Abstract
Given the increasing prevalence of chronic diseases in the aging population, it is of great importance to gain an understanding of how changes in body composition affect health outcomes. Handgrip strength (HGS) serves as a valuable proxy for overall muscle strength, while relative HGS (RHGS) adjusts for body size, providing a more accurate assessment of the relationship between muscle strength and metabolic disease. Lipid accumulation products (LAP) are an indicator that can reflect visceral lipid accumulation. Based on previous studies, the relationship between LAP and RHGS has not been explored. This study aims to address this gap in the literature and provide insights for public health recommendations. Data was collected and extracted from the 2011–2014 National Health and Nutrition Examination Survey (NHANES) database. LAP was calculated from the arithmetic product of waist circumference (WC) and fasting plasma triglyceride (TG), the calculation as following formulas: for females, [WC (cm) − 58] × [TG (mmol/l)], and for males, [WC (cm) − 65] × [TG (mmol/l)]; RHGS was derived from the HGS to BMI ratio. The correlation between the variables was initially explored using multivariate linear regression. Secondly, smoothed-fitted curves were used to investigate the non-linear relationship between the variables. The inflection point values were determined based on the results of the threshold effect analysis. Subgroup analyses were also conducted to assess the stability of the relationship between the variables in different populations. The study analyzed 3990 patients. After accounting for different covariates, multivariate linear regression analysis demonstrated a significant negative correlation between increased levels of LAP and increased levels of RHGS (beta coefficient = -0.0020; 95% confidence interval CI: -0.0023 to -0.0017; P < 0.0001). The interaction test did not have a statistically significant effect on this association. Furthermore, curve fit and threshold effect analysis demonstrated a non-linear relationship with a breakpoint at 49.8083 cm·mmol/L. The results of this study demonstrate an inverse relationship between LAP and RHGS in various populations in the United States. These findings provide compelling evidence of the clinical significance of LAP as a predictor of RHGS, offering valuable insights for developing early intervention strategies in high-risk populations.
Similar content being viewed by others
Introduction
In the rapidly aging global population, there is a consequential rise in the prevalence, morbidity, and mortality of chronic diseases, underscoring the urgent need for research into the multifaceted aspects of aging and its impact on health1. Notably, aging is intricately associated with alterations in body composition and an increase in the sarcopenic obesity phenotype, where the interplay between diminished skeletal muscle mass and increased fat accumulation presents significant health risks2. Sarcopenic obesity, characterized by the coexistence of sarcopenia and obesity, notably central or visceral obesity, has been implicated in the pathogenesis of various cardiometabolic diseases and frailty, highlighting the critical role of muscle mass reduction and fat accumulation in chronic disease mechanisms during aging3. The clinical landscape offers a plethora of methodologies for measuring muscle mass, strength, and obesity. Dual-energy X-ray absorptiometry is commonly employed for quantifying muscle and fat mass, providing insights into body composition dynamics essential for diagnosing conditions like sarcopenia or central obesity. However, these methods fall short of assessing muscle strength, particularly for screening purposes4. In this regard, handgrip strength (HGS) has emerged as a valuable, sensitive, and cost-effective proxy for overall muscle strength, despite its interpretation being influenced by body composition5,6. Given the varying methodologies in HGS measurement across studies, the need for a standardized approach to HGS interpretation is evident. Some research calculates absolute HGS by summing the maximum HGS of both hands or using the maximal or average HGS of the dominant hand. In contrast, others adjust for body size by dividing absolute HGS by body mass index (BMI) to derive relative HGS (RHGS)7,8. This adjustment provides a more accurate representation of muscle strength relative to body size, offering valuable insights for screening sarcopenic obesity. Due to its non-invasive nature, simplicity, and cost-effectiveness, RHGS has gained prominence in epidemiological and clinical research in recent years.
The use of lipid accumulation products (LAP) as biomarkers enables the estimation of the degree of lipid accumulation within the body9. This estimation is derived through the calculation of waist circumference (WC) and fasting plasma triglyceride (TG) levels. The waist circumference is a surrogate marker for abdominal fat, while triglyceride levels reflect the levels of circulating lipids. Both factors have been identified as significant predictors of metabolic syndrome and related conditions, thus establishing the association of LAP with metabolic diseases including type 2 diabetes10, metabolic dysfunction-associated fatty liver disease11 and metabolic syndrome9. Furthermore, LAP has emerged as a critical parameter in lipidology, with ongoing research over recent years exploring its broader clinical applications, including its role in osteoarthritis12, atherosclerosis13, and testosterone deficiency14. However, no studies have yet reported on the relationship between LAP and RHGS, a potential indicator of muscle waste.
