, the surface chemistry of thermoplastics can be altered easily using methods such as O2 plasma or UV/O3, which induces the formation of carboxylic acid groups enhancing surface wettability26. We employed FIB milling and photolithography to create a single silicon master mold, which was then used to create resin stamps that enabled the production of replicas via nano-injection molding (see Fig. S1 for overview of fabrication process). This approach substantially decreases device cost and enhances production rates27,28. We fabricated functional nanofluidic devices with dimensions ≤ 30 nm. This technique offers high production rates (i.e., thousands of devices per day) at low inter-device variability (< 5% RSD)28.
We used the dual in-plane nanopore sensor to detect, differentiate, and approximate the shape of model proteins based on RPS event characteristics and the ToF. Through our nanofluidic platform, we characterized proteins using four variables: event amplitude, dwell time, ToF, and event shape. To the best of our knowledge, no previous work has demonstrated thermoplastic in-plane nanopore devices for protein differentiation and shape approximation.
Methods
Protein samples
In this study, a variety of chemicals and materials acquired from reputable sources were utilized without the need for further purification. Affinity-purified Streptavidin was obtained from Thermo Fisher Scientific, Waltham, MA, while the Human CD19 antibody/Mouse IgG1 was sourced from R&D Systems, Minneapolis, MN. Myoglobin (M5696) and Cytochrome C (C2037) were purchased from Sigma-Aldrich, Saint Louis, MO.
Other reagents and materials
S1813 photoresist and MF319 developer were acquired from MicroChemicals in Germany, while KOH pellets were obtained from Fisher Scientific in Waltham, MA. IPA was purchased from Sigma-Aldrich in St. Louis, MO, and HF, TPGDA, TMPTA, and photoinitiator (2,2-Dimethoxy-2-phenylacetophenone) were sourced from the same supplier. NOA72 was procured from Norland Products in Neuchâtel, Switzerland, and Si wafers were obtained from WaferPro in Santa Clara, CA. PET with a thickness of 250 µm was sourced from Goodfellow in Coraopolis, PA, while Si wafers (<100> orientation) were acquired from University Wafers in Boston, MA. UV-curable polyurethane resin was purchased from Chansang Co. COP (Type 1060R) sheets were acquired from STRATEC SE in Birkenfeld, Germany. COC (Type 8007) was obtained from TOPAS Advanced Polymers in Florence, KY. Finally, molecular biology-grade water was obtained from Thermo Fisher in Waltham, MA.
Manufacturing and assembly of the nanofluidic device
The Si master mold was created using Si wafers coated with a 100 nm thick silicon oxide layer on each side. To form the microchannels, photolithography and wet-chemical etching processes were employed. Initially, a layer of S1813 photoresist was evenly spread onto the wafer and then exposed to UV light. After exposure, it was developed, and the silicon oxide layer was etched away to create an opening. Subsequently, the wafer underwent an etching process using a KOH solution resulting in the formation of microchannels with a depth of 10 µm. FIB milling was utilized to construct the nanostructures, including nanofunnels, nanochannels, and nanopores. To replicate the structures of the silicon master mold (see Fig. S1), a resin stamp was produced by dispensing drops of a UV-curable resin solution called MD 700 against the Si master mold and curing it using UV light. The molded UV-resin/Polyethylene terephthalate (PET) backbone was removed from the Si master mold and used to make another resin mold in MD700 on the PET backbone. Finally, the resin mold with positive structures was produced on a stainless-steel block, and used for the injection molding process28. The substrate (COP) of the device was fabricated using nano-injection molding, and the injection molded nanopore devices were subsequently characterized using scanning electron microscopy (SEM) and atomic force microscopy (AFM)28. To form enclosed fluidic structures, the injection molded COP substrate was themal fusion bonded to a COC 8007 cover plate. The bonding process was performed after subjecting both the substrate and cover plate to UV/O3 treatment at 50 W for 3.5 min, following the procedure outlined by Uba et al.29 Please see Fig. S1 in Supporting Information (SI) for more information on the fabrication process and Fig. S2 for the dual in-plane nanopore sensor layout and metrology. In Fig. S3 is shown AFM images of the in-plane nanopores showing a pore depth of ~ 30 nm for an unbonded chip (i.e., does not contain a cover plate). We should note that cover plate bonding can result in an approximate 66% reduction in pore dimensions25. As noted in our previous manuscript, injection molding and cover plate assembly produced a relative standard deviation of inter-device production of 5.4%30.
Surface activation by UV/O3 treatment
The thermoplastic surfaces underwent UV/O3 treatment using a UV/O3 Cleaner manufactured by Jelight (USA). This cleaner featured a low-pressure mercury vapor grid lamp operating at 1 atmospheric pressure and emitting 10 W/cm2 at a wavelength of 185 and 254 nm. The UV lamps were positioned approximately 35 mm away from the samples.
Water contact angle measurements
The hydrophilicity of the native and UV/O3 treated COP surfaces was assessed using a VCA Optima instrument from AST Products. This evaluation involved measuring the water contact angle through the sessile drop method. Sections of 1.5 mm thick COP sheets measuring 2.0 cm × 2.0 cm, were prepared. A volume of 2.0 µL of nanopure water with a pH of 7.5 was carefully deposited onto the surface. The sessile water contact angle was then analyzed using the software provided by the manufacturer and images were recorded throughout the procedure. For each position of the COP substrate, the mean and standard deviation of three separate drops were documented. The results of these measurements are presented in Fig. S4.
Single-molecule protein detection using dual in-plane nanopore sensor
These experiments utilized a COP dual in-plane nanopore sensor to conduct experiments involving Streptavidin, IgG, Cytochrome C, and Myoglobin proteins. Initially, the device was filled with a 1 × PBS buffer containing 1 M KCl at a pH of 7.4. Subsequently, the open pore current was measured by applying a 1 V potential using the Axopatch Digidata 1440B (Molecular Devices) operating at 10 kHz bandwidth. After this, the buffer solution was replaced in one reservoir with a 1 nM protein solution in 1 M KCl, and Ag/AgCl electrodes were positioned in the device’s reservoirs on either side of the pores (see Fig. S2d). As a note, the 1 nM concentration was selected to keep the probability of double occupancy low (< 0.01%) to make sure the event peak pairs arose from single molecules. Once again, a 1 V potential was applied, and data was recorded using the data acquisition software Clampfit 11.1. This procedure was repeated for all proteins using different devices fabricated under the same molding and bonding conditions. All data were recorded with a sampling frequency of 250 kHz and a low-pass filter of 10 kHz. The data collection process took approximately 5 min, and the device was placed in a Faraday cage during both the open pore current measurements and the protein experiments. For the cationic protein (cytochrome C), the anode was on the cis side of the device (protein migrated with the electroosmotic flow, EOF), and for the anionic proteins, the cathode was placed on the cis side of the device (proteins migrated against the EOF).
