Abstract
Preeclampsia (PE) is a pregnancy-specific disorder characterized by multi-system involvement, leading to increased perinatal morbidity and mortality as well as long-term cardiovascular damage in both mother and fetus. This study aimed to investigate the alterations and potential role of high temperature requirement factor A4 (Htra4) in early-onset PE. We conducted a comparative analysis of the baseline data between patients with early-onset PE and normal controls, as well as analyzed the correlation of Htra4 levels in the peripheral blood with pregnancy outcomes. Additionally, we investigated the impact of recombinant protein Htra4 on human umbilical vein endothelial cells (HUVECs). Patients with early-onset PE patients exhibited cardiac, hepatic, and renal impairment, as well as elevated levels of Htra4 in peripheral blood but not in umbilical cord blood. Further correlation analysis reveals a significant association between peripheral blood Htra4 levels and adverse pregnancy outcomes in patients with early-onset PE, particularly a strong correlation between maternal blood Htra4 levels and systolic and diastolic blood pressure, suggesting that Htra4 may be associated with impaired vascular function. Histopathological examination revealed inadequate trophoblast cell invasion in the decidua of early-onset PE patients, along with unsuccessful uterine artery remodeling. Additionally, the spiral arterial lumen in the decidua exhibited narrower and irregularly shaped remodeling. Immunofluorescence localization demonstrated the expression of Htra4 in trophoblasts of villous as well as extra-villous lineages. We observed intensified Htra4 staining in the placenta and decidua tissues of patients with early-onset PE. In combination with the pathological alterations of decidual vessels, it is hypothesized that Htra4 might be related to the dysfunction of endothelial cells. Subsequent research indicated that Htra4 induce the onset of oxidative stress, and inflammatory response in HUVECs, suggesting a potential association between the elevated Htra4 levels and vascular endothelial injury in patients with early-onset PE. This study identified a correlation between Htra4 and adverse pregnancy outcomes in patients with early-onset PE, potentially leading to vascular endothelial injury through elevated levels of Htra4.
Similar content being viewed by others
Introduction
PE is a pregnancy-specific multisystem disorder, affecting approximately 5–10% of pregnancies globally each year. This condition is the