The National Health and Nutrition Examination Survey (NHANES), conducted by the National Center for Health Statistics under the auspices of the Centers for Disease Control and Prevention, has emerged as a pivotal tool for assessing and monitoring the health status of U.S. citizens, owing to its comprehensive data scope and continuous updates15. Consequently, the objective of this study is to analyze data from the NHANES to explore the possible association between LAP and RHGS among adults in the United States. The aim is to offer evidence-based advice for public health suggestions.
Methods
Survey description and study population
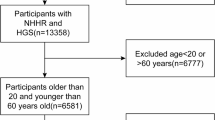
This study utilized data collected from two biennial survey cycles, encompassing the years between NHANES 2011 and 2014. To enhance the accuracy and dependability of the research findings, rigorous exclusion criteria were implemented as follows: (1) Participants younger than 20 years were excluded (N = 8,602). (2) Individuals who lacked RHGS data or had undergone previous hand surgery were also excluded (N = 1,859). (3) Participants missing LAP data were excluded (N = 5,140). (4) Those without data on the covariates were excluded (N = 340).
Assessment of LAP and RHGS
Consistent with previous research utilizing data from NHANES, the calculation of the LAP index is based on the following formulas: for females, [WC (cm) − 58] × [TG (mmol/l)], and for males, [WC (cm) − 65] × [TG (mmol/l)]11,16. In addition, RHGS is calculated using the following formula: RHGS = Absolute Grip Strength (kg)/ BMI (kg/m2)7,17. Consistent with our previous reports, the three groups of LAP and RHGS were divided into three equal groups based on the number of people18,19.
Covariate definitions
Based on previously published articles7,20,21,22,23,24, the following potential factors that may influence RHGS outcomes were selected as confounders for this study: gender (male, female), age, race (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other race), marital status (married or single), educational status (less than high school, more than high school), Family poverty-to-income ratio (PIR), drinking habits (yes, no), smoking habits (yes, no), presence of hypertension (yes, no) and diabetes (yes, no), vigorous daily activity (yes, no), normal daily activity (yes, no), smoking habits (current, former, never), presence of hypertension (yes, no) and diabetes (yes, no), strenuous daily activities versus normal daily activities (yes, no). Concerning the aforementioned covariates, the participants were classified into two groups based on their smoking status: non-smokers and smokers. This was determined by inquiring about the number of cigarettes smoked in their lifetime and whether they currently smoked. Similarly, subjects were categorized into two groups based on whether they had consumed 12 cups of alcohol in their lifetime25. Disease status and activity data were collected through questionnaires. Participants were asked to indicate whether they had been informed of a disease by a doctor or other health professional and whether they engaged in strenuous or moderate physical activity regularly.
Statistical analysis
Following our previously reported research methods25,26,27,28, all statistical analyses were conducted using the R software (version 4.2.2) and EmpowerStats (Version 2.0), with the significance level set at P < 0.05. Firstly, the mean ± standard deviation was employed to describe continuous variables. Subsequently, a Kruskal-Wallis test was conducted to assess any differences, which will be derived using Fisher exact probability test if the number of valid data is insufficient. Categorical variables were described using frequencies and percentages, and differences were assessed using chi-square tests. To investigate the possible relationship between LAP and RHGS, multivariable linear regression analysis was performed. In this study, three different models were used to elucidate the association between LAP and RHGS. Model 1 was unadjusted and served as the baseline. Model 2 was revised for demographic variables such as gender, age, race, marital status, and PIR. Model 3, our core model, extended Model 2 by including additional variables such as educational status, diabetes status, hypertension status, hyperlipidemia status, alcohol consumption status, smoking status, moderate work, recreational activity, vigorous work and recreational activity. To further explore potential nuances within specific demographics, the same statistical methodology was applied to subgroups defined by age, BMI, and diabetes status. Smooth fitting curves were generated using the R language (mgcv package) to explore the non-linear relationship between LAP and RHGS. Inflection points were identified using a piecewise regression model combined with the second-order derivative method, offering deeper insights into their association29. Additionally, acknowledging potential discrepancies in the LAP calculation formula across genders, a gender-specific sensitivity analysis was conducted.
Results
Population baseline characteristics
Figure 1 illustrates the specifics of the patient inclusion process, a total of 3990 individuals met the inclusion criteria and were included in the analysis. Table 1 lists the baseline characteristics of the study participants who were triple categorized by RHGS. The mean age of participants was 48.10 ± 17.48 years, and 50.08% were female. In terms of racial demographics, 43.56% of respondents identified as non-Hispanic white, 21.28% as non-Hispanic black, 11.20% as Mexican Americans, 8.87% as people from other Hispanic backgrounds, and 15.09% as members of other racial groupings. In terms of educational status, 20.63% of the participants had not graduated from high school, while 79.37% of the participants had an educational status higher than high school. Table 1 divides the number of patients into three equal parts according to the RHGS distribution. As the RHGS level, the LAP level decreased significantly. Additionally, the data were trichotomized based on the exposure indicator LAP, and the results for the LAP trichotomies and all participants are presented in Fig. S1. The results indicate that the higher RHGS level exhibited a decreasing trend as the lower LAP level.
