Abstract
This study investigates the role of CALCR, a G-protein-coupled receptor, in gastric cancer (GC) progression and its interaction with ANTXR1. A total of 121 patients with gastric cancer were enrolled from the Department of General Surgery, Anyang Tumor Hospital, Anyang City, Henan Province, China. 218 tumor tissues and corresponding para-carcinoma tissues were collected from 109 patients, while adjacent tissues were retained from the remaining 12 cases. Kaplan–Meier analysis evaluated the prognostic value of m6A-related genes in GC. Immunohistochemistry (IHC) was conducted to evaluate CALCR expression. Quantitative real-time PCR (qRT-PCR), Western blot analysis, CCK-8 assays, flow cytometry and transwell assays were used to assess CALCR’s role in cell proliferation, apoptosis, migration, and invasion. Co-immunoprecipitation experiments were performed to explore the interaction between CALCR and ANTXR1. Statistical analyses were conducted using SPSS 25.0 and GraphPad Prism 8.0, with p < 0.05 considered significant. IHC staining revealed that 53.2% (n = 58) of the tumor tissues exhibited high CALCR expression, compared to only 6.6% (n = 8) of the para-carcinoma tissues (p < 0.001). CALCR knockdown in GC cell lines significantly reduced proliferation (p < 0.01), increased apoptosis (p < 0.01), and inhibited migration and invasion (p < 0.001). In a nude mouse model, CALCR knockdown resulted in significantly reduced tumor growth and metastasis (p < 0.05). Co-immunoprecipitation showed that CALCR interacts with ANTXR1, leading to decreased AKT phosphorylation. CALCR is a crucial factor in GC progression, presenting a potential prognostic marker and therapeutic target. Targeting the CALCR-ANTXR1 axis and AKT pathway offers new avenues for GC treatment.
Similar content being viewed by others
Introduction
Gastric carcer (GC) is the fifth most common malignant tumor and the third leading cause of cancer-related deaths worldwide1. Despite advancements in surgical techniques, chemotherapy, and targeted therapy, treatment outcomes for advanced gastric cancer remain suboptimal, with an overall five-year survival rate 40%. The complex biological characteristics of gastric cancer, including genetic predisposition2, contribute to the difficulty in achieving complete cures and accurate prognostication based solely on clinicopathological data3.
Calcitonin (CT) a 32-amino-acid polypeptide hormone, primarily synthesized by the thyroid, regulates calcium homeostasis under hypercalcemic conditions4,5. The calcitonin receptor (CALCR), a member of G-protein-coupled receptor, is the sole receptor for CT, functioning as the signaling protomer for the heteromeric amylin receptor (CTR/RAMP-1). Upon binding to calcitonin, CALCR activates adenylate cyclase through the stimulatory Gα protein subunit, increasing cyclic AMP (cAMP) levels and subsequently activating downstream signaling pathways involving protein kinase A (PKA) and Exchange Protein Activated by cAMP (Epac)6. Additionally, CALCR is implicated in intracellular calcium release and phosphorylation of extracellular signal-regulated kinases (ERK), contributing to diverse cellular roles including bone metabolism, calcium flux, and cancer cell proliferation7.
CALCR expression is observed in specific cell types involved in wound healing and various malignant tissues, such as breast cancer8, brain tumors9, medullary thyroid carcinoma6 and prostate cancer10. However, its role in malignant tissues is not well characterized, with effects reported across tumor types. For instance, wild-type CALCR inhibits Ras-mediated transformation of astrocytes in glioblastoma, whereas the CT/CALCR axis promotes malignancy in prostate cancer11,12.
Anthrax toxin receptor 1 (ANTXR1), also known as tumor endothelial marker 8 (TEM8), is a highly conserved transmembrane glycoprotein overexpressed in tumor vasculature13 and various cancers, including gastric, breast, colon, and pancreatic tumors14,15,16. ANTXR1 facilitates anthrax toxin entry into cells and has emerged as a promising target for cancer immunotherapy, with studies demonstrating the efficacy of anti-ANTXR1 therapies in preclinical models of triple-negative breast cancer and gastric cancer14,15,16,17,18. The role of ANTXR1 in tumor angiogenesis, progression, invasion, and metastasis underscores its potential as a therapeutic target, although its specific mechanisms in gastric cancer remain underexplored.
This study aims to investigate the expression, clinical significance, biological function, and regulatory mechanisms of CALCR in gastric cancer, focusing on its interaction with ANTXR1 and the involvement of the AKT signaling pathway. The findings will provide insights into the potential of CALCR as a prognostic marker and therapeutic target for improving gastric cancer management and outcomes.
Methods
Patients and tissue specimens
A total of 121 patients with gastric cancer were enrolled at the Department of General Surgery, Anyang Tumor Hospital, Anyang City, Henan Province, China. For 109 of them, we collected tumor tissues along with corresponding adjacent non-tumor tissues. In 12 additional cases, the pathologic type was identified via gastroscopy prior to treatment. However, post-surgery, the cancer lesions in these cases were too small to sample, so only adjacent tissues were retained. These collected tissues were subsequently subjected to immunohistochemistry analysis.
Organisms and cell lines
All organisms and cell lines used in this study were obtained from Procell Life Science & Technology Co., Ltd. (Wuhan, China). Male BALB/c nu/nu mice were purchased from the company and housed in sterile mouse facilities. Human gastric cancer cell lines (BGC-823, AGS, SGC-7901, MGC-803) were also acquired from Procell Life Science & Technology Co., Ltd. Cells were cultured in RPMI-1640 medium under a 5% CO₂ atmosphere at 37 °C.
