Abstract
Diabetic retinopathy (DR) is characterized by progressive retinal vascular damage that ultimately causes vision loss. The prognostic nutritional index (PNI), which integrates albumin and lymphocytes, serves as an indicator of an individual’s inflammatory response, nutritional condition, and immune system function. This research aimed to explore the possible association between PNI and DR. This was a cross-sectional study utilizing data from the National Health and Nutrition Examination Survey (NHANES) between 2001 and 2018. Weighted logistic regression analyses were employed to assess the relationship between PNI and DR prevalence. A total of 4791 adults aged 20 years and older were included in the analysis. Results indicated a statistically significant negative correlation between PNI and DR prevalence. In the fully adjusted model, a one-unit rise in PNI corresponded to a 7% reduction in the probability of DR prevalence. Quartile analysis consistently indicated that individuals in the highest PNI quartile had notably lower odds of DR prevalence compared to those in the lowest quartile. Additionally, smooth curve fitting suggested a nonlinear relationship between PNI and DR. Subgroup analysis reinforced the strength of the inverse association between PNI and DR (all p for interaction > 0.05). This nationally representative study demonstrated a significant inverse relationship between PNI levels and DR prevalence among diabetic adults in the United States. Our findings emphasize the potential role of maintaining optimal PNI values in preventing the development of DR.
Similar content being viewed by others
Introduction
Diabetic retinopathy (DR), frequently observed as a complication of diabetes, involves the gradual deterioration of blood vessels within the retina, ultimately culminating in the loss of vision1. It has become a primary cause of visual impairment2. In 2019, the International Diabetes Federation reported that diabetes affected 463 million people globally, with projections indicating a potential increase to 700 million by 20453.Studies have shown that nearly one-third of those with diabetes worldwide suffer from DR4. With the increasing prevalence of diabetes, the associated impact of diabetic retinopathy (DR) is anticipated to grow substantially in the years ahead1.Consequently, DR has become a critical global public health issue, highlighting the importance of identifying modifiable risk factors to manage and prevent DR effectively.
The prognostic nutritional index (PNI) serves as a straightforward and reliable indicator to evaluate an individual’s nutritional health, immune system activity, and inflammatory state. Its calculation is derived from measurements of serum albumin concentration and lymphocyte count5. Initially introduced in 1984, PNI was first applied to evaluate cancer prognosis6. Since then, growing evidence has demonstrated its utility across a wide range of health conditions, including predicting outcomes in colorectal cancer7, hepatocellular carcinoma and cervical cancer8, mortality in type 2 diabetes mellitus (T2DM) patients9, migraine10, albuminuria5. The growing range of clinical uses for PNI has attracted considerable attention in the medical community, emphasizing its significance as a promising biomarker for integration into standard clinical procedures.
Existing studies have highlighted a connection between higher PNI levels and a reduced prevalence of diabetic kidney disease9. Additionally, a cohort study revealed that serum albumin is associated with diabetic chronic microvascular complications, emphasizing its potential role in both the development and management of diabetes-related conditions11. Despite these findings, there remains a paucity of research exploring the direct association between PNI and DR. A cross-sectional investigation conducted on Indian T2DM patients offered preliminary evidence regarding the relationship between PNI and DR12. However, the study’s insights were constrained due to its single-center design and limited participant size, underscoring the necessity of broader, more diverse research to substantiate these findings. In response to this research gap, the current study examined the correlation between PNI and DR in a US-based diabetic population using data sourced from the National Health and Nutrition Examination Survey (NHANES). The aim was to deepen the understanding of the correlation between PNI and the prevalence of DR, with the goal of providing meaningful clinical insights and practical guidance for the treatment and management of DR.
Materials and methods
Study population
The National Center for Health Statistics (NCHS) in the USA developed the NHANES to perform a nationally representative cross-sectional survey, gathering insights on Americans’ nutrition and health for effective health management and early detection of emerging public health issues. To ensure a representative sample of the non-institutionalized civilian population across the United States, participants were selected through a sophisticated, stratified, multistage probability cluster sampling approach. Ethical oversight for the NHANES study was provided by the Ethics Review Committee of NCHS, which approved the study protocol. Prior to enrollment, all participants signed written informed consent forms. This investigation concentrated on individuals aged 20 and above, utilizing data spanning NHANES cycles from 2001 to 2018.