Associations between LAP and RHGS
As shown in Table 2, Model 1 (without any covariate adjustment) showed that the higher level in LAP was associated with the lower level in RHGS. This negative correlation remained even after adjusting for demographic and socio-economic variables in Model 2 (β = -0.0024; 95% CI = -0.0027 to -0.0021; P < 0.0001) and was still significant after further adjustment for health-related behaviors and conditions in Model 3 (β = -0.0020; 95% CI = -0.0023 to -0.0017; P < 0.0001). In addition, trend analyses of LAP tertiles confirmed that higher levels of LAP were consistently associated with lower RHGS (P for trend values < 0.0001).
Subgroup analysis
Table 3 showed the correlation between LAP and RHGS in various subgroups of demographic and health-related variables. The results showed that the negative correlation between LAP and RHGS remained stable across populations (all P<0.05).
Smooth curve fitting and threshold effect analysis
A non-linear relationship between the LAP index and RHGS was found using smooth curve fitting as shown in Fig. 2A. Table 4 showed that 49.8083 cm·mmol/L cut-off point was obtained using the threshold effect analysis. There was also a significant correlation between LAP and RHGS to the left of the cut-off point (β = -0.0227; 95% CI = -0.0246 to -0.0208; P < 0.0001). Conversely, on the right side of the cut-off point, there was no statistically significant correlation between LAP and RHGS (β = -0.0002; 95% CI = -0.0007 to -0.0002; P = 0.3238). In addition, further comparations were made with different age groups, as shown in Fig. 2B, the negative correlation between LAP and RHGS remained stable, but with slightly different inflection points. The inflection point for elderly patients was 87.7784 cm·mmol/L, which was significantly higher than that for young and middle-aged patients.
Sensitivity analysis
Given the differing methodologies employed for calculating LAP in males and females, separate multiple linear regression analyses were conducted for males and females, respectively, to further elucidate the relationship between LAP and RHGS in the two distinct sex groups. The results presented in Table 5 indicate that in Model 1, a negative relationship between LAP and RHGS was present in both males and females (95% CI: -0.0049 to -0.0037, P < 0.0001; 95% CI: -0.0023 to -0.0017, P < 0.0001). The results of which were comparable for Model 2 (95% CI: -0.0043 to -0.0033, P < 0.0001; 95% CI: -0.0018 to -0.0013, P < 0.0001). The results of Model 3 (95% CI: -0.0039 to -0.0028, P < 0.0001; 95% CI: -0.0015 to -0.0010, P < 0.0001) indicate the significant negative correlation between LAP and RHGS. In addition, trend analyses of LAP tertiles also confirmed that higher LAP levels were associated with lower RHGS, with the most pronounced decrease in the third tertile. A statistically significant negative association between LAP and RHGS was found in three different adjusted models (all P < 0.05). As shown in Fig. 3(A, B, C and D), a non-linear relationship between LAP index and RHGS was found in both males and females by smoothing curve fitting. As shown in Table S2, the inflection point of 53.5805 cm·mmol/L for the male group and 58.3712 cm·mmol/L for the female group was obtained by threshold effect analysis.
Discussion
This cross-sectional study, involving a cohort of 3990 eligible participants from NHANES 2011–2014, revealed a significant negative association between LAP and RHGS. The robustness of this correlation was maintained following rigorous triangulation of LAP and meticulous adjustment for relevant confounders in the comprehensively adjusted model. The subgroup analyses further corroborate the stability of the negative correlation across diverse populations. Additionally, smooth-fit curves and threshold effect analyses substantiated the negatively correlated nonlinear relationship between LAP and RHGS. We also calculated the turning point of LAP to be 49.8083 cm·mmol/L. Gender-based sensitivity analyses also underscored the consistency of this negative correlation. These findings highlight the critical importance of LAP in understanding the likelihood of diminished RHGS.
The LAP has emerged over the past decade as a significant marker, capturing the convergence of visceral fat accumulation and dysregulated lipid metabolism. Considering its association with heightened risks for diabetes and cardiovascular disease, particularly within the metabolically obese normal-weight phenotype, Du et al.30 evaluated data from normal-weight subjects from the 2009 nationwide China Health and Nutrition Survey. Their analysis concluded that LAP provides a more robust metric than traditional anthropometric measurements, including BMI, waist circumference, waist-to-hip ratio, and waist-to-height ratio. Mazidi et al.31 validated the LAP index as an accessible, cost-effective, and reasonably precise surrogate for insulin resistance detection in the American adult populace, employing the homeostatic model assessment. They found that LAP boasts greater predictive capability than other conventional screening tools, such as TG and high-density lipoprotein. However, no studies have investigated the correlation between LAP and RHGS, a useful indicator of total body muscle strength and function.