Data analysis and statistics
The raw data obtained from Clampfit software were imported into MATLAB R2022b, provided by MathWorks, Inc., to generate a data report that included the peak characteristics of individual RPS events. Events were scored when their amplitude exceeded a threshold condition set by the open pore current trace to keep the false positive rate equal to 0. These characteristics comprised peak amplitudes for pore 1 and pore 2, dwell times for pore 1 and pore 2, and ToF. Subsequently, histograms were created for these variables for each protein to represent the data distribution visually. All histograms were created using Origin Pro 2015; OriginLab Corp. For the one-way analysis of variance (ANOVA), we employed Prism developed by GraphPad Software, Inc., to ascertain whether there were any statistically significant differences among the means of each variable. Please see Fig. S5 for event characteristics. As seen, each single molecule that is transported through the sensor giving rise to a pair of RPS events (i.e., peak pair) from which the ToF could be deduced.
Results and discussion
Scaling effects and noise characteristics for RPS detection of single proteins using in-plane plastic nanopores
The signal-to-noise ratio (SNR) in RPS is defined as the ratio of event amplitude (ΔI) to the root mean square (RMS) noise in the open pore current (IRMS). The open pore current (I0) is the current measured with the carrier electrolyte only. Most biological pores have a very narrow opening (~ 1 nm), which restricts the passage of intact proteins31. However, larger pores, such as Cytolysin A (~ 3.5 nm) and those formed by the self-assembly of complement component 9 (C9), result in a diameter of ~ 10 nm, which can allow for protein RPS detection32. Although the size of our sensing nanopore is larger (apparent diameter ~ 10 nm following thermal fusion bonding assembly; sensing volume ~ 392 nm3 based on the physical dimensions of the pore and assuming a 1/2 cylinder shape)25. than the aforementioned pores, biological pores achieved a lower SNR (4–15) compared to our plastic in-plane pores (45–90) for protein RPS sensing (Fig. 1)32,33,34. Studies conducted using solid-state out-of-plane nanopores35 reported SNRs for proteins ~ 40, which was limited by the noise characteristics of the pore near the bandwidth of the electronics (~ 10 kHz)36. The SNR improvements for our plastic in-plane pores were attributed to their distinct structural characteristics and the dielectric properties of the COP substrate, resulting in a lower IRMS in spite of their larger effective pore diameter, as well as better mechanical stability generating low 1/f noise characteristics32,37.
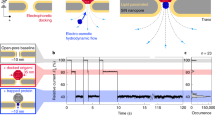
(a) RPS current traces that were digitally low pass filtered at 10 kHz with a duration of 2 s for myoglobin (1 nM) translocating through the dual in-plane nanopore sensor at voltages that ranged from 600 to 1600 mV ([KCl] = 1 M, 1 × PBS, pH = 7.4). (b) Current blockage histograms as a function of applied voltage in the range of 400 to 1600 mV. The number of translocation events at 400, 600, 800, 1000, 1200, 1400 and 1600 mV were 101,120,125, 135, 88, 72, and 52, respectively. (c) Current amplitude medians (mean ± standard deviation) as a function of sensor applied voltage. (d) The capture rate (mean ± standard deviation) as a function of applied voltage. The histogram was fit to a Gaussian function to describe the relationship between capture rate and voltage. Scale bars represent 1 s and 0.8 nA (ΔI). The RPS amplitudes were normalized with respect to the open-pore current (I0) to account for variations in pore sizes.
The dual in-plane nanopore sensor included two microchannels, two funnel-like structures, two nanopores, and one nanochannel serving as the electrophoresis flight tube from which the ToF was deduced. The two in-plane pores were positioned on either side of the flight tube (Fig. S2). An input funnel served to enhance sampling efficiency by extending the driving electric field into the adjoining microchannel37. Microchannels, funnel-like structures, and the nanochannel are known as “guide structures,” which help to stabilize the ionic current and background noise associated with the in-plane pores38. Moreover, positioning two nanopores in series can lead to nonlinearities in ion distributions at each pore, enhancing RPS signal amplitude. This configuration can create unique interactions between the pores by altering ion flow and potentially generating concentration gradients. As ions are accumulated or are depleted near the pores, the resulting changes in electric fields enhance signal intensity with minimal increases in IRMS, thereby improving the SNR in the RPS measurement39,40.
There are several reasons for the low IRMS in our in-plane plastic nanopores. Among the various noise sources for nanopore systems, 1/f noise (flicker noise) predominates in the ≲ 100 Hz range.36 The mechanical stability of our in-plane pore system is greater than that of pores suspended on thin membranes, such as those made from two-dimensional (2D) materials like graphene or silicon nitride; mechanical stability helps to reduce 1/f noise.41 Additionally, we observed low noise characteristics in the mid-frequency range (< 1000 Hz) as determined from the power spectral density (PSD) analysis of the open pore current traces from the dual in-plane sensor (Fig. S6a). We also found reduced dielectric noise (1–10 kHz) due to the electrical properties of the thermoplastic and capacitive noise (> 10 kHz). The small capacitor plate associated with our in-plane pores results in low parasitic capacitance. Comparisons of the power spectral density for our in-plane nanopores and out-of-plane pores clearly show that the out-of-plane pores have significantly higher noise characteristics near 10 kHz (see Fig. S6a,b).42.
RPS of single protein molecules using plastic in-plane pores.
RPS of intact proteins using our in-plane pores was investigated using myoglobin (negative charge at pH 7.4 in 1 M KCl and 1X PBS, pH = 7.4) by applying various bias voltages ranging from 400 to 1600 mV. As depicted in Fig. 1a, we observed events at all voltages. However, upon close examination of the RPS traces, noticeable changes in ΔI/I0 and CR (number of events per second) were observed as a function of applied voltage. For comparison purposes, we generated histograms of normalized peak amplitudes (ΔI/I0) for each voltage. As seen in Fig. 1b, increases in ΔI/I0 were observed as a function of increased voltage resulting in a linear dependence of ΔI/I0 with respect to voltage (Fig. 1c). Conversely, CR initially increased from 400 to 1000 mV but decreased when the applied voltage was > 1000 mV (Fig. 1d).