Mice euthanasia was performed by administering an overdose of Pentobarbital (100 mg/kg) to the mice. All procedures were conducted in accordance with ethical guidelines and approved by the institutional animal care and use committee.
RNAi lentiviral vector construction
To create RNAi lentiviral vectors for CALCR knockdown, we first designed multiple 19-21nt RNAi target sequences using the CALCR gene as a template. After evaluation, the sequences selected for Human-CALCR-1, Human-CALCR-2, and Human-CALCR-3 were GAGGAATGAACCAGCCAACAA, TGGGTCATTCTTTGTCAATTT, and CAAATCAAACCTATCCAACAA, respectively. Short hairpin RNA (shRNA) interference sequences were then designed based on these target sequences.
The shRNA sequences were synthesized and cloned into the BR-V108 vector by adding restriction endonuclease sites at both ends. The vector was constructed with TTTTT as the termination signal at the 3’ end of the forward strand and the complementary sequence at the 5’ end of the reverse strand. The synthesized single-stranded DNA oligo was dissolved in annealing buffer (final concentration 100 μM) and annealed to form double-stranded DNA with sticky ends.
A 50 μL reaction system was prepared using the New England BioLabs (NEB) protocol, which included BR-V108 vector, CutSmart Buffer, and Age I/EcoR I to linearize the vector. The double-stranded DNA oligo was then ligated into the linearized vector using a 20 μL reaction system prepared according to the Fermentas T4 DNA Ligase instructions. The ligation products were transformed into competent E. coli cells (TIANGEN Cat. #CB104-03), and clones carrying the target gene were identified by PCR and sequencing.
Positive clones were cultured overnight in 150 ml of Luria–Bertani broth (LB) liquid medium containing ampicillin (Amp) antibiotics. Plasmids were extracted using the EndoFree Maxi Plasmid Kit, and those passing quality control were used for subsequent experiments.
To achieve effective knockdown, lentiviral particles were prepared and used to infect target cells. Cells were plated and incubated overnight at 37 °C with 5% CO2 until they reached 80% confluence. The lentiviral particle solution was added to the cell suspension, and fresh media were replaced after 18–20 h. Infection efficiency was evaluated after 72 h by observing fluorescence under a microscope.
Cell culture
Human gastric cancer cell lines BGC-823, AGS, SGC-7901, MGC-803 were obtained and cultured in RPMI 1640 Complete Medium supplemented with 2 mM L-Glutamine and 10% U.S. origin fetal bovine serum (FBS).
Transfections
Target cells were resuscitated and incubated in 6-cm plates at 37 °C, 5% CO2 overnight. The cells should be approximately 80% confluent. Lentiviral particle solution was then added to the cell suspension. Fresh media were changed 18–20 h after infection. And after 72 h, the fluorescence were observed under microscope and the infection efficiency was calculated.
Tumorigenicity assay in nude mice
Male balb/c nu/nu mice (6–8 weeks old) were purchased and housed two per cage under standard conditions of 12-h light/dark cycles, fed on a standard autoclaved laboratory diet, and quarantined for 1 week prior to the use in the study. Target cells were transplanted to prepared nude mice by tail-vein injection. After a week, the nude mice were euthanatized and the xenograft was removed for further analysis by In Vivo Imaging System.
Real-time quantitative PCR analysis
Total RNAs were extracted from sorted or cultured cells using the Trizol Kit (Sigma, T9424-100 m) according to the protocol, followed by reverse transcription into single-stranded cDNA using HiScript Q RT SuperMix for qPCR (Vazyme), prior to real-time quantitative PCR amplification.
Western blotting analysis
Cell samples were pelleted, washed with PBS, and lysed in a lysis buffer. Protein was estimated and loaded on SDS-PAGE. Then the proteins were transferred to polyvinylidene difluoride (PVDF) membranes after SDS-PAGE. The PVDF membrane was incubated using prepared blocking buffer (5% skim milk), corresponding primary antibody, and horseradish peroxidase (HRP)-conjugated IgG antibody in order. Immobilon Western Chemiluminescent HRP Substrote Kit (Millipore RPN2232) was used for immunoreactive protein visualization.
Immunohistochemistry (IHC)
For immunohistochemical studies, xylene-based removal of paraffin was performed on sections from paraffin-embedded tissue, followed by heat-induced epitope retrieval. After blocking with 3% hydrogen peroxide, sections were stained with primary antibodies at 4˚C overnight, and then reacted with secondary antibodies.
Immunohistochemical staining was evaluated using the immunoreactive score (IRS) system, which incorporates two parameters: the percentage of positive cells and the staining intensity. The percentage of positive cells was scored as follows: 0 for no positive cells, 1 for < 10% positive cells, 2 for 10–50% positive cells, 3 for 51–80% positive cells, and 4 for > 80% positive cells; staining intensity was scored as 0 for no color reaction, 1 for a mild reaction, 2 for a moderate reaction, and 3 for an intense reaction. The final IRS was calculated by multiplying the scores of these two parameters, yielding a range from 0 to 12, which was subsequently categorized as negative (0–1), mild expression (2–3), moderate expression (4–8), and strong expression (9–12) 19. In this study, high expression was typically defined as a high proportion of positive cells (a score of 3 or higher) combined with a staining intensity of 3, resulting in an IRS of 9 or above.
Fluorescence-activated cell sorting (FACS) analysis
eBioscience Annexin V Apoptosis Detection Kit was used for staining of apoptotic cells. In brief, apoptosis was induced when the cells in 6-well plates of each experimental group were grown to approximately 70% confluence. The apoptotic cells were pelleted by centrifugation (1300 RPM, 5 min, RT), and washed by D-Hanks (pH = 7.2 ~ 7.4) at 4 °C. The cells were resuspended in 1 × binding buffer, followed by Annexin V-APC staining at RT without light exposure. Then, 400 μL of 1 × binding buffer was added to the sample, which was tested by flow cytometry.