As far as we know, this investigation stands as the inaugural cross-sectional study to scrutinize the association between LAP and RHGS, marking a novel venture into understanding the nexus between metabolic health and physical function. Previous research has explored the relationship of RHGS with various health outcomes across different populations, employing a myriad of epidemiological approaches. In a study involving individuals aged ≥ 45 years from the China Health and Retirement Longitudinal Study (CHARLS) cohort (2011–2012), He et al.32 observed a potential correlation between reduced RHGS and heightened diabetes risk, particularly among obese subjects. Within the Korean Genome and Epidemiology Study (2013–2014) in Anseong, Gyeonggi Province, Park et al.33 observed that individuals in the intermediate and high RHGS groups exhibited a 27% and 55% lower incidence of metabolic syndrome, respectively. This suggests that there may be a more pronounced relationship between metabolic syndrome and RHGS than previously thought. In Riyadh, Saudi Arabia, Alkahtani et al.34 revealed that RHGS had a more substantial association with Metabolic Syndrome than HGS alone, especially in males, highlighting the importance of relative strength measures. Luo et al.35, analyzing data from participants aged ≥ 45 from the 2011 CHARLS, concluded that enhanced relative muscle strength serves as an independent protective factor against hypertension, underlining the potential benefits of improving muscle strength in hypertension management. Chi et al.36 implemented an examination from the Korean NHANES and found RHGS to be a superior predictor of myocardial infarction and angina than absolute HGS, with this association being notably stronger among females. Similarly, Wu et al.37 conducted a study that discovered that each incremental increase in RHGS was associated with a 9.2% reduction in Parkinson’s disease risk. Kuo et al.38, with the sample from the UK Biobank, confirmed the protective effect of RHGS against all dementia types. Zhang et al.39 explored female NHANES participants (2011–2014) and found an inverse relationship between RHGS levels and the risk and severity of stress urinary incontinence. Furthermore, Yang et al.40 conducted a study of females (average age 48.6 years) who participated in self-referred health screenings between April 2015 and December 2019 and reported a positive correlation between decreased grip strength and urinary ailments such as frequency, nocturia, urgency, and incontinence. Taken together, these studies underscore the pivotal function of RHGS as a readily available and non-invasive biomarker for evaluating an individual’s comprehensive health risk and condition, beyond a mere assessment of muscle mass. This body of evidence strongly supports the integration of RHGS into clinical evaluations and public health initiatives, aimed at monitoring and improving bodily functions and overall well-being.
The precise mechanism of the link between LAP and RHGS remains elusive. To further expand on the potential mechanisms between elevated LAP levels and reduced RHGS, it is critical to consider the multifactorial pathways through which lipids may influence muscle physiology and function41,42. Excessive lipid accumulation within skeletal muscle fibers plays a critical role in the pathogenesis of muscle atrophy and insulin resistance, a phenomenon underpinned by several interrelated mechanisms. At the cellular level, lipids serve as an essential energy source, with lipid oxidation accounting for a significant portion of resting skeletal muscle energy production43. However, beyond a certain threshold, lipid accumulation can lead to lipotoxic effects, characterized by the detrimental accumulation of lipid intermediates such as diacylglycerols and ceramides44,45. These intermediates disrupt normal insulin signaling pathways by activating serine/threonine kinases that impair insulin receptor activity, leading to decreased glucose transporter 4 translocation and reduced glucose uptake into muscle cells46. This cascade fosters insulin resistance within skeletal muscle tissues. Moreover, the excessive uptake of fatty acids can overwhelm mitochondrial capacity, leading to increased production of mitochondrial reactive oxygen species further exacerbating insulin resistance47. The dysregulation of fatty acid uptake, mediated by proteins such as fatty acid-binding protein 3 and cluster of differentiation 36, contributes to this process by facilitating the entry of FAs into muscle cells beyond their oxidative capacity48. This imbalance between fatty acid uptake and beta-oxidation initiates a series of metabolic disturbances, including impaired insulin action. Intramyocellular lipid droplets, coated by proteins like perilipins (PLINs), play a pivotal role in buffering against lipid excess by storing triglycerides. However, the pathological increase in LDs, while sometimes protective, can also signify an overwhelmed system, especially evident in conditions like sarcopenia and diabetes, where PLINs expression and function may be dysregulated. Notably, PLIN2 and PLIN5 have emerged as key players in muscle lipid metabolism, with PLIN2 overexpression linked to exacerbated lipotoxic effects and insulin signaling impairments, while PLIN5 appears to protect against lipotoxicity by promoting lipid storage in LDs and facilitating their oxidation49,50.