The electric field drives protein translocation through the nanopores and flight tube due to its electrophoretic mobility43. Electroosmotic flow (EOF), hydrodynamic drag forces, and permselectivity can impede the transport process, especially for negatively charged proteins when the surface charge of the pore is also negative as we see here for our thermoplastic pores that have been UV/O3 activated (see Fig. S4)43,44. As the voltage increases, both EOF and electrophoretic mobility increase. However, the electrophoretic mobility can increase at a higher rate compared to the EOF45. Consequently, more molecules enter the pore increasing CR at higher voltages. When the electrophoretic force is strong enough to overcome forces inhibiting their entry into the pore, CR will increase with higher voltage. Additionally, permselectivity can affect CR as well but is voltage independent (i.e., depends on bulk ion concentration only). The exponential increase in CR with applied voltage has been observed experimentally for protein translocation using out-of-plane pores46.
When the voltage exceeded 1000 mV, CR decreased. We suspect this was attributed to bandwidth limitations. As the voltage was increased to a point where the inverse event dwell time exceeds the system’s intrinsic bandwidth, which is set by the current amplifier (10 kHz), this will cause event clipping resulting in a reduction in the apparent CR due to events that fall below the leading-edge event threshold. This ultimately results in a smaller apparent CR15,47. Our results demonstrate an inverse relationship between the median dwell time and applied voltage (see Fig. S7, which supports this observation).
Transient current responses are generated when a molecule translocates through the nanopore arising from a partial current blockage (ΔI) compared to a pore that is unoccupied (I0). While formalisms have been generated to describe ΔI, these have been produced using a single pore. To describe ΔI and I0, we started with the equations described for a single isolated pore and took into account the presence of two pores in series (see SI)48. This led to the equations shown below for our 2 pores in series.
where \(I_{F}\) is given by;
In Eqs. (1–3), \(A\) is a constant and given by the expression \(\frac{8{h}_{t}}{\pi {d}_{t}^{2}}\) where \({h}_{t}\) and \({d}_{t}\) refer to the flight tube’s length and diameter, respectively. Also, \(\sigma\) is the solution conductance, \(V\) is the sensor applied voltage, \({h}_{eff}\) is the effective length of the nanopore (incudes pore 1 and 2), \({d}_{m}\) is the diameter of the molecule, and \(d\) is the diameter of pores 1 or 2. Based on Eq. (2), ΔI is proportional to \(V\) indicating a linear dependence between ΔI and the applied voltage. According to Shi et al.49, as the applied voltage increases stronger electric fields form around the particle and nanopore obstructing more ions from passing through the nanopore. Consequently, the ion blockage effect induced by the analyte is enhanced; this is clearly seen in the data of Fig. 1c. Inspection of Eq. (2) would indicate that RPS characteristics for pores 1 and 2 should be similar as seen in the histograms for dwell times and normalized peak amplitudes (see Fig. S8).
Electrokinetic transport of proteins through the dual in-plane nanopore sensor
We next employed the dual in-plane nanopore sensor to determine the nanoscale electrokinetic transport behavior of four different proteins: IgG, streptavidin, cytochrome c, and myoglobin (see Table 1). These proteins were selected based on differences in their size, shape, and charge. The electrokinetic transport through this sensor consists of two transport processes: (1) translocation through the in-plane pores; and (2) electrophoretic motion through the nanochannel flight tube.
We used 1X PBS supplemented with 1 M KCl as the electrolyte solution. We observed a higher ΔI for the RPS events of the proteins when we supplemented PBS with 1 M KCl (data not shown). According to Smeets et al.50, high salt concentrations in the carrier electrolyte can lead to an increase in I0 as well as a higher value for ΔI. Despite the higher salt concentration and larger I0 in the presence of 1 M KCl, we did not observe a significant difference in IRMS with or without KCl added to the PBS buffer, which therefore resulted in improved SNR for the RPS events.
Intact proteins were analyzed in terms of three RPS characteristics ΔI, dwell time (tD), as well as the inter-pulse time, which is the ToF34. In Fig. 2a we show the current trace obtained for a blank, which was comprised of 1X PBS with 1 M KCl. As anticipated, in the absence of analyte, no RPS events were observed with the associated threshold condition utilized. Figure 2b illustrates RPS current traces for 1 nM solutions of IgG, streptavidin, cytochrome c, and myoglobin using the dual in-plane nanopore sensor. RPS events were found to produce downward spikes, indicating that the passage of a single protein molecule through the nanopore transiently reduced the conductance within the pore, irrespective of the charge on the protein15. IgG and streptavidin are larger proteins with approximately similar isoelectric point ranges, but they differ in shape; IgG has an oblate shape while streptavidin is spherical (Table 1)51. In contrast, cytochrome C and myoglobin are smaller proteins with different isoelectric points and shapes; cytochrome C is highly positively charged and cylindrical52, while myoglobin is negatively charged and nearly spherical (see Table 1)53.
(a) RPS current trace obtained for a blank solution (1X PBS, 1 M KCl, pH = 7.4). (b) RPS current traces (time duration = 2 s) obtained for the translocation of 1 nM solutions (with 1X PBS, 1 M KCl) of IgG, streptavidin, cytochrome C, and myoglobin. Downward spikes in the current traces represent individual translocation events for each protein. The molecules were translocated through two in-plane nanopores with an effective diameter of ~ 10 nm. Refer to Table 1 for detailed information on the four proteins. All traces, including the blank, were digitally low pass filtered at 10 kHz and acquired under a driving voltage of 1 V. Histograms displaying the distribution of normalized peak amplitude (ΔI/I0) (c), time-of-flight (ToF) (d), and dwell time, tD (e) values derived from resistive pulse recordings of IgG, streptavidin, cytochrome C, and myoglobin. Numbers in the histograms represent the median ± interquartile range. RPS current traces were acquired for a duration of 2 s and obtained from the translocation of 1 nM solutions (with 1X PBS, 1 M KCl) of each protein. Results of a one-way analysis of variance (ANOVA) and Tukey’s multiple comparisons test for each RPS event characteristic of IgG, streptavidin, cytochrome C and myoglobin; ΔI/I0 (f), tD (g) and ToF (h). Results from three different translocation experiments (n = 3) were considered for the analysis (f). For ANOVA and Tukey’s multiple comparisons tests: The overall p values for the ΔI/I0, tD and ToF were 0.0003, 0.0002 and 0.0004, respectively. For ΔI/I0, *p = 0.0151, **p = 0.0011, ***p = 0.0002, ns (not significant) = 0.2036. For tD, *p = 0.0496, **p = 0.0065, ***p = 0.0007, ns = 0.2676. For ToF, *p = 0.0381, **p = 0.0091, ***p = 0.0002, ns = 0.9664.