Celigo cell counting assay
Each experimental group of cells in the logarithmic growth phase were digested by trypsin and resuspended in complete medium. Equal number of cells were placed in a 96-well plate and kept in incubator (37 °C, 5% CO2). Celigo machine (Nexcelom) was used to measure the number of the fluorescent cells.
Colony formation assay
The colony-forming assay was conducted in 6-well plates. Cells were seeded in medium with agar at a density of 1000 cells per well and incubated at 37 °C, 5% CO2 for 14 days, with the medium changed and cell status observed every 3 days. After the incubation, the photographs of the colonies growing in the plates were taken under a fluorescent microscope. The cell colonies were washed by PBS and fixed with 4% polyformaldehyde. After PBS washing, the cells were stained with 1 × GIEMSA stain for 20 min, followed by ddH2O washing for several times. Once the cells had been dried out, photographs were taken using a digital camera for further calculation.
Transwell assay
To evaluate the migratory and invasive abilities of cells, Transwell chambers (Corning 3422, Corning, NY, USA) with an 8-μm pore polycarbonate membrane insert (CLS3422-48EA, Sigma Aldrich) were used. Cells were seeded (12,000 cells/well) in the upper chamber of the transwell containing 100 μL serum free DMEM F-12 medium and the lower chambers containing 600 μL RPMI 1640 medium with 30% FBS as a chemoattractant. The system was incubated at 37 °C, 5% CO2, and after 24 h, the invaded or migrated cells in the lower chambers from upper chambers were fixed and stained with 1% Crystal Violet. Images were captured under a fluorescence microscope (Olympus IX 73, Tokyo, Japan) and cells were counted.
Wound-healing assay
96-well wound-healing assays (VP scientific) enabling high-throughput measurement of cellular migration capabilities were performed. Around 3 × 104 transfected cells were added to each well, followed by cellular confluency of over 90% next day. The medium was changed to low-level serum. The monolayer of cells was wounded with a sterile pipette tip lightly pushed up from the center of the lower end. The cells were then washed twice using serum-free medium, before 0.5% FBS was added to the medium. Next, the cells were incubated for additional 24 h at an incubator (Thermo 371) at 37 °C, 5% CO2, with images captured at 0 h, 8 h, 16 h, 24 h under a fluorescence microscope (Olympus IX 73, Tokyo, Japan). The number of cells migrated into the wound in each group was determined, and cellular migration rate was calculated.
Co-immunoprecipitation
For immunoprecipitation assays, cells were lysed in IP lysis buffer and 1 mg of extracts were incubated with 1 μg indicated antibody at 4 °C on a rotating rack overnight. Then 20 μL of beads were added for a 2-h incubation at 4 °C on a rotating rack. Samples were collected by centrifugation (2000 g, 1 min, 4 °C) and washed with IP lysis buffer, separated by SDS-PAGE, and immunoblotted with indicated antibodies.
Statistical analysis
Data were analyzed using SPSS 25.0 and GraphPad Prism 8.0 software. Comparisons between two groups were conducted using Student’s t-test, while multiple group comparisons were made using one-way ANOVA. Kaplan–Meier survival analysis was performed using the log-rank test. p < 0.05 was considered statistically significant.
Results
CALCR is highly related to human gastric cancer
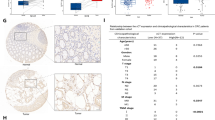
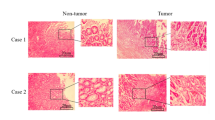
In clinical practice, we initially observed that the expression of CALCR in gastric cancer tissues was significantly higher than that in para-carcinoma tissues through immunohistochemical (IHC) staining (Fig. 1A). To further investigate this observation, we analyzed a total of 230 clinical samples of gastric cancer and para-carcinoma tissues. The IHC staining results revealed that 53.2% (n = 58) of the tumor tissues exhibited high CALCR expression, whereas only 6.6% (n = 8) of the para-carcinoma tissues showed high CALCR expression (Fig. 1B).
High expression of CALCR in gastric cancer is associated with tumor growth and predicts a poor prognosis for GC. (A) Representative IHC staining of CALCR in TMA of GC patients (cancer (CA) or para-carcinoma tissue (PT)). (B) Percentages of different tissues expressing high level of CALCR. (C) Volcano plot shows the differential gene expression between tumor and para-carcinoma tissues. (D) Principal component analysis (PCA) shows the difference of CALCR concentration between para-carcinoma tissues. (E) The expression profiling of CALCR in TCGA analysis. (Normal) and tumor tissues (Tumor). (F) Kaplan–Meier survival curves for overall survival and disease-free survival in patients with different expression of CALCR in gastric cancer tissues.
We also performed bioinformatics analysis of the TCGA database for gastric cancer. As expected, the analysis revealed numerous differentially expressed genes between Normal (paraneoplastic) and Tumor (cancer) tissues (Fig. 1C). Principal component analysis (PCA) was used for quality control, demonstrating that Normal and Tumor tissues could be clearly separated in PC1. This separation indicated that the data from different batches of samples exhibited high stability, with the intra-group differences being smaller than the inter-group differences (Fig. 1D). Among the differentially expressed genes, a significant difference in the expression of CALCR between Normal and Tumor tissues was also observed (P < 0.001) (Fig. 1E).