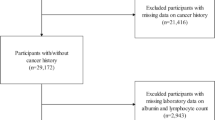
Initially, 91,351 eligible participants were recruited. However, several exclusions were applied: 85,170 participants who could not complete a diabetic retinopathy evaluation, 710 with missing PNI data, and 680 either lacking full covariate information or identified as pregnant. Following these exclusions, the final analysis incorporated 4791 participants who satisfied all study criteria (Fig. 1).
Exposure variables and outcome definition
In this study, PNI was employed as the primary exposure variable and computed using the formula: PNI = 10 × albumin (g/dL) + 0.005 × absolute lymphocyte count (103cells/µL). To further reflect the dose-response relationship between PNI and DR, PNI was divided into four quartiles. Serum albumin levels were determined through the bromocresol purple dye method, while absolute lymphocyte counts were measured using a Beckman Coulter DxH 800 analyzer.
Diabetes mellitus was identified if one of the following criteria was satisfied: (1) glycohemoglobin levels ≥ 6.5%; (2) fasting glucose concentration ≥ 7.0 mmol/L; or (3) a self-reported prior diabetes diagnosis from a physician. Participants were labeled as having DR if they responded affirmatively to the question: ‘Has a doctor ever told you that diabetes has affected your eyes or that you had retinopathy?’.
Covariates
Variables were selected based on established confounding factors identified in previous research and medical practices. To more accurately assess the relationship between PNI and diabetic retinopathy, the analysis included the following covariates: age (categorized as 20–40, ≥ 40), gender, race, marital status, education level, smoking status, hypertension, alcohol consumption, poverty-to-income ratio (PIR), healthy eating index-2015 (HEI-2015), physical activity level, last time had pupils dilated for exam, diabetic medications, obesity, total calorie intake, diabetic nephropathy (DN). HEI-2015 is an index used to measure whether the dietary habits of the U.S. population align with the Dietary Guidelines for Americans, with higher scores indicating healthier dietary habits13. The formula for calculating weekly physical activity time is the time spent in moderate-intensity physical activity plus twice the time spent in high-intensity physical activity, and participants with weekly physical activity time ≥ 150 min are considered physically active14,15. Obesity is defined as a body mass index ≥ 30 kg/m². Participants with a urinary albumin creatinine ratio ≥ 30 mg/g are considered to have DN16,17.
Statistical analysis
This study adhered to the NHANES guidelines for statistical analysis, applying survey weights to address the intricacies of the sampling structure. Participant characteristics were summarized using descriptive statistics, categorized by the presence or absence of DR. Continuous variables were analyzed using a weighted linear regression model, whereas categorical variables were evaluated through a weighted chi-square test. To investigate the relationship between DR prevalence and PNI, weighted logistic regression analysis was performed. PNI was categorized into quartiles and the first quartile designated as the reference.
Three regression models were constructed to examine the data: Model 1 was an unadjusted crude model; Model 2 accounted for adjustments in age, gender, and race; Model 3 incorporated additional adjustments for marital status, education level, smoking behavior, hypertension, alcohol drinking, PIR, HEI-2015, physical activity level, last time had pupils dilated for exam, diabetic medications, obesity, total calorie intake, DN. To assess the potential non-linear relationship between PNI and the prevalence of DR, a smoothing curve fitting was incorporated into the fully adjusted analytical model.
Subgroup analyses, alongside interaction tests, were employed to investigate potential differences across various population groups. In the fully adjusted model, participants were categorized based on age, gender, race, marital status, education level, smoking behavior, hypertension, alcohol use, and PIR. To assess differences in effects across these subgroups, the interaction between PNI and potential modifying factors was analyzed (p for interaction).
We conducted two sensitivity analyses following established methodologies to validate result reliability. First, we re-examined the PNI-DR association using unweighted logistic regression, excluding adjustments for NHANES complex sampling design. Second, after excluding participants with extreme PNI values (< 5th or > 95th percentiles), we analyzed this relationship using both weighted and unweighted logistic regression models to ensure methodological consistency across different analytical frameworks.
All statistical analyses were performed utilizing the EmpowerStats software suite in conjunction with R software (version 4.2.0). The significance threshold for these analyses was established at a P-value of less than 0.05.
Results
Baseline characteristics
From 2001 to 2018, a total of 4791 participants were assessed for inclusion in the study. The average age of the participants was 61.95 ± 13.08 years, with males comprising 52.52% of the cohort, and the overall prevalence of diabetic retinopathy was 21.75%. As shown in Table 1, the PNI values of participants without DR were notably higher than those with DR (P < 0.01). Additionally, the DR group showed a higher prevalence of hypertension compared to the non-DR group (P < 0.01).