Furthermore, the vicious cycle between local musculoskeletal degeneration and muscle insulin resistance can now interact with local adipose inflammation, forming a larger pathological loop that culminates in an inflammatory storm51. Elevated levels of TNF-α and IL-6 activate the ubiquitin-proteasome system, particularly upregulating ubiquitin ligases such as muscle RING finger 1, which accelerates muscle protein degradation, leading to muscle atrophy and strength loss52,53,54. Additionally, chronic inflammation exacerbates mitochondrial dysfunction, increasing reactive oxygen species production, disrupting mitophagy, and impairing mitochondrial biogenesis, ultimately depleting ATP supply54,55. This energy deficit directly compromises cross-bridge cycling efficiency, reduces muscle fiber contractility, and ultimately impairs overall muscle strength and motor function.
Clinical Implications
Our study reveals that LAP can serve as an early warning indicator for muscle strength decline, potentially enabling timely interventions. Healthcare providers can leverage this metric to develop personalized prevention strategies targeting lifestyle modifications, nutritional interventions, and exercise prescriptions. Moreover, the simplicity of measuring LAP and RHGS makes this approach particularly attractive for large-scale screening programs, especially in aging populations where muscle strength and metabolic health are critical indicators of overall well-being. The potential for early detection and proactive management could significantly reduce healthcare costs and improve patient outcomes by addressing metabolic and muscular decline before they progress to more serious health complications.
Strengths and limitations
Based on a large sample from NHANES, this cross-sectional study demonstrates several strengths including robust statistical power and generalizability due to its extensive cohort size. The study design incorporated rigorous triangulation of LAP and detailed adjustment for numerous potential confounders, ensuring the stability and reliability of the identified negative association between LAP and RHGS. The use of diverse analytical strategies, such as subgroup analyses, smooth-fit curves, and threshold effect analyses, provided comprehensive insights into the non-linear dynamics of the association, enhancing the depth and robustness of the findings. Additionally, gender-based sensitivity analyses bolstered confidence in the consistency of this correlation across different demographics.
However, as a cross-sectional analysis, it cannot inherently establish causality, only permitting the identification of associations. Consequently, it must be confirmed by future perspectives and experimental studies. Despite meticulous adjustments for confounders, the potential for residual confounding by unmeasured variables remains, which could skew the results. Moreover, the reliance on subgroup analyses, while illuminating, necessitates cautious interpretation due to the increased risk of type I errors arising from multiple comparisons. Lastly, the applicability may be constrained by the specifics of the NHANES dataset, limiting their extrapolation to other populations or geographic settings.
Conclusion
This study yielded intriguing insights into the inverse relationship between LAP and RHGS in diverse populations in the United States. It is acknowledged that there are still considerable gaps in our knowledge regarding the use of LAP as a predictor of muscular dystrophy. Further research is required to address this issue in greater depth. The findings of this study potentially represent a significant contribution to the growing body of evidence demonstrating the clinical utility of LAP as a predictor of muscular dystrophy and provide valuable insights into the development of early intervention strategies for potentially atrophic populations.
Data availability
The data underlying this article are available in the NHANES at https://wwwn.cdc.gov/Nchs/Nhanes/.
Abbreviations
- BMI:
-
Body mass index
- CHARLS:
-
China Health and Retirement Longitudinal Study
- CI:
-
Confidence interval
- HGS:
-
Handgrip strength
- LAP:
-
Lipid Accumulation Products
- NHANES:
-
National Health and Nutrition Examination Survey
- PIR:
-
Poverty-to-income ratio
- RHGS:
-
Relative handgrip strength
- TG:
-
Plasma triglyceride
- WC:
-
Waist circumference
References
The burden of diseases, injuries, and risk factors by state in the USA, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet (London England) 404, 2314–2340. https://doi.org/10.1016/s0140-6736(24)01446-6 (2024).
Kim, Y. M., Kim, S., Won, Y. J. & Kim, S. H. Clinical manifestations and factors associated with osteosarcopenic obesity syndrome: A Cross-Sectional study in Koreans with obesity. Calcif Tissue Int. 105, 77–88. https://doi.org/10.1007/s00223-019-00551-y (2019).
Cooper, C. et al. Frailty and sarcopenia: definitions and outcome parameters. Osteoporos. Int. 23, 1839–1848. https://doi.org/10.1007/s00198-012-1913-1 (2012).
Kelly, T. L., Wilson, K. E. & Heymsfield, S. B. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One 4, e7038. https://doi.org/10.1371/journal.pone.0007038 (2009).
Beaudart, C. et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 16, 170. https://doi.org/10.1186/s12877-016-0349-4 (2016).
Hardy, R. et al. Body mass index, muscle strength and physical performance in older adults from eight cohort studies: the halcyon programme. PLoS One. 8, e56483. https://doi.org/10.1371/journal.pone.0056483 (2013).