Furthermore, we conducted a comprehensive analysis of the demographic and clinical characteristics of gastric cancer patients with different CALCR expression (Table 1). A significant correlation was found between CALCR expression and the degree of tumor infiltration in gastric cancer patients (P < 0.01). Additionally, Kaplan–Meier survival analysis indicated that CALCR expression was significantly associated with overall survival (P = 0.001) and disease-free survival (P = 0.001) in gastric cancer patients (Fig. 1F). Patients with higher CALCR expression in gastric cancer tissues had much lower survival rates.
CALCR promotes tumorigenesis by regulating cell proliferation and migration
To determine whether CALCR plays a role in regulating cell proliferation and migration, we designed shRNA constructs to inhibit CALCR expression (referred to as ‘shCALCR’). AGS and SGC-7901 cells, which are commonly used human gastric cancer cell lines, showed significantly reduced CALCR protein levels in the shCALCR group (Fig. 2A), indicating successful knockdown of the CALCR gene.
CALCR promotes tumorigenesis by regulating cell proliferation and migration. (A) Reduced CALCR protein expression in AGS and SGC-7901 cells in the shCALCR group. (B) Knockdown of CALCR inhibits proliferation in both AGS and SGC-7901 cell lines. (C) In vivo imaging system showing tumorigenesis in negative control (NC) mice and CALCR knockout (KD) mice. (D) Total and average radiation efficiency of NC mice (#1 to #10) and KD mice (#11 to #20). (E) Tumor volume and tumor mass over time in KD mice and NC mice. (F) Representative immunohistochemical staining images and relative expression levels of ki-67 in KD and NC mouse groups. Experiments were repeated at least three times. Asterisks (*) indicate a significant difference compared with control groups (*P < 0.05, **P < 0.01 and ***P < 0.001). NC, negative control. KD, knockdown. (G, H) Fluorescence activated cell sorting (FACS) showed the difference of cell apoptosis percentage between different groups. (I, J) transwell assay showed the difference of migration rate between different groups. (K) Western blot analysis showed the level of CALCR and AKT pathway-associated protein in MGC-803 cell line from shCALCR and AKT activator groups. (L) Association of CALCR with apoptosis-related gene expression. (M) Changes in expression of apoptotic genes associated with the CALCR gene as shown by Western Blot. Experiments were repeated at least three times. Asterisks (*) indicate a significant difference compared with control groups (*P < 0.05, **P < 0.01 and ***P < 0.001). NC, negative control. KD, knockdown. (N) CALCR mediated expression of inflammatory factors in vitro. Experiments were repeated at least three times. Asterisks (*) indicate a significant difference compared with control groups (*P < 0.05, **P < 0.01 and ***P < 0.001).
Next, we conducted a series of functional assays to elucidate the role of CALCR in cell proliferation and migration. Celigo cell counting assays demonstrated that the proliferation rates of AGS cells (P < 0.001, fold change = -2.4) and SGC-7901 cells (P < 0.01, fold change = − 1.5) in the shCALCR group were significantly reduced (Fig. 2B). Flow cytometry analysis revealed a significant increase in apoptosis rates in both AGS cells (P < 0.01, fold change = 1.9) and SGC-7901 cells (P < 0.01, fold change = 2.3) in the shCALCR group (Fig. 2C). Additionally, cell cycle analysis showed an increased proportion of cells in the G2 phase in both cell lines in the shCALCR group compared to the shCtrl group (P < 0.001) (Fig. 2D).
To assess cell migration ability, we performed wound-healing (Fig. 2E) and transwell assays (Fig. 2F). Both assays showed decreased migration rates in the shCALCR group for AGS and SGC-7901 cells.
Furthermore, we divided the MGC-803 cells into two groups: the Adenovirus short hairpin RNA targeting CALCR (shCALCR) group and the Adenovirus empty vector (as a control short hairpin RNA, shCTRL) group. A subset of cells from each group was treated with an exogenous AKT activator (shCALCR + AKT and shCTRL + AKT) to further validate our findings.
Flow cytometry results indicated that the apoptosis rate was significantly lower in the shCALCR + AKT group compared to the shCALCR group (P < 0.001), while the apoptosis rate was higher in the shCALCR + AKT group compared to the shCTRL + AKT group (P < 0.001) (G, H). Transwell assay results showed that the migration rate increased significantly in the shCALCR + AKT group compared to the shCALCR group (P < 0.01), but decreased compared to the shCTRL + AKT group (P < 0.001) (Fig. 2I, J).
Western blot analysis revealed that AKT expression was upregulated in the shCALCR group compared to the NC group, while CALCR and p-AKT expression were downregulated. The addition of an exogenous AKT activator led to the downregulation of CALCR and PI3K expression and upregulation of p-AKT expression compared to corresponding control groups (shCTRL + AKT vs. NC, shCALCR + AKT vs. shCALCR). No p-PI3K expression was detected in any group (Fig. 2K).
We also examined the association of CALCR with the expression of apoptosis-related genes. The expression of genes such as Bad, Bax, Caspase8, CD40, CD40L, cytoC, Fas, FasL, HSP60, HTRA, IGFBP-3, IGGBP-6, p27, p53, etc., was significantly up-regulated in the shCALCR group (P < 0.05) (Fig. 2L). Conversely, the expression of P-mTOR, CDK1, CDK6, and PIK3CA was downregulated in the shCALCR group (P < 0.05), while mTOR expression remained unchanged (Fig. 2M).