Association between PNI and DR
Weighted logistic regression analyses were conducted to explore the association between PNI and DR. As shown in Table 2, PNI was negatively associated with DR prevalence. Across all models, including the unadjusted model (Model 1, OR = 0.93, 95% CI: 0.91, 0.96, p < 0.001), the partially adjusted model (Model 2, OR = 0.93, 95%CI: 0.90, 0.95, p < 0.001), and the fully adjusted model (Model 3, OR = 0.93, 95%CI: 0.91, 0.95, p < 0.001), a consistent negative correlation was observed. The findings suggest that a one-unit rise in PNI corresponds to a 7% decrease in the prevalence of DR. Additionally, individuals in the highest PNI quartile (Q4) demonstrated a 53% reduced chance of developing DR relative to those in the lowest quartile (Q1)(Table 2, Model 3, OR = 0.47, 95%CI: 0.36, 0.62, p for trend < 0.001).
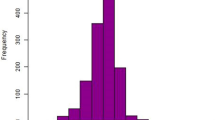
Smoothed curve fitting, applied within the fully adjusted model, revealed further insights into the nonlinear relationship between PNI and DR (Fig. 2).
Subgroup and sensitivity analyses
The PNI demonstrated a negative correlation with DR across all subgroups (all OR < 1). As shown in Table 3, subgroup analysis suggested that the negative correlation between PNI and DR was more pronounced among participants aged over 40 years (OR = 0.93, 95%CI: 0.90, 0.96, p < 0.001), female participants (OR = 0.92, 95%CI: 0.88, 0.95, p < 0.001), participants of Mexican American (OR = 0.90, 95%CI: 0.86, 0.95, p < 0.001), participants never married (OR = 0.90, 95%CI: 0.83, 0.98, p = 0.012), participants with hypertension (OR = 0.92, 95%CI: 0.90, 0.95, p < 0.001), non-drinking participants(OR = 0.88, 95%CI:0.82, 0.95, p = 0.001), participants with PIR ≤ 1 (OR = 0.91, 95%CI: 0.87, 0.95, p < 0.001), participants without DN (OR = 0.94, 95%CI: 0.91, 0.97, p < 0.001) .
The results of two sensitivity analyses consistently found a negative correlation between PNI and DR prevalence, further supporting the reliability of our findings (Supplementary Table 1, Supplementary Tables 2, and Supplementary Table 3).
Discussion
As far as we know, this is the first cross-sectional study using the NHANES database to analyze the relationship between PNI and DR. Using a nationally representative of diabetic adults from the United States, our findings demonstrated a notable inverse relationship between PNI levels and the prevalence of DR. After controlling for potential confounders, each unit increase in PNI corresponds to a 7% reduction in the prevalence of DR. In the subgroup analysis, we observed that hypertension significantly influenced the relationship between PNI and DR, with the negative correlation being more pronounced among individuals with hypertension. The results imply that individuals with lower PNI values may benefit from early interventions and targeted strategies aimed at enhancing nutritional health and mitigating inflammation, potentially reducing the incidence of DR.
PNI is a simple and readily accessible composite index with substantial clinical implications. Previous research highlights a strong link between PNI and onset, development, and chronic microvascular complications of diabetes. For instance, Zhang et al. conducted a cohort study exploring the association between PNI, all-cause mortality, and the emergence of diabetic nephropathy in individuals with T2DM. Their findings indicated that elevated PNI levels correlate with reduced risks of all-cause mortality and diabetic nephropathy9. Likewise, a cross-sectional study identified an inverse association between PNI and all-cause mortality among gestational diabetic patients18. Another cross-sectional study involving 128 patients with T2DM reported that lower PNI values are common in patients with DR12. These findings underscore PNI’s potential utility as a clinical marker for identifying diabetes-related vascular complications, including diabetic retinopathy.
PNI is derived from two key components: albumin and lymphocytes, which together provide an assessment of inflammation, nutritional status, and immune function. The specific physiological mechanisms that explain the connection between PNI and DR are not yet clearly understood and require additional research. However, several physiological mechanisms may explain the inverse association between PNI and DR.