Lawman, H. G. et al. Associations of relative handgrip strength and cardiovascular disease biomarkers in U.S. Adults, 2011–2012. Am. J. Prev. Med. 50, 677–683. https://doi.org/10.1016/j.amepre.2015.10.022 (2016).
Lee, W. J., Peng, L. N., Chiou, S. T. & Chen, L. K. Relative handgrip strength is a simple indicator of cardiometabolic risk among Middle-Aged and older people: A nationwide Population-Based study in Taiwan. PLoS One. 11, e0160876. https://doi.org/10.1371/journal.pone.0160876 (2016).
Li, Y. et al. Association between four anthropometric indexes and metabolic syndrome in US adults. Front. Endocrinol. (Lausanne). 13, 889785. https://doi.org/10.3389/fendo.2022.889785 (2022).
Cho, H. E. et al. The relationship between the lipid accumulation product and Beta-cell function in Korean adults with or without type 2 diabetes mellitus: the 2015 Korea National health and nutrition examination survey. Endocr. Res. 47, 80–88. https://doi.org/10.1080/07435800.2022.2053540 (2022).
Li, H., Zhang, Y., Luo, H. & Lin, R. The lipid accumulation product is a powerful tool to diagnose metabolic dysfunction-associated fatty liver disease in the united States adults. Front. Endocrinol. (Lausanne). 13, 977625. https://doi.org/10.3389/fendo.2022.977625 (2022).
Huang, J. et al. Association between lipid accumulation products and osteoarthritis among adults in the united States: A cross-sectional study, NHANES 2017–2020. Prev. Med. 180, 107861. https://doi.org/10.1016/j.ypmed.2024.107861 (2024).
Zheng, L. et al. Association between visceral obesity and 10-year risk of first atherosclerotic cardiovascular diseases events among American adults: National health and nutrition examination survey. Front. Cardiovasc. Med. 10, 1249401. https://doi.org/10.3389/fcvm.2023.1249401 (2023).
Liu, Q., Huang, Y., Wang, M., Jiang, H. & Zhang, X. Association of lipid accumulation products with testosterone deficiency in adult American men: A cross-sectional study. Andrology 11, 551–560. https://doi.org/10.1111/andr.13355 (2023).
Curtin, L. R. et al. National Health and Nutrition Examination Survey: sample design, 2007–2010. Vital and health statistics. Series 2, Data evaluation and methods research, 1–23 (2013).
Yan, S. et al. Associations of serum carotenoids with visceral adiposity index and lipid accumulation product: a cross-sectional study based on NHANES 2001–2006. Lipids Health Dis. 22, 209. https://doi.org/10.1186/s12944-023-01945-6 (2023).
La, R. et al. Is inflammation a missing link between relative handgrip strength with hyperlipidemia? Evidence from a large population-based study. Lipids Health Dis. 23. https://doi.org/10.1186/s12944-024-02154-5 (2024).
Xu, B. et al. Is systemic inflammation a missing link between cardiometabolic index with mortality? Evidence from a large population-based study. Cardiovasc. Diabetol. 23, 212. https://doi.org/10.1186/s12933-024-02251-w (2024).
Wu, Q. et al. Association of reproductive lifespan and age at menopause with depression: data from NHANES 2005–2018. J. Affect. Disord. 356, 519–527. https://doi.org/10.1016/j.jad.2024.04.077 (2024).
Ji, C., Xia, Y., Tong, S., Wu, Q. & Zhao, Y. Association of handgrip strength with the prevalence of metabolic syndrome in US adults: the National health and nutrition examination survey. Aging (Albany NY). 12, 7818–7829. https://doi.org/10.18632/aging.103097 (2020).
Wen, Z. et al. Handgrip strength and muscle quality: results from the National health and nutrition examination survey database. J. Clin. Med. 12. https://doi.org/10.3390/jcm12093184 (2023).
Weng, L., Xu, Z., Chen, Y. & Chen, C. Associations between the muscle quality index and adult lung functions from NHANES 2011–2012. Front. Public. Health. 11, 1146456. https://doi.org/10.3389/fpubh.2023.1146456 (2023).
Williams, E. R., Wilson, H. K., Ross, R. E. & Gregory, C. M. Relative handgrip strength as a vitality measure in US stroke survivors. Disabil. Rehabil. 1–7. https://doi.org/10.1080/09638288.2024.2327488 (2024).
Xie, Z. et al. Mediation of 10-Year cardiovascular disease risk between inflammatory diet and handgrip strength: base on NHANES 2011–2014. Nutrients 15. https://doi.org/10.3390/nu15040918 (2023).
Yan, Y. et al. The association between remnant cholesterol and rheumatoid arthritis: insights from a large population study. Lipids Health Dis. 23, 38. https://doi.org/10.1186/s12944-024-02033-z (2024).