Additionally, we found that downregulation of CALCR expression was associated with altered expression of inflammatory factors. In the shCALCR group, OCT4 expression decreased, while IL-17A, Nanog, and ICAM1 expression increased. However, TLR4 and SOX2 expression did not change significantly between the two groups (Fig. 2N). These results indicate that CALCR may play a key role in cell carcinogenesis by activating the AKT pathway, affecting cell survival, growth, proliferation, migration and related gene expression, enhancing cell proliferation and migration, and altering the expression of cellular inflammatory factors.
CALCR knockdown reduces tumor growth in vivo
To investigate the effects of CALCR gene inhibition in vivo, we constructed a CALCR knockdown (KD) mouse model and conducted tumorigenicity experiments. The in vivo imaging system clearly showed tumorigenesis in negative control (NC) mice and CALCR knockdown (KD) mice (Fig. 3A). Compared with NC mice (# 1 to # 10), KD mice (# 11 to # 20) had significantly lower total and average radiation efficiency (P < 0.01) (Fig. 3B).
Knockdown of CALCR suppresses tumor growth and metastasis in vivo. (A) In vivo imaging system showing tumorigenesis in negative control (NC) mice and CALCR knockout (KD) mice. (B) Total and average radiation efficiency of NC mice (#1 to #10) and KD mice (#11 to #20). (C) Tumor volume and tumor mass over time in KD mice and NC mice. (D) Representative immunohistochemical staining images and relative expression levels of ki-67 in KD and NC mouse groups. Experiments were repeated at least three times. Asterisks (*) indicate a significant difference compared with control groups (*P < 0.05, **P < 0.01 and ***P < 0.001). NC, negative control. KD, knockdown.
Tumor volume and mass measurements were performed to assess the impact of CALCR knockdown on tumor growth. It was determined that the tumor growth rate was significantly reduced in KD mice compared to NC mice (Fig. 3C). Specifically, KD mice exhibited smaller tumor volumes (P < 0.05) and masses (P < 0.05) at each time point. Additionally, the expression of CALCR in the tumors of NC mice was significantly higher than that in KD mice, confirming the successful knockdown of CALCR in vivo (Fig. 3D).
The significant reduction in tumor growth following CALCR knockdown highlights its potential as a therapeutic target for gastric cancer treatment.
CALCR can bind to ANTXR1 and affect ANTXR1 expression
Genome-wide gene expression microarray analysis of shCtrl and shCALCR was performed in AGS cell lines to obtain the differentially expressed genes of shCtrl and shCALCR (Fig. 4A). To further explore the correlation between CALCR and KEGG signaling pathway, we constructed a protein–protein interaction network (Fig. 4B)20,21,22.
CALCR can bind to ANTXR1 and affect ANTXR expression. (A) Genome-wide gene expression microarray analysis showing the differentially expressed genes of shCtrl and shCALCR. (B) Protein interaction network between CALCR and KEGG signaling pathway20,21,22. (C) mRNA expression levels of relevant downstream genes in the shCALCR group relative to the shCtrl group. (D) Western Blot demonstrating protein expression levels of relevant downstream genes in the shCALCR group relative to the shCtrl group. (E) ANTXR1 was co-immunoprecipitated with CALCR. Bound proteins were eluted and analyzed by Western blot using an anti-CALCR antibody.
Among these downstream genes, the expression of ETS1, ANTXR1, IL1A, IL1B, EGR1, IL6, SKA1, CCND1, PLAU, RBL1, E2F1, and SOX2 was significantly down-regulated in the shCALCR group compared to the shCtrl group (P < 0.05) (Fig. 4C), Notably, the expression of ANTXR1 exhibited the most substantial decrease in response to reduced CALCR expression (Fig. 4D).
Immunoprecipitation experiments confirmed a direct interaction between CALCR and ANTXR1 (Fig. 4E). This interaction suggests that CALCR knockdown significantly alters the function of ANTXR1 and that ANTXR1 is a direct functional partner of CALCR. The downregulation of ANTXR1 and other key signaling molecules further underscores the role of CALCR in regulating critical pathways involved in cell cycle progression, apoptosis, and tumorigenesis.
ANTXR1 enhances CALCR’s role in cell proliferation and migration, and promotes gastric carcinogenesis
Subsequently, to investigate the interaction between CALCR and ANTXR1, we designed a gene rescue assay using several human gastric cancer cell lines, including MGC-803, MKN-45, and HGC-27. We observed that CALCR was significantly less expressed in MGC-803 cells (P < 0.001) (Fig. 5A). Among various shRNAs, shCALCR-1 showed the highest knockdown efficiency in MGC-803 cells (Fig. 5B). In our experiments, over 80% of the cells were successfully infected with lentivirus, and the cells remained in good condition (Fig. 5C).
Co-immunoprecipitation assay and Gene rescue assays showed close relationship between ANTXR1 and CALCR. (A) The results of qRT-PCR showed different mRNA expression of CALCR among MGC-803, MKN-45, HGC-27 cell lines. (B) Relative mRNA level of CALCR was detected by qRT-PCR in MGC-803 cell line after CALCR expression was interfered by different RNAi. (C) Cells infected by lentivirus were observed under bright-field microscope and fluorescent microscope. (D) Relative mRNA level of ANTXR1 and CALCR in different experimental groups after lentivirus infection. (E) The expression of protein level of ANTXR1 and CALCR in different experimental groups after lentivirus infection. (F) Whole-cell imaging and analysis was performed using Celigo in 4 different groups. (G) Cell sorting and profiling in 4 different groups were performed using fluorescence activated cell sorting (FACS). The apoptosis rate in 4 different groups was reviewed. (H) Colony formation was observed and cell colony number was calculated in 4 different groups. (I) Photographs of wound-healing assay probing collective cell migration in two dimensions in 4 different groups were taken at 48 h, and migration rate was then calculated. (J) Photographs of transwell assay performed in 4 different groups was taken under microscope, and migration rate was then calculated. Experiments were repeated at least three times. Asterisks (*) indicate a significant difference compared with control groups (*P < 0.05, **P < 0.01 and ***P < 0.001).