First, serum albumin may delay the onset of DR due to its antioxidative and anti-inflammatory properties. Albumin, being the most prevalent serum protein produced by the liver, significantly contributes to minimizing oxidative stress and controlling inflammation—two critical factors in the development of diabetic retinopathy. Its antioxidant and anti-inflammatory properties may help protect retinal cells from damage and reduce the progression of vascular complications19,20. Excessive oxidative stress plays a pivotal role in the onset of insulin resistance, a fundamental process contributing to the development of diabetes. Serum albumin, with its potent antioxidant properties—such as its capacity for multiple ligand binding and trapping free radicals—may play a pivotal role in mitigating oxidative stress. These properties of serum albumin could be instrumental both the onset and development of diabetes, while also aiding in lowering the risk and slowing the progression of diabetic retinopathy, a common vascular complication of diabetes21,22. Lower serum albumin levels have been linked to elevated inflammatory markers23, with cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 being closely associated with this reduction24. It is worth noting that normal physiological concentrations of albumin are capable of suppressing the TNF-α-induced expression of vascular cell adhesion molecules, thus mitigating inflammation. This anti-inflammatory property of serum albumin may play a protective role in vascular health, particularly in conditions like DR, where inflammation is a key pathological factor25. In recent decades, growing evidence has highlighted the critical roles of oxidative stress and chronic inflammation in the development of T2DM and DR. Oxidative stress can impair insulin signaling, promote vascular damage, and accelerate the progression of DR, while chronic inflammation contributes to endothelial dysfunction and retinal damage through the release of pro-inflammatory cytokines. Together, these mechanisms underline the importance of targeting oxidative stress and inflammation in the prevention and management of T2DM and its complications26,27. By reducing oxidative stress, trapping free radicals, and inhibiting the expression of pro-inflammatory cytokines and vascular adhesion molecules, albumin helps mitigate the inflammatory and oxidative processes that contribute to the vascular and retinal damage seen in DR. These protective properties suggest that maintaining optimal serum albumin levels could be beneficial in preventing or slowing the onset and development of DR.
Second, higher PNI may reflect lower levels of endothelial dysfunction, which contribute to retinal microvascular damage28. A cross-sectional study demonstrated that PNI levels are significantly inversely associated with albuminuria5. Albuminuria is a critical indicator of endothelial dysfunction, disrupting the delicate balance between vascular constrictors like angiotensin II and dilators such as nitric oxide. This disruption fosters vasoconstriction, modifies vascular permeability, and exacerbates endothelial impairment. Moreover, the presence of albumin in urine may trigger inflammatory responses in vascular endothelial cells, leading to apoptosis and weakening the endothelial barrier’s structural integrity29,30,31. The specific pathophysiological mechanisms involved still require further study.
DN and DR are both microvascular complications of diabetes. DN may affect serum albumin, an important factor in the calculation of PNI, thereby influencing the credibility of the negative association between PNI and DR. To further confirm the impact of DN on the observed negative association, we conducted subgroup analysis and interaction tests based on whether the participants had DN. The analysis results showed a stable negative association (OR < 1) between PNI and DR regardless of whether the participants had DN, and DN did not alter this association (p for interaction > 0.05). These analyses further validate the credibility of our findings.
The significant strengths of the study consist of its extensive and representative sample size, which improves the applicability of the results to larger populations. Additionally, the adjustment for potential confounding variables helps ensure that the observed associations are less likely to be influenced by other factors, thereby providing more accurate and reliable results. Furthermore, a subgroup analysis was conducted to evaluate the consistency of the relationship between PNI and DR across across different populations. These methodological strengths strengthen the study’s validity and support its conclusions regarding the relationship between PNI and DR prevalence. Our results underscore the potential role of maintaining high levels of PNI in preventing diabetic retinopathy, particularly among diabetic individuals with hypertension. This study further provides evidence supporting the potential role of controlling PNI through proper nutrition and inflammation control as a strategy to reduce the burden of DR.
There are a number of limitations that need to be taken into account when evaluating the findings. First, the cross-sectional study design measures exposure and outcome factors simultaneously, making it impossible to establish temporality and thus leaving the causal relationship between these factors uncertain. Secondly, our study relies on self-reported data and diagnostic codes from NHANES, which may introduce biases or inaccuracies. Thirdly, given the observational design of this study, the possibility of residual confounding from factors that were not measured cannot be excluded. Despite adjusting for numerous confounders, certain critical factors, including medications and conditions affecting albumin levels, were not controlled. These unmeasured variables might affect the observed association between PNI and albuminuria. Future longitudinal studies are warranted to ensure better control of confounding variables through improved experimental design and data analysis. Moreover, as a static indicator, PNI represents an individual’s current nutritional status but fails to capture its temporal changes. As PNI is subject to variation based on health conditions and lifestyle behaviors, a single measurement may fail to accurately represent long-term nutritional patterns or their effects on health. Future research should adopt large-scale longitudinal designs with multiple time-point data collection to better understand the dynamic relationship between PNI and DR.