We observed that the mean ΔI/I0 for these proteins followed a trend with IgG, the largest protein, exhibiting the largest median ΔI/I0 and cytochrome C, the smallest protein, displaying the smallest median ΔI/I0 (see histograms in Figs. 2c and S9 for a plot of ΔI/I0 versus protein volume). This trend can be partially explained by Huo et al.54; larger proteins contain more volume and thus exclude more carrier electrolyte ions resulting in larger ΔI/I0 compared to proteins with smaller sizes. However, there were exceptions to the aforementioned trend. For instance, there was little difference between cytochrome C and myoglobin ΔI/I0 values despite their size differences. Again, this indicates that the volume exclusion model cannot be used solely to explain a current blockage ΔI/I0 value for our sensor. Another consideration is off-axis effects55. Molecules can translocate through the pore in two primary modes: centerline or off-axis (e.g., away from the centerline). Off-axis translocation of molecules can result in higher pulse amplitudes compared to centerline translocations. The increase in pulse amplitude for off-axis translocation can be attributed to the higher electric potential near the pore wall leading to larger ionic fluxes and current densities resulting in a larger ΔI compared to centerline translocation55. Although cytochrome C has a smaller volume compared to myoglobin, its positive charge causes it to electrostatically interact with the negatively charged pore wall and thus, cytochrome C is more likely to undergo off-axis translocation, resulting in higher pulse amplitudes compared to the negatively charged myoglobin. This effect can be pronounced in our in-plane nanopore system due to the asymmetrical shape of the pores. Our pores are U-shaped (see Fig. S3), which creates a non-homogeneous electric field unlike symmetrical pores, such as encountered for solid-state out-of-plane pores51.
When comparing ΔI/I0 (Fig. 2f), statistical differences were seen for all proteins (p-values ranged from 0.0151 to 0.0002) except for the cytochrome C/myoglobin pair as well as the myoglobin/streptavidin pair. In addition, we noticed a broader distribution of ΔI/I0 values for cytochrome C compared to the other proteins (see Fig. 2c). This broader distribution is attributed to the expected higher interaction with the nanopore walls for cytochrome C, which can influence the local concentration of ions within the nanopore and alter the electric field distribution. Changes in the conductivity of the electrolyte solution due to protein translocation and wall interactions is correlated with changes in the peak amplitude of the signal as noted above56.
The direction and velocity of protein motion through the dual in-plane nanopore sensor, whether translocation or electrophoretic motion, are controlled by three factors: (i) electrophoresis (EP); (ii) electrochromatography (EC); and (iii) electroosmosis (EOF). Thermoplastics, including COP and COC, generate an EOF from anode-to-cathode when activated using UV/O3. This occurs due to the deprotonation of -COOH groups on the surface of the plastic57,58. For the proteins investigated herein, cytochrome C moved in the same direction as the EOF while the other proteins, which are negatively charged, moved in the opposite direction of the EOF.
The effect of protein charge on dwell times
Dwell time (tD) represents the duration of time that a protein takes to pass through a single nanopore (pore length ~ 10 nm); it was measured as the full width at half-maximum (FWHM) of a blockage event to ensure consistent data for the duration of each event59. Negatively charged proteins should move moderately slow through the nanopore due to the opposing effects of the EOF. In the case of cytochrome C and its positive charge, it should move with the EOF and thus display the shortest tD. As shown in Fig. 2d, cytochrome C exhibited a longer tD than the other three negatively charged proteins and showed a larger variance. This most likely could be attributed to increased electrostatic interactions with the negatively charged walls within the nanopore60. As shown in Fig. 2d and the statistical analysis in Fig. 2g, cytochrome C exhibited a significantly longer tD than the other three negatively charged proteins (p values ranged from 0.0065 to 0.0007).
In terms of the negatively charged proteins, myoglobin showed the shortest tD (0.048 ± 0.010 ms) with a very narrow distribution (see Fig. 2d,g) due to it possessing the highest negative charge (− 7.3) of these three proteins while IgG and streptavidin showed no statistical difference in their tD values. In addition, myoglobin possesses a 3D structure that is spherical, and of the negatively charged proteins, has the smallest volume (20 nm3; 154 amino acid residues) and thus, less constricted in terms of its translocation through the in-plane nanopores compared to the other two negatively charged proteins (i.e., IgG and streptavidin). We note that 1/tD for myoglobin is slightly lower than the bandwidth of the current amplifier circuitry (10 kHz) and as such, there could be errors in this determination due to event clipping. Finally, in spite of differences in both size and charge between IgG and streptavidin, there was no statistical difference (p = 0.2676) between their tD values, which could have arisen from differences in their shape (Table 1).
While the amplifier circuitry we are using is limited to 10 kHz (3 dB point) with a single pole, which indicates that higher bandwidth signals can pass but may be perturbed by the limiting nature of the TIA amplifier circuitry. We have calculated the operational bandwidth of the pore itself in a previous publication1, which yielded a pore bandwidth for COP of 130 MHz that is much higher than the bandwidth set by the TIA circuitry.
Deducing the ToF using the dual in-plane nanopore sensor
The motivation for placing two in-plane nanopores in series with an intervening flight tube into our sensor was to measure the ToF by observing the time difference between a pair of RPS events for a single molecule, which is directly related to its apparent mobility.20 Fig. 2e shows histograms for the ToF of the model proteins (note—ToF values are shown as absolute values and thus do not indicate the direction of motion).
Drawing from the findings of Firnkes et al.61, we hypothesized that at pH 7.4, negatively charged IgG, myoglobin, and streptavidin would move against the EOF, while positively charged cytochrome C would move in the same direction as the EOF. Our results confirmed this hypothesis. Due to the low dosing of UV/O3 light (3.5 min @ 22 mW/cm2) to the plastic and 1 M KCl added to the carrier electrolyte, µeo was measured as 3.7 × 10–6 cm2/Vs for our sensor and as such µeo was smaller than µep for the negatively charged proteins. As such, cytochrome C’s electrokinetic transport through the nanosensor was anode-to-cathode and in the same direction as the EOF, while the negatively charged proteins moved in a direction opposed to the EOF that would normally indicate longer ToFs for the negatively charged proteins. We note that the general trend observed for these proteins in terms of the ToF was very similar to that seen for the tD values.