The gene rescue experiments utilized qRT-PCR (Fig. 5D) and Western blot analysis (Fig. 5E) to reveal the intrinsic connection between CALCR and ANTXR1. Compared to the NC (OE + KD) group, the mRNA level of ANTXR1 was reduced in the shCALCR + NC-ANTXR1 group, where only CALCR was knocked down (P < 0.001). In the ANTXR1 + shCALCR group, which backfilled ANTXR1, the CALCR mRNA levels were significantly higher compared to the shCALCR + NC-ANTXR1 group with lower CALCR levels. However, overexpressing ANTXR1 alone in the ANTXR1 + NC-shCALCR group did not significantly change CALCR mRNA levels compared to the NC (OE + KD) group. These findings suggest that down-regulation of CALCR decreases ANTXR1 expression, and restoring ANTXR1 expression at low CALCR levels reverses this effect. Overexpression of ANTXR1 alone does not increase CALCR expression, confirming that ANTXR1 is downstream of CALCR.
In terms of cell proliferation and migration, the Celigo cell counting assay showed that cells in the ANTXR1 + NC-shCALCR group proliferated faster (P < 0.05) compared to the NC (OE + KD) group, while cells in the shCALCR + NC-ANTXR1 group proliferated slower (P < 0.001) (Fig. 5F). Additionally, cells in the ANTXR1 + shCALCR group proliferated slower compared to the ANTXR1 + NC-shCALCR group (P < 0.01), and faster compared to the shCALCR + NC-ANTXR1 group (P < 0.001). Flow cytometry (Fig. 5G) and colony formation assays (Fig. 5H) supported these results.
The wound-healing assay (Fig. 5I) and transwell assay (Fig. 5J) indicated an increased migration rate in the ANTXR1 + NC-shCALCR group and a decreased migration rate in the shCALCR + NC-ANTXR1 group compared to the NC (OE + KD) group. Moreover, the migration rate was reduced in the ANTXR1 + shCALCR group compared to the ANTXR1 + NC-shCALCR group, and increased compared to the shCALCR + NC-ANTXR1 group.
Based on these comprehensive results, we conclude that CALCR plays a critical role in the development and progression of gastric cancer by regulating ANTXR1. The CALCR-ANTXR1 axis significantly influences cellular proliferation and migration, thereby promoting gastric carcinogenesis. This relationship underscores the importance of CALCR as a potential therapeutic target, offering new insights into gastric cancer treatment strategies aimed at disrupting this pathway.
Discussion
This study investigates the role of the calcium signal receptor CALCR in the development of gastric cancer (GC), particularly its potential impact on tumor metastasis and prognosis.
Tumor metastasis is recognized as a primary cause of mortality in advanced gastric cancer (GC) patients. Despite extensive research, the mechanisms driving this process remain elusive23. Current treatment options for metastatic gastric cancer are limited, contributing to generally poor prognosis24.
G-protein-coupled receptors (GPCRs) are seven-transmembrane receptors responsible for regulating and transmitting extracellular signals, thereby initiating various intracellular signaling pathways25. It has been reported that they participate in the development of various cancers11,26. As a member of the GPCR family, CALCR plays a central role in calcium ion channel-related cellular signaling processes. CALCR (formerly known as CTR) is located on chromosome 7q21.3 and encodes the calcitonin receptor protein27. Acting as a calcitonin receptor and an extracellular calcium ion sensor27, the role of CALCR in GC progression and metastasis has not been fully evaluated.
This study initially observed high expression of CALCR in gastric cancer tissues and confirmed this through analysis of 230 gastric cancer and adjacent tissue samples (Fig. 1B). This suggests that CALCR may play an important role in the development of gastric cancer. Furthermore, principal component analysis (PCA) confirmed significant differences in CALCR expression between gastric cancer and non-tumor tissues (Fig. 1C, D). Additionally, CALCR expression was found to significantly correlate with overall survival and disease-free survival in gastric cancer patients, indicating its potential role as a prognostic indicator (Fig. 1F).
In vitro experiments involved specific knockdown of CALCR expression, revealing significantly decreased proliferation rates and increased apoptosis rates in gastric cancer cell lines (AGS and SGC-7901), alongside reduced cell migration and invasion capabilities (Fig. 2). These results demonstrate that CALCR plays a crucial role in the survival, proliferation, and migration processes of gastric cancer cells.
Our in vivo experiments using nude mouse models further confirmed that CALCR knockdown significantly inhibited tumor growth and metastasis (Fig. 3), consistent with the results of in vitro experiments, emphasizing the potential value of targeting CALCR in gastric cancer treatment strategies.
Recent studies have identified ANTXR1 (tumor endothelial marker 8, TEM8) as a key factor in extracellular matrix homeostasis28, angiogenesis29, and cell migration30, closely associated with tumor occurrence and metastasis development. In 2020, Huang et al. identified ANTXR1 as a prognostic biomarker associated with GC progression and an activator of the PI3K/AKT/mTOR signaling pathway31. The protein–protein interaction network we constructed revealed that CALCR could indirectly regulate the cell cycle through interactions with THBS1 and CCNB1. Additionally, CALCR-related downstream genes were found to be involved in the PI3K/AKT signaling pathway, ERK/MAPK signaling pathway, NF-κB signaling pathway, and G1/S checkpoint regulatory pathway (Fig. 4A, B). Specifically, we confirmed through immunoprecipitation experiments the interaction between CALCR and ANTXR1 (Fig. 4E), extensively exploring this interaction’s role in gastric cancer progression. In vitro experiments revealed that ANTXR1 overexpression promotes CALCR expression, while CALCR downregulation decreases ANTXR1 expression. This confirmed ANTXR1 as a direct functional target of CALCR, with the CALCR/ANTXR1 axis regulating GC cell proliferation, migration, invasion, and apoptosis.