Our findings highlight several directions for future research. First, longitudinal cohort studies are essential to determine the timing and progression relationship between PNI and DR. Second, clinical trials should investigate various strategies aimed at modulating PNI to prevent or manage DR. Third, exploring the underlying pathophysiological processes is vital for discovering new biomarkers and therapeutic targets.
Conclusions
In conclusion, this research, based on a nationally representative cohort of diabetic adults in the United States, found an inverse association between PNI and the prevalence of DR. Compared to individuals with low PNI levels, the prevalence of DR is lower in those with high PNI levels, especially among individuals with hypertension. These findings suggest that maintaining an optimal PNI may play a role in preventing DR. Future longitudinal research is needed to verify the causal direction and uncover the mechanisms involved. Public health initiatives aimed at optimizing PNI levels could help mitigate the impact of DR.
Data availability
The original data presented in the study are openly available at https://www.cdc.gov/nchs/nhanes/index.htm (accessed in 2024). The data have not been previously presented orally or by poster at scientific meetings.
Abbreviations
- DR:
-
Diabetic retinopathy
- DN:
-
Diabetic nephropathy
- PNI:
-
Prognostic nutritional index
- T2DM:
-
Type 2 diabetes mellitus
- NHANES:
-
National Health and Nutrition Examination Survey
- NCHS:
-
National Center for Health Statistics
- PIR:
-
Poverty-to-income ratio
- TNF-α:
-
Tumor necrosis factor-α
References
Yau, J. W. & Rogers, S. L. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 35 (3), 556–564 (2012).
Bourne, R. R. et al. Causes of vision loss worldwide, 1990–2010: A systematic analysis. Lancet Glob. Health. 1 (6), e339–349 (2013).
Sun, H. et al. IDF diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119 (2022).
Teo, Z. L. et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and Meta-analysis. Ophthalmology 128 (11), 1580–1591 (2021).
Jiang, Z., Zhu, X., Jiang, H., Zhao, D. & Su, F. Prognostic nutritional index and albuminuria in adults aged 20 years and above: A cross-sectional analysis in the united States. Front. Nutr. 11, 1462789 (2024).
Onodera, T., Goseki, N. & Kosaki, G. Prognostic nutritional index in Gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 85 (9), 1001–1005 (1984).
Wang, D. et al. Prognostic nutritional index and systemic Immune-Inflammation index predict the prognosis of patients with HCC. J. Gastrointest. Surg. 25 (2), 421–427 (2021).
Tokunaga, R. et al. Comparison of systemic inflammatory and nutritional scores in colorectal cancer patients who underwent potentially curative resection. Int. J. Clin. Oncol. 22 (4), 740–748 (2017).
Zhang, J., Chen, Y., Zou, L. & Gong, R. Prognostic nutritional index as a risk factor for diabetic kidney disease and mortality in patients with type 2 diabetes mellitus. Acta Diabetol. 60 (2), 235–245 (2023).
Peng, C. et al. Association between the prognostic nutritional index and severe headache or migraine: A population-based study. Nutr. Neurosci. 26 (12), 1202–1211 (2023).
Zhu, Y. et al. Serum albumin, but not bilirubin, is associated with diabetic chronic vascular complications in a Chinese type 2 diabetic population. Sci. Rep. 9 (1), 12086 (2019).
Kurtul, B. E., Koca, S. & Yilmaz, M. O. Prognostic nutritional index as a novel marker for diabetic retinopathy in individuals with type 2 diabetes mellitus. Saudi J. Ophthalmol. 36 (3), 322–326 (2022).
Krebs-Smith, S. M. et al. Update of the healthy eating index: HEI-2015. J. Acad. Nutr. Diet. 118 (9), 1591–1602 (2018).
Divney, A. A. et al. Diabetes prevalence by Leisure-, Transportation-, and Occupation-Based physical activity among racially/ethnically diverse U.S. Adults. Diabetes Care. 42 (7), 1241–1247 (2019).
Meng, F. et al. Effects of neutrophils on the relationship between physical activity and depression: Evidence from cross-sectional study and Mendelian randomization analysis. J. Affect. Disord. 375, 406–411 (2025).