Accordingly, cytochrome C should exhibit the shortest ToF because its EP was in the same direction as the EOF. However, it showed the highest ToF value. We attribute this observation to electrostatic interactions with the wall, which was similar to what we observed for the tD values (Fig. 2h)62,63. Among the negatively charged proteins, myoglobin exhibited the shortest ToF, which was primarily due to its high negative charge at pH 7.4 (− 7.3)53. Despite variations in size and shape, IgG and streptavidin exhibited comparable ToFs likely because of similarities in their zeta potentials61. Myoglobin showed a significantly shorter ToF compared to IgG and streptavidin.
Apparent mobilities of proteins within the nanosensor
The apparent mobilities (µapp) of the proteins within the nanopore and nanochannel flight tube was calculated using the dwell time (tD) and ToF, respectively, and the equation below;
where L is the length of the nanochannel or nanopore, Δt is ToF or tD, and E is the electric field strength across the nanochannel or nanopore, which was determined from a COMSOL simulation of this sensor we have previously published (Fig. 3b).37 Notably, µapp in the nanopore for the proteins were significantly smaller than those in the nanochannel flight tube (Fig. 3c). This indicates that the translocation of proteins through a nanopore and their electrophoretic motion in a nanochannel are different because of several reasons. For example, the electric field strength is 1000 times higher in the in-plane nanopores compared to the nanochannel flight tube,37 which significantly affects the apparent mobility of all proteins in the nanopore according to Eq. (4). Considering the dimensions of the two environments, the nanopore has a width and depth of ~ 10 nm, while the nanochannel dimensions are 50 nm × 50 nm (Fig. 3a). Because the nanopore has a more confined environment compared to the nanochannel, the EOF is larger in the nanopore. This is due to the nanopore having a higher surface-to-volume ratio, which increases the contribution of surface charge to the EOF64. In terms of contributions of electroviscosity to the EOF, it will be minimal due to the low surface charge of the device employed herein because of the low UV/O3 dosing we employed. We calculated the electrical double layer (EDL) thickness (Debye length) using the Debye-Hückel approximation and it was ~ 0.28 nm. Therefore, given the ~ 10 nm apparent pore diameter, there is not significant EDL overlap within the nanopore. However, there is a higher likelihood of protein molecules interacting with the EDL inside the nanopore compared to the nanochannel. Additionally, exclusion effects (ion selectivity by the diffuse layer of the EDL) for negatively charged proteins can be more pronounced when traveling through a nanopore compared to the nanochannel, potentially slowing down translocation in the nanopore65. At the same time, although positively charged protein molecules move with the EOF, translocation through a narrower constriction increases wall interactions with the negatively charged pore wall via electrostatics, which slows down the translocation process compared to the electrokinetic motion through the flight tube.
(a) Schematic representation of dual in-plane nanopore sensor. The nanopore and nanochannel walls were negatively charged, leading to the development of an electrical double layer (EDL) on the wall surfaces, which induced an electroosmostic flow (EOF). Negatively charged proteins such as IgG, streptavidin, and myoglobin traveled against the EOF, while positively charged cytochrome C traveled with the EOF. lpore = length of the pore (~ 10 nm), Wpore = width of the pore (~ 10 nm), μep = electrophoretic mobility, μeo = electroosmotic flow. (b) Length, voltage drop, and electric field strength within the nanopore and nanochannel flight tube. Voltage drops were calculated using a COMSOL simulation applied for the entire sensor fluidic network. (c) Apparent mobilities of the four model proteins through the nanopore and nanochannel flight tube. Negative values represent apparent mobilities of proteins that travel against the EOF, while positive values represent the apparent mobilities of proteins that travel in the same direction as the EOF. Equation (5) was used to calculate the apparent mobilities. Apparent mobilities were calculated assuming there is no chromatographic effect on the movement of protein molecules through both the nanopore and the nanochannel.
Comparison of the RPS temporal profiles of proteins with different shapes
Houghtaling et al.51 and others66,67,68 have demonstrated that the shape asymmetry of protein molecules can influence the RPS event temporal profile during their passage through a solid-state nanopore as a function of the orientation while symmetrically-shaped proteins elicit current responses irrespective of orientation. These current fluctuations provide a method for obtaining information concerning the molecule’s orientation and approximate shape while resident within the nanopore. Sha et al.67 and Houghtaling et al.51 analyzed the distribution of resistive pulse magnitude and temporal profile and introduced the concept of the electrical shape factor, denoted as Ɣ. This factor connects the particle’s alignment with respect to the electric field. While spherical particles have a consistent Ɣ value during translocation through the nanopore, non-spherical particles can exhibit a range of Ɣ values, varying from the completely perpendicular (Ɣmax) to perfectly parallel (Ɣmin) orientation within the nanopore (see Fig. 4).
(a) Current transient peak shape for an oblate shaped protein (IgG) that can translocate through a nanopore in two extreme orientations, either with its major axis orthogonal to the direction of flow (1) or parallel to the flow direction (2). (b) RPS current trace for a spherically shaped protein (streptavidin) as a function of translocation time through the in-plane nanopore. Also shown is the anticipated current transient event, which adopts a square-type shape. (c) Different orientational directions for proteins translocating through the in-plane pore.
As shown in Fig. 5a, we examined individual translocation events of IgG, streptavidin, cytochrome C, and myoglobin each possessing a unique 3D conformational shape (see Fig. 5b). Notably, proteins with a spherical shape, such as streptavidin and myoglobin, tend to produce rectangular-shaped RPS events. In contrast, non-spherical proteins like IgG and cytochrome C yielded events with diverse shapes. For instance, IgG’s RPS events consisted of multiple sub-peaks. These amplitude fluctuations can be attributed to changes in Ɣ due to the rotational motion of this asymmetrically shaped protein while translocating through the pore51,63,66. Ɣ is influenced by the angle (θ) formed between the electric field and the pore axis (Fig. 4c)66. θ can range between 0° and 90°, where 0° and 90° correspond to Omin (orientation that minimizes current blockage) and Omax (orientation that maximizes current blockage), respectively. Each event involving IgG primarily consists of two distinct current levels, Omax and Omin51,67. These observations are substantiated based on the protein’s rotational time, which is on the nanoseconds to microseconds69,70, and shorter than proteins’ tD indicating that these molecules can complete their entire rotational movement within the nanopore during their residence.
(a) Examples of RPS current traces for the translocation of single IgG, streptavidin, cytochrome C and myoglobin through the in-plane nanopores. Scale bars represent 1 ms and 1.6 nA. (b) Comparison of the estimated shapes of four proteins with the temporal profiles of individual translocation events. Scale bar represents 5 nm.