We also demonstrated CALCR’s involvement in the PI3K-AKT pathway, where ANTXR1 acts as a downstream gene crucial in regulating GC biological functions. This discovery provides new directions for future research, suggesting that CALCR may activate the AKT signaling pathway through interaction with ANTXR1, thereby promoting tumor growth and migration.
Additionally, besides CALCR itself, the tumor microenvironment plays a critical role in gastric cancer progression. Tumor metastasis is driven not only by accumulated intrinsic changes in malignant cells but also significantly regulated by various immune and stromal components in the tumor microenvironment32,33. CALCR expression, activation, and interactions with other cells may influence cellular behaviors related to tumor microenvironment regulation, thereby affecting tumor growth and dissemination.
While our study reveals the significant role of CALCR in gastric cancer development and its potential as a therapeutic target, we acknowledge several limitations, such as the detailed molecular mechanisms linking the CALCR/ANTXR1 axis to the AKT signaling pathway and its specific functions in different gastric cancer subtypes. Moreover, limitations of in vivo models need to be overcome through further experiments. Nevertheless, the CALCR/ANTXR1 axis can still be considered an effective biomarker influencing GC patients.
In summary, our study identifies CALCR as a crucial factor in the progression and metastasis of gastric cancer. The interaction between CALCR and ANTXR1 activates the AKT signaling pathway, promoting tumor growth and metastatic potential. These findings highlight the potential of CALCR as a prognostic marker and a therapeutic target in GC. By targeting the CALCR-ANTXR1 axis, novel therapeutic strategies could be developed to improve the prognosis and treatment outcomes for GC patients.
Further research should focus on understanding the detailed molecular mechanisms of CALCR in GC and exploring its interactions within the tumor microenvironment. Clinical trials evaluating the efficacy of CALCR-targeted therapies could pave the way for new, effective treatments for gastric cancer. Our study provides a foundation for future investigations into the role of GPCRs in cancer and their potential as targets for cancer therapy.
Data availability
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the small possibility of compromising the individual privacy of patients.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. https://doi.org/10.3322/caac.21492 (2018).
Guo, Q. et al. Analysis of a cytoskeleton-associated kinase PEAK1 and E-cadherin in gastric cancer. Pathol. Res. Pract. 210, 793–798. https://doi.org/10.1016/j.prp.2014.09.013 (2014).
Ilson, D. H. Advances in the treatment of gastric cancer. Curr. Opin. Gastroenterol. 35, 551–554. https://doi.org/10.1097/MOG.0000000000000577 (2019).
Masi, L. & Brandi, M. L. Calcitonin and calcitonin receptors. Clin. Cases Miner. Bone Metab. 4, 117–122 (2007).
Turner, A. G. et al. The role of the calcitonin receptor in protecting against induced hypercalcemia is mediated via its actions in osteoclasts to inhibit bone resorption. Bone 48, 354–361. https://doi.org/10.1016/j.bone.2010.09.013 (2011).
Cappagli, V. et al. Calcitonin receptor expression in medullary thyroid carcinoma. PeerJ 5, e3778. https://doi.org/10.7717/peerj.3778 (2017).
Terra, S. R. et al. STC1 interference on calcitonin family of receptors signaling during osteoblastogenesis via adenylate cyclase inhibition. Mol. Cell. Endocrinol. 403, 78–87. https://doi.org/10.1016/j.mce.2015.01.010 (2015).
Nakamura, M., Han, B., Nishishita, T., Bai, Y. & Kakudo, K. Calcitonin targets extracellular signal-regulated kinase signaling pathway in human cancers. J. Mol. Endocrinol. 39, 375–384. https://doi.org/10.1677/JME-07-0036 (2007).
Wookey, P. J. et al. The expression of calcitonin receptor detected in malignant cells of the brain tumour glioblastoma multiforme and functional properties in the cell line A172. Histopathology 60, 895–910. https://doi.org/10.1111/j.1365-2559.2011.04146.x (2012).
Sabbisetti, V. S. et al. Calcitonin increases invasiveness of prostate cancer cells: role for cyclic AMP-dependent protein kinase A in calcitonin action. Int. J. Cancer 117, 551–560. https://doi.org/10.1002/ijc.21158 (2005).
Pal, J. et al. Loss-of-function mutations in calcitonin receptor (CALCR) identify highly aggressive glioblastoma with poor outcome. Clin. Cancer Res. 24, 1448–1458. https://doi.org/10.1158/1078-0432.CCR-17-1901 (2018).
Kale, A., Aldahish, A. & Shah, G. Calcitonin receptor is required for T-antigen-induced prostate carcinogenesis. Oncotarget 11, 858–874. https://doi.org/10.18632/oncotarget.27495 (2020).
Chaudhary, A. et al. TEM8/ANTXR1 blockade inhibits pathological angiogenesis and potentiates tumoricidal responses against multiple cancer types. Cancer Cell 21, 212–226. https://doi.org/10.1016/j.ccr.2012.01.004 (2012).
Rmali, K. A. et al. Tumour endothelial marker 8 (TEM-8) in human colon cancer and its association with tumour progression. Eur. J. Surg. Oncol. 30, 948–953. https://doi.org/10.1016/j.ejso.2004.07.023 (2004).