La, R. et al. Association between oxidative balance score and rheumatoid arthritis in female: a cross-sectional study. BMC Women’s Health. 24, 225. https://doi.org/10.1186/s12905-024-03066-3 (2024).
Yan, Y. et al. The association between triglyceride glucose index and arthritis: a population-based study. Lipids Health Dis. 22. https://doi.org/10.1186/s12944-023-01899-9 (2023).
Ding, W. et al. Associations between neutrophil percentage to albumin ratio and rheumatoid arthritis versus osteoarthritis: a comprehensive analysis utilizing the NHANES database. Front. Immunol. 16, 1436311. https://doi.org/10.3389/fimmu.2025.1436311 (2025).
Perperoglou, A., Sauerbrei, W., Abrahamowicz, M. & Schmid, M. A review of spline function procedures in R. BMC Med. Res. Methodol. 19, 46. https://doi.org/10.1186/s12874-019-0666-3 (2019).
Du, T., Yu, X., Zhang, J. & Sun, X. Lipid accumulation product and visceral adiposity index are effective markers for identifying the metabolically obese normal-weight phenotype. Acta Diabetol. 52, 855–863. https://doi.org/10.1007/s00592-015-0715-2 (2015).
Mazidi, M., Kengne, A. P., Katsiki, N., Mikhailidis, D. P. & Banach, M. Lipid accumulation product and triglycerides/glucose index are useful predictors of insulin resistance. J. Diabetes Complications. 32, 266–270. https://doi.org/10.1016/j.jdiacomp.2017.10.007 (2018).
He, Y. Y., Jin, M. L., Fang, X. Y. & Wang, X. J. Associations of muscle mass and strength with new-onset diabetes among middle-aged and older adults: evidence from the China health and retirement longitudinal study (CHARLS). Acta Diabetol. https://doi.org/10.1007/s00592-024-02265-6 (2024).
Park, D., Rho, J., Kim, Y. & Kim, E. Comparison of absolute and relative grip strength to predict incidence of metabolic syndrome: Eight-Year Follow-Up study in Korea. Metab. Syndr. Relat. Disord. 22, 123–132. https://doi.org/10.1089/met.2023.0206 (2024).
Alkahtani, S. A. et al. Association between handgrip strength and metabolic syndrome in relation to gender and adiposity among middle aged and older Saudi populations. Aging Male. 27, 2325146. https://doi.org/10.1080/13685538.2024.2325146 (2024).
Luo, J. H., Zhang, T. M., Yang, L. L., Cai, Y. Y. & Yang, Y. Association between relative muscle strength and hypertension in middle-aged and older Chinese adults. BMC Public Health 23, 2087. https://doi.org/10.1186/s12889-023-17007-6 (2023).
Chi, J. H. & Lee, B. J. Association of relative hand grip strength with myocardial infarction and angina pectoris in the Korean population: a large-scale cross-sectional study. BMC Public. Health. 24, 941. https://doi.org/10.1186/s12889-024-18409-w (2024).
Wu, K. M. et al. Association of grip strength and walking Pace with the risk of incident Parkinson’s disease: a prospective cohort study of 422,531 participants. J. Neurol. https://doi.org/10.1007/s00415-024-12194-7 (2024).
Kuo, K. et al. Associations of grip strength, walking Pace, and the risk of incident dementia: A prospective cohort study of 340212 participants. Alzheimers Dement. 19, 1415–1427. https://doi.org/10.1002/alz.12793 (2023).
Zhang, N. et al. Association between grip strength and stress urinary incontinence of NHANES 2011–2014. BMC Womens Health. 23, 521. https://doi.org/10.1186/s12905-023-02628-1 (2023).
Yang, S. J. et al. Association of decreased grip strength with lower urinary tract symptoms in women: a cross-sectional study from Korea. BMC Womens Health. 21, 96. https://doi.org/10.1186/s12905-021-01241-4 (2021).
Szekeres, Z., Nagy, A., Jahner, K. & Szabados, E. Impact of selected Glucagon-like Peptide-1 receptor agonists on serum lipids, adipose tissue, and muscle Metabolism-A narrative review. Int. J. Mol. Sci. 25. https://doi.org/10.3390/ijms25158214 (2024).
Watt, M. J. & Hoy, A. J. Lipid metabolism in skeletal muscle: generation of adaptive and maladaptive intracellular signals for cellular function. Am. J. Physiol. Endocrinol. Metab. 302, E1315–1328. https://doi.org/10.1152/ajpendo.00561.2011 (2012).
Ranallo, R. F. & Rhodes, E. C. Lipid metabolism during exercise. Sports Med. 26, 29–42. https://doi.org/10.2165/00007256-199826010-00003 (1998).