Cytochrome C exhibited a “V” shaped RPS event (Fig. 5a). This globular protein closely resembles a cylinder in its 3D shape as determined by its unit cell parameters71. The event shape depends on the extent of rotation within the pore, which may explain the observed “V” shape resulting from varying rotation angles during its residence within the pore. The lowest point in the peak corresponds to Omax, while other points arise from different rotation angles between 0° and 90°. Furthermore, the curved shape or the shallow blockage observed in the later part of the peak can be attributed to the interaction of the protein molecule with the pore wall as it approaches the exit of the pore72. Due to its positive charge, cytochrome C is attracted to the negatively charged pore wall so when the protein exits the pore, one end of the molecule may extend outside the pore while the other end remains attached to the surface of the nanopore’s interior73.
In the case of IgG, the ionic current experiences variations based on its orientation67,73. This effect primarily occurs because the mean cross-sectional area changes as it rotates within the nanopore as previously mentioned. However, spherical proteins like streptavidin and myoglobin, despite their rotation while passing through the nanopore, do not induce significant changes in the ionic current due to their symmetrical nature and thus gives “rectangular” shaped RPS events.67.
Conclusions
The dual in-plane nanopore sensor reported herein is unique compared to other nanopore sensors primarily due to the incorporation of two in-plane nanopores that flank a nanochannel flight tube, which can secure in addition to the RPS signal characteristics, the apparent electrophoretic mobility of the target as well. The nanosensor was made in a plastic via nano-injection molding, which allows for the wide-scale distribution of the technology into the general community due to the high-scale production of the nanosensor unlike solid-state sensors embedded in this thin membrane layer, which require primarily ion or electron milling. This nanosensor was used to detect, differentiate, and approximate the shapes of four model proteins using the RPS technique. Despite its unique format, the dual in-plane nanosensor can secure data similar to that obtained using nanopores embedded within a thin membrane, such as deducing the shape of the protein from the temporal profile of the RPS signal74,75. Collecting multiple RPS variables in conjunction with pore-independent data can be useful for identifying molecules when a single variable is not sufficient37. For instance, the ΔI/I0 values of cytochrome c and myoglobin were not significantly different, but the dwell time and ToF resulted in statistically significant differences. Peak amplitude and dwell time depend on several variables including protein size, charge, and their interactions with the walls of nanopores and channels. We also examined the electrical shape factor of proteins during translocation revealing insights into their rotational motion within the nanopore. Non-spherical proteins exhibited diverse peak shapes reflecting their varying orientations during passage through the nanopore. In contrast, spherical proteins displayed rectangular peak shapes due to their uniform cross-sectional area.
Data availability
All data associated with this manuscript is detailed in the form of figures and tables included in the manuscript as well as material shown in the Supplementary Information. The data presented in all figures and tables can be made available to the readership upon request to the corresponding author of this manuscript.
Change history
04 July 2025
The original online version of this Article was revised: The Acknowledgments section in the original version of this Article was incomplete. “The authors thank the NIH for financial support of this work via NIBIB funded Center of Biomodular Multiscale Systems for Precision Medicine (CBMM, P41-EB020594), NIBIB: R01-EB031579; NCI: R33 CA235597, NCI: R21-CA277351, and COBRE Kansas Institute for Precision Medicine (KIPM, P20 GM130423). The authors also acknowledge the KU Nanofabrication Facility (P20GM103638).” It now reads: "The authors thank the NIH for financial support of this work via NIBIB funded Center of Biomodular Multiscale Systems for Precision Medicine (CBMM, P41-EB020594), NIBIB: R01-EB031579; NCI: R33 CA235597, NCI: R21-CA277351, and COBRE Kansas Institute for Precision Medicine (KIPM, P20 GM130423). The authors also acknowledge the KU Nanofabrication Facility’s P20 grant (P20GM103638) that funded a previous phase—during the work cited in the article, the facility was funded by a new P30 grant (5P30GM145499).” The original Article has been corrected.
References
Gooding, J. J. & Gaus, K. Single-molecule sensors: Challenges and opportunities for quantitative analysis. Angew. Chem. Int. Ed. 55(38), 11354–11366 (2016).
Holzmeister, P. et al. Quantum yield and excitation rate of single molecules close to metallic nanostructures. Nat. Commun. 5(1), 5356 (2014).
Varongchayakul, N., Song, J., Meller, A. & Grinstaff, M. W. Single-molecule protein sensing in a nanopore: A tutorial. Chem. Soc. Rev. 47(23), 8512–8524 (2018).
Picotti, P. & Aebersold, R. Selected reaction monitoring–based proteomics: Workflows, potential, pitfalls and future directions. Nat. Methods 9(6), 555–566 (2012).
Howorka, S. & Siwy, Z. Nanopore analytics: Sensing of single molecules. Chem. Soc. Rev. 38(8), 2360–2384 (2009).
Wei, R., Gatterdam, V., Wieneke, R., Tampé, R. & Rant, U. Stochastic sensing of proteins with receptor-modified solid-state nanopores. Nat. Nanotechnol. 7(4), 257–263 (2012).
Luo, Y., Wu, L., Tu, J. & Lu, Z. Application of solid-state nanopore in protein detection. Int. J. Mol. Sci. 21(8), 2808 (2020).
Kasianowicz, J. J., Brandin, E., Branton, D. & Deamer, D. W. Characterization of individual polynucleotide molecules using a membrane channel. Proc. Natl. Acad. Sci. U. S. A. 93(24), 13770–13773 (1996).
Laszlo, A. H. et al. Detection and mapping of 5-methylcytosine and 5-hydroxymethylcytosine with nanopore MspA. Proc. Natl. Acad. Sci. U. S. A. 110(47), 18904–18909 (2013).
Wei, R., Martin, T. G., Rant, U. & Dietz, H. DNA origami gatekeepers for solid-state nanopores. Angew. Chem. Int. Ed. 51(20), 4864–4867 (2012).
Wendell, D. et al. Translocation of double-stranded DNA through membrane-adapted phi29 motor protein nanopores. Nat. Nanotechnol. 4(11), 765–772 (2009).
Lee, K. et al. Recent progress in solid-state nanopores. Adv. Mater. 30(42), e1704680 (2018).
Li, J. et al. Ion-beam sculpting at nanometre length scales. Nature 412(6843), 166–169 (2001).
Han, A. et al. Sensing protein molecules using nanofabricated pores. Appl. Phys. Lett. 88, 093901 (2006).