McGrath, K. & Edi, R. Diabetic kidney disease: Diagnosis, treatment, and prevention. Am. Fam. Phys. 99 (12), 751–759 (2019).
Chen, X., Lin, Y., Dong, W., Wen, X. & Zuo, Y. Exploring the relationship between grip strength and diabetic nephropathy among U.S. Adults with type 2 diabetes mellitus: A cross-sectional NHANES analysis. BMC Endocr. Disord. 25 (1), 35 (2025).
Cao, J., Bu, X., Chen, J. & Zhang, X. Associations of prognostic nutritional index with risk of all-cause and cardiovascular disease mortalities in persons with gestational diabetes mellitus: A NHANES-based analysis. Diab Vasc. Dis. Res. 21 (5), 14791641241284409 (2024).
Arques, S. Human serum albumin in cardiovascular diseases. Eur. J. Intern. Med. 52, 8–12 (2018).
Don, B. R. & Kaysen, G. Serum albumin: Relationship to inflammation and nutrition. Semin. Dial. 17 (6), 432–437 (2004).
Njajou, O. T. et al. Association between oxidized LDL, obesity and type 2 diabetes in a population-based cohort, the health, aging and body composition study. Diabetes Metab. Res. Rev. 25 (8), 733–739 (2009).
Siddiqui, A. et al. Association of oxidative stress and inflammatory markers with chronic stress in patients with newly diagnosed type 2 diabetes. Diabetes Metab. Res. Rev. 35 (5), e3147 (2019).
Zhang, J. et al. The level of serum albumin is associated with renal prognosis in patients with diabetic nephropathy. J. Diabetes Res. 2019, 7825804 (2019).
Moshage, H. J., Janssen, J. A., Franssen, J. H., Hafkenscheid, J. C. & Yap, S. H. Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J. Clin. Invest. 79 (6), 1635–1641 (1987).
Zhang, W. J. & Frei, B. Albumin selectively inhibits TNF alpha-induced expression of vascular cell adhesion molecule-1 in human aortic endothelial cells. Cardiovasc. Res. 55 (4), 820–829 (2002).
Jha, J. C., Ho, F., Dan, C. & Jandeleit-Dahm, K. A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clin. Sci. (Lond). 132 (16), 1811–1836 (2018).
Ferrucci, L. & Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15 (9), 505–522 (2018).
Wang, W. & Lo, A. C. Y. Diabetic retinopathy: Pathophysiology and treatments. Int. J. Mol. Sci. 19(6) (2018).
Ochodnicky, P., Henning, R. H., van Dokkum, R. P. & de Zeeuw, D. Microalbuminuria and endothelial dysfunction: Emerging targets for primary prevention of end-organ damage. J. Cardiovasc. Pharmacol. 47 (Suppl 2), S151–162 (2006). discussion S172-156.
Stehouwer, C. D., Lambert, J., Donker, A. J. & van Hinsbergh, V. W. Endothelial dysfunction and pathogenesis of diabetic angiopathy. Cardiovasc. Res. 34 (1), 55–68 (1997).
Paisley, K. E. et al. Endothelial dysfunction and inflammation in asymptomatic proteinuria. Kidney Int. 63 (2), 624–633 (2003).
Acknowledgements
This research was funded by Startup Fund for scientific research, Fujian Medical University, grant number 2021QH1309. Natural Science Foundation of Fujian Province, grant number 2024J08261.
Funding
This research was funded by Startup Fund for scientific research, Fujian Medical University, grant number 2021QH1309. Natural Science Foundation of Fujian Province, grant number 2024J08261.
Author information
Authors and Affiliations
Contributions
Original draft preparation, methodology, data curation, L.Z.X. and X.L.L.; review and editing, T.L.; revision, L.Z.X. and X.L.L.; conceptualization, review, editing, and supervision, J.P.W. and G.C. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
This study did not require Consent Statement because it involved the use of publicly available data.
Institutional review board statement
The NHANES was approved by the Institutional Review Committee of the National Centre for Health Statistics. Due to the present study based on the secondary analysis, additional ethics approval was not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, L., Lin, X., Li, T. et al. Association between prognostic nutritional index and diabetic retinopathy among U.S. diabetic adults in NHANES. Sci Rep 15, 12986 (2025). https://doi.org/10.1038/s41598-025-96582-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-96582-7
Keywords
This article is cited by
-
The diagnostic value of prognostic nutritional ındex and controlling nutritional status score in differentiating retinal vein occlusion
International Ophthalmology (2026)