Szot, C. et al. Tumor stroma-targeted antibody-drug conjugate triggers localized anticancer drug release. J. Clin. Invest. 128, 2927–2943. https://doi.org/10.1172/JCI120481 (2018).
Davies, G. et al. Elevated levels of tumour endothelial marker-8 in human breast cancer and its clinical significance. Int. J. Oncol. 29, 1311–1317 (2006).
Byrd, T. T. et al. TEM8/ANTXR1-specific CAR T cells as a targeted therapy for triple-negative breast cancer. Can. Res. 78(2), 489–500 (2018).
Sotoudeh, M. et al. ANTXR1 (TEM8) overexpression in gastric adenocarcinoma makes the protein a potential target of immunotherapy. Cancer Immunol. Immunother. 68, 1597–1603. https://doi.org/10.1007/s00262-019-02392-y (2019).
Remmele, W. & Stegner, H. E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 8, 138–140 (1987).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951. https://doi.org/10.1002/pro.3715 (2019).
Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Ishiguro-Watanabe, M. KEGG: Biological systems database as a model of the real world. Nucl. Acids Res. 53, D672-d677. https://doi.org/10.1093/nar/gkae909 (2025).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucl. Acids Res. 28, 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Hugen, S., Thomas, R. E., German, A. J., Burgener, I. A. & Mandigers, P. J. J. Gastric carcinoma in canines and humans, a review. Vet. Comp. Oncol. 15, 692–705. https://doi.org/10.1111/vco.12249 (2017).
Seeneevassen, L. et al. Gastric cancer: Advances in carcinogenesis research and new therapeutic strategies. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22073418 (2021).
Nugent, A. & Proia, R. L. The role of G protein-coupled receptors in lymphoid malignancies. Cell Signal 39, 95–107 (2017).
He, T. & Ling, F. CALCR knockdown inhibits the development and progression of non-small-cell lung cancer. Carcinogenesis 42, 1390–1398. https://doi.org/10.1093/carcin/bgab076 (2021).
Jurado, L. A. P., Li, X. & Francke, U. The human calcitonin receptor gene (CALCR) at 7q21.3 is outside the deletion associated with the Williams syndrome. Cytogenetics Cell Genetics 70(3–4), 246–249 (1995).
Hu, K., Olsen, B. R. & Besschetnova, T. Y. Cell autonomous ANTXR1-mediated regulation of extracellular matrix components in primary fibroblasts. Matrix Biol. 62, 105–114. https://doi.org/10.1016/j.matbio.2016.12.002 (2017).
Li, M. et al. N-Myc promotes angiogenesis and therapeutic resistance of prostate cancer by TEM8. Med. Oncol. 38, 127. https://doi.org/10.1007/s12032-021-01575-x (2021).
Sergeeva, O. A. & van Der-Goot, F. G. Converging physiological roles of the anthrax toxin receptors. F1000Res 8, 1415 (2019).
Cai, C. et al. Anthrax toxin receptor 1/tumor endothelial marker 8 promotes gastric cancer progression through activation of the PI3K/AKT/mTOR signaling pathway. Cancer Sci. 111, 1132–1145. https://doi.org/10.1111/cas.14326 (2020).
Bubnovskaya, L. & Osinsky, D. Tumor microenvironment and metabolic factors: contribution to gastric cancer. Exp. Oncol. 42, 2–10. https://doi.org/10.32471/exp-oncology.2312-8852.vol-42-no-1.14056 (2020).
Rojas, A., Araya, P., Gonzalez, I. & Morales, E. Gastric tumor microenvironment. Adv. Exp. Med. Biol. 1226, 23–35. https://doi.org/10.1007/978-3-030-36214-0_2 (2020).
Acknowledgements
The authors wish to thank Anyang Tumor Hospital for their dedicated efforts to provide gastric cancer tissues from patients.
Funding
This research was funded by the CAMS (Chinese Academy of Medical Sciences) Innovation Fund for Medical Sciences (CIFMS; 2023-I2M-C&T-B-026).
Author information
Authors and Affiliations
Contributions
Methodology: H.L. and Q.G. Software: J.L. and Z.Y. (Zihan Yang). Validation: H.L., J.L., and Q.G. Formal Analysis: H.L. and Q.G. Investigation: H.L., L.L. (Lele Lin), D.S. (Dike Shi), Y.C. (Yiming Chu), and Y.C. (Yanna Cai). Resources: B.L. (Baozhong Li). Data Curation: H.L. and Z.Y. Writing—Original Draft Preparation: Z.Y. and J.H. (Jingbo Huang). Writing—Review and Editing: H.L., J.H., and Q.G. Visualization: J.L. and H.L. Supervision: D.W. (Dan Wu), J.L., and Q.G. Project Administration: Q.G.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Anyang Tumor Hospital (protocol code 2023WZ01K01). The animal study protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine (protocol code AIRB-2023–0513).
Consent to participate
Informed consent was obtained from all subjects involved in the study. The authors confirm that all experiments were performed in accordance with relevant guidelines and regulations. The authors also confirm that this study has been reported in accordance with the ARRIVE guidelines.
Consent for publication
All authors have read and agreed to the published version of the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hongbo Li, Zihan Yang, Jingbo Huang have shared first authorship.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, H., Yang, Z., Huang, J. et al. CALCR interaction with ANTXR1 drives gastric tumor growth and metastasis via AKT signaling pathway. Sci Rep 15, 11826 (2025). https://doi.org/10.1038/s41598-025-96310-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-96310-1