Larkin, J., Henley, R. Y., Muthukumar, M., Rosenstein, J. K. & Wanunu, M. High-bandwidth protein analysis using solid-state nanopores. Biophys. J. 106(3), 696–704 (2014).
Oukhaled, A., Bacri, L., Pastoriza-Gallego, M., Betton, J.-M. & Pelta, J. Sensing proteins through nanopores: Fundamental to applications. ACS Chem. Biol. 7(12), 1935–1949 (2012).
Si, W. & Aksimentiev, A. Nanopore sensing of protein folding. ACS Nano 11(7), 7091–7100 (2017).
Chen, Q. & Liu, Z. Fabrication and applications of solid-state nanopores. Sensors (Basel) 19(8), 1886 (2019).
Zhang, M. et al. In-plane, in-series nanopores with circular cross sections for resistive-pulse sensing. ACS Nano 16(5), 7352–7360 (2022).
Choi, J. et al. Label-free identification of single mononucleotides by nanoscale electrophoresis. Small 17(42), 2102567 (2021).
Yang, L. & Yamamoto, T. Quantification of virus particles using nanopore-based resistive-pulse sensing techniques. Front. Microbiol. 7, 1500 (2016).
Angeli, E. et al. Simultaneous electro-optical tracking for nanoparticle recognition and counting. Nano Lett. 15(9), 5696–5701 (2015).
Haywood, D. G., Harms, Z. D. & Jacobson, S. C. Electroosmotic flow in nanofluidic channels. Anal. Chem. 86(22), 11174–11180 (2014).
Piotter, V., Hanemann, T., Ruprecht, R. & Haußelt, J. Injection molding and related techniques for fabrication of microstructures. Microsyst. Technol. 3(3), 129–133 (1997).
Athapattu, U. S. et al. Tailoring thermoplastic in-plane nanopore size by thermal fusion bonding for the analysis of single molecules. ACS Sens. 6(8), 3133–3143 (2021).
Weerakoon-Ratnayake, K. M., O’Neil, C. E., Uba, F. I. & Soper, S. A. Thermoplastic nanofluidic devices for biomedical applications. Lab Chip 17(3), 362–381 (2017).
Zhang, N., Byrne, C. J., Browne, D. J. & Gilchrist, M. D. Towards nano-injection molding. Mater. Today 15(5), 216–221 (2012).
Shiri, F. et al. Nano-injection molding with resin mold inserts for prototyping of nanofluidic devices for single molecular detection. Lab Chip 23(22), 4876–4887 (2023).
Uba, F. I., Hu, B., Weerakoon-Ratnayake, K., Oliver-Calixte, N. & Soper, S. A. High process yield rates of thermoplastic nanofluidic devices using a hybrid thermal assembly technique. Lab Chip 15(4), 1038–1049 (2015).
Shiri, F. et al. Nano-injection molding with resin mold inserts for prototyping of nanofluidic devices for single molecular detection. Lab Chip 23, 4876–4887 (2023).
Crnković, A., Srnko, M. & Anderluh, G. Biological nanopores: Engineering on demand. Life 11(1), 27 (2021).
Soskine, M. et al. An engineered ClyA nanopore detects folded target proteins by selective external association and pore entry. Nano Lett. 12(9), 4895–4900 (2012).
Zernia, S., van der Heide, N. J., Galenkamp, N. S., Gouridis, G. & Maglia, G. Current blockades of proteins inside nanopores for real-time metabolome analysis. ACS Nano 14(2), 2296–2307 (2020).
Huang, G. et al. Electro-osmotic vortices promote the capture of folded proteins by PlyAB nanopores. Nano Lett. 20(5), 3819–3827 (2020).
Fologea, D., Ledden, B., McNabb, D. S. & Li, J. Electrical characterization of protein molecules by a solid-state nanopore. Appl. Phys. Lett. 91(5), 539011–539013 (2007).
Fragasso, A., Schmid, S. & Dekker, C. Comparing current noise in biological and solid-state nanopores. ACS Nano 14(2), 1338–1349 (2020).
Rathnayaka, C. et al. Detection and identification of single ribonucleotide monophosphates using a dual in-plane nanopore sensor made in a thermoplastic via replication. Lab Chip 24(10), 2721–2735 (2024).
Lee, K. et al. Enhancing the sensitivity of DNA detection by structurally modified solid-state nanopore. Nanoscale 9(45), 18012–18021 (2017).
Wanunu, M., Morrison, W., Rabin, Y., Grosberg, A. Y. & Meller, A. Electrostatic focusing of unlabelled DNA into nanoscale pores using a salt gradient. Nat. Nanotechnol. 5(2), 160–165 (2010).
Rivas, F., DeAngelis, P. L., Rahbar, E. & Hall, A. R. Optimizing the sensitivity and resolution of hyaluronan analysis with solid-state nanopores. Sci. Rep. 12(1), 4469 (2022).
Zhou, Z. et al. DNA translocation through hydrophilic nanopore in hexagonal boron nitride. Sci. Rep. 3, 3287 (2013).
Nunes, P., Ohlsson, P., Ordeig, O. & Kutter, J. Cyclic olefin polymers: Emerging materials for lab-on-a-chip applications. Microfluid. Nanofluid. 9, 145–161 (2010).
Wu, L. et al. Electrically facilitated translocation of protein through solid nanopore. Nanoscale Res. Lett. 9(1), 140 (2014).
Pandey, D., Bhattacharyya, S. & Ghosal, S. A numerical study of the selectivity of an isolated cylindrical or conical nanopore to a charged macro-ion. Biomicrofluidics https://doi.org/10.1063/1.5124132 (2019).
Charcosset, C. Electrophoretic mobility. In Encyclopedia of Membranes (eds Drioli, E. & Giorno, L.) 658–659 (Springer, 2016). https://doi.org/10.1007/978-3-662-44324-8_208.
Cressiot, B. et al. Protein transport through a narrow solid-state nanopore at high voltage: Experiments and theory. ACS Nano 6(7), 6236–6243 (2012).
Hartel, A. J. W. et al. High bandwidth approaches in nanopore and ion channel recordings - A tutorial review. Anal Chim Acta 1061, 13–27 (2019).
Wanunu, M. et al. Rapid electronic detection of probe-specific microRNAs using thin nanopore sensors. Nat. Nanotechnol. 5, 807–814 (2010).
Shi, L. et al. The influence of electric field intensity and particle length on the electrokinetic transport of cylindrical particles passing through nanopore. Micromachines 11(8), 722. https://doi.org/10.3390/mi11080722 (2020).