Bakke, S. S. et al. Myotubes from severely obese type 2 diabetic subjects accumulate less lipids and show higher lipolytic rate than myotubes from severely obese non-diabetic subjects. PLoS One 10, e0119556. https://doi.org/10.1371/journal.pone.0119556 (2015).
Biltz, N. K. et al. Infiltration of intramuscular adipose tissue impairs skeletal muscle contraction. J. Physiol. 598, 2669–2683. https://doi.org/10.1113/jp279595 (2020).
Summers, S. A., Garza, L. A., Zhou, H. & Birnbaum, M. J. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell. Biol. 18, 5457–5464. https://doi.org/10.1128/mcb.18.9.5457 (1998).
Zhang, L., Keung, W., Samokhvalov, V., Wang, W. & Lopaschuk, G. D. Role of fatty acid uptake and fatty acid beta-oxidation in mediating insulin resistance in heart and skeletal muscle. Biochim. Biophys. Acta. 1801, 1–22. https://doi.org/10.1016/j.bbalip.2009.09.014 (2010).
Bonen, A., Dyck, D. J. & Luiken, J. J. Skeletal muscle fatty acid transport and transporters. Adv. Exp. Med. Biol. 441, 193–205. https://doi.org/10.1007/978-1-4899-1928-1_18 (1998).
Bosma, M. et al. Perilipin 2 improves insulin sensitivity in skeletal muscle despite elevated intramuscular lipid levels. Diabetes 61, 2679–2690. https://doi.org/10.2337/db11-1402 (2012).
Harris, L. A. et al. Perilipin 5-Driven lipid droplet accumulation in skeletal muscle stimulates the expression of fibroblast growth factor 21. Diabetes 64, 2757–2768. https://doi.org/10.2337/db14-1035 (2015).
Kim, K. H. et al. Hepatic FXR/SHP axis modulates systemic glucose and fatty acid homeostasis in aged mice. Hepatology 66, 498–509. https://doi.org/10.1002/hep.29199 (2017).
Wu, J. et al. Macrophage phenotypic switch orchestrates the inflammation and repair/regeneration following acute pancreatitis injury. EBioMedicine 58, 102920. https://doi.org/10.1016/j.ebiom.2020.102920 (2020).
DeFuria, J. et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc. Natl. Acad. Sci. U S A. 110, 5133–5138. https://doi.org/10.1073/pnas.1215840110 (2013).
Li, C. W. et al. Circulating factors associated with sarcopenia during ageing and after intensive lifestyle intervention. J. Cachexia Sarcopenia Muscle. 10, 586–600. https://doi.org/10.1002/jcsm.12417 (2019).
Scott, D. et al. Associations of sarcopenic obesity and dynapenic obesity with bone mineral density and incident fractures over 5–10 years in Community-Dwelling older adults. Calcif. Tissue Int. 99, 30–42. https://doi.org/10.1007/s00223-016-0123-9 (2016).
Acknowledgements
The authors would like to express their gratitude to all the participants and staff of the National Health and Nutrition Examination Survey and the National Centre for Environmental Health for their valuable contributions.
Funding
This research was funded by the National Natural Science Foundation of China (No. 82102619), the Jiangsu Provincial Traditional Chinese Medicine Science and Technology Development Project (No. ZD202232).
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology: Qian Wu and Yicheng Ling. Investigation and formal analysis: Yicheng Ling, Guiqiang Yuan, and Rui La. Data curation: Bin Xu, Erkai Pang, Chuan Lin, Zhanghuan Chen and Zhigang Zhang. Visualization and writing - original draft: Qian Wu, Yicheng Ling, and Guiqiang Yuan. Writing - review & editing: Lixin Huang, Dinghua Jiang, Jiangnan Xu and Yubo Mao.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study used publicly available pooled data; no ethical approval was required. And all subjects involved in this study from the NHANES database were treated by the ethical principles set out in the Declaration of Helsinki and the database was approved by the National Centre for Health Statistics (NCHS) Research Ethics Review Board. At the same time, at the time of participation in the database data collection, patients/participants had provided written informed consent to participate in the data collection and other related aspects.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, Q., Ling, Y., Yuan, G. et al. Association between lipid accumulation products and relative handgrip strength: a large population-based study. Sci Rep 15, 11562 (2025). https://doi.org/10.1038/s41598-025-96481-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-96481-x
Keywords
This article is cited by
-
Association of lipid accumulation product with the prevalence and incidence of sarcopenia: a nationwide study in Chinese
Nutrition & Metabolism (2025)
-
Prognostic nutritional index, sarcopenia, and risk of mortality: a national population-based study
Nutrition & Metabolism (2025)
-
Muscle strength and the risk of Multimorbidity in Middle-aged and older Chinese adults: A prospective cohort study
Aging Clinical and Experimental Research (2025)