Smeets, R. M. M. et al. Salt dependence of ion transport and DNA translocation through solid-state nanopores. Nano Lett. 6(1), 89–95 (2006).
Houghtaling, J. et al. Estimation of shape, volume, and dipole moment of individual proteins freely transiting a synthetic nanopore. ACS Nano 13(5), 5231–5242 (2019).
Hüttemann, M. et al. The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: From respiration to apoptosis. Mitochondrion 11(3), 369–381 (2011).
Alsenaidy, M. A. Aggregation and conformational stability evaluation of myoglobin in the presence of ionic surfactant. Saudi Pharm. J. 26(4), 515–519 (2018).
Huo, M.-Z., Li, M.-Y., Ying, Y.-L. & Long, Y.-T. Is the volume exclusion model practicable for nanopore protein sequencing? Anal. Chem. 93(33), 11364–11369 (2021).
Jodeyri, Z. & Taghipoor, M. Multivariate analysis of nanoparticle translocation through a nanopore to improve the accuracy of resistive pulse sensing. Phys. Chem. Chem. Phys. 26(6), 5097–5105 (2024).
Wilson, J., Sarthak, K., Si, W., Gao, L. & Aksimentiev, A. Rapid and accurate determination of nanopore ionic current using a steric exclusion model. ACS Sens. 4(3), 634–644 (2019).
O’Neil, C. E. et al. Characterization of activated cyclic olefin copolymer: effects of ethylene/norbornene content on the physiochemical properties. Analyst 141, 6521–6532 (2016).
van Dorp, S., Keyser, U. F., Dekker, N. H., Dekker, C. & Lemay, S. G. Origin of the electrophoretic force on DNA in solid-state nanopores. Nat. Phys. 5(5), 347–351 (2009).
Pedone, D., Firnkes, M. & Rant, U. Data analysis of translocation events in nanopore experiments. Anal. Chem. 81(23), 9689–9694 (2009).
Tripathi, P. et al. Electrical unfolding of cytochrome c during translocation through a nanopore constriction. Proc. Natl. Acad. Sci. 118(17), e2016262118 (2021).
Firnkes, M., Pedone, D., Knezevic, J., Döblinger, M. & Rant, U. Electrically facilitated translocations of proteins through silicon nitride nanopores: Conjoint and competitive action of diffusion, electrophoresis, and electroosmosis. Nano Lett. 10(6), 2162–2167 (2010).
Weerakoon-Ratnayake, K. M., Uba, F. I., Oliver-Calixte, N. J. & Soper, S. A. Electrophoretic separation of single particles using nanoscale thermoplastic columns. Anal. Chem. 88(7), 3569–3577 (2016).
Wang, X. et al. Nanopore detection using supercharged polypeptide molecular carriers. J. Am. Chem. Soc.145(11), 6371–6382 (2023).
Haywood, D. G., Saha-Shah, A., Baker, L. A. & Jacobson, S. C. Fundamental studies of nanofluidics: Nanopores, nanochannels, and nanopipets. Anal. Chem. 87(1), 172–187 (2015).
Vlassiouk, I., Smirnov, S. & Siwy, Z. Ionic selectivity of single nanochannels. Nano Lett. 8(7), 1978–1985 (2008).
Yusko, E. C. et al. Real-time shape approximation and fingerprinting of single proteins using a nanopore. Nat. Nanotechnol. 12(4), 360–367 (2017).
Sha, J. et al. Identification of spherical and nonspherical proteins by a solid-state nanopore. Anal. Chem. 90(23), 13826–13831 (2018).
Golibersuch, D. C. Observation of aspherical particle rotation in Poiseuille flow via the resistance pulse technique. I. Application to human erythrocytes. Biophys. J. 13(3), 265–280 (1973).
Haridasan, N., Kannam, S. K., Mogurampelly, S. & Sathian, S. P. Rotational diffusion of proteins in nanochannels. J. Phys. Chem. B 123(23), 4825–4832 (2019).
Loman, A., Gregor, I., Stutz, C., Mund, M. & Enderlein, J. Measuring rotational diffusion of macromolecules by fluorescence correlation spectroscopy. Photochem. Photobiol. Sci. 9(5), 627–636 (2010).
Mirkin, N., Jaconcic, J., Stojanoff, V. & Moreno, A. High resolution X-ray crystallographic structure of bovine heart cytochrome c and its application to the design of an electron transfer biosensor. Proteins 70(1), 83–92 (2008).
Wu, H. et al. Translocation of rigid rod-shaped virus through various solid-state nanopores. Anal. Chem. 88(4), 2502–2510 (2016).
Niedzwiecki, D. J., Grazul, J. & Movileanu, L. Single-molecule observation of protein adsorption onto an inorganic surface. J. Am. Chem. Soc. 132(31), 10816–10822 (2010).
Schmid, S., Stömmer, P., Dietz, H. & Dekker, C. Nanopore electro-osmotic trap for the label-free study of single proteins and their conformations. Nat. Nanotechnol. 16, 1244–1250 (2021).
Houghtaling, J. et al. Estimation of shape, volume, and dipole moment of individual proteins freely transiting a synthetic nanopore. ACS Nano 19, 5231–5242 (2019).
Acknowledgements
The authors thank the NIH for financial support of this work via NIBIB funded Center of Biomodular Multiscale Systems for Precision Medicine (CBMM, P41-EB020594), NIBIB: R01-EB031579; NCI: R33 CA235597, NCI: R21-CA277351, and COBRE Kansas Institute for Precision Medicine (KIPM, P20 GM130423). The authors also acknowledge the KU Nanofabrication Facility’s P20 grant (P20GM103638) that funded a previous phase—during the work cited in the article, the facility was funded by a new P30 grant (5P30GM145499).
Author information
Authors and Affiliations
Contributions
S.S.: Investigation, Writing—original draft, Writing—revising and editing; M.C.: Investigation, Writing—original draft; C.M.: Investigation; M.V.: Investigation, Writing; J.C.: Investigation, Writing; S.P.: Resources, Writing; F.S.: Resources; A.R.H.: Writing—revising and editing; S.A.S.: Conceptualization, Supervision, Funding Acquisition, Writing—revising and editing.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shivanka, S., Shiri, F., Chibuike, M. et al. Insights on using plastic-based dual in-plane nanopore sensors for differentiation and shape determinations of single protein molecules. Sci Rep 15, 13742 (2025). https://doi.org/10.1038/s41598-025-96232-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-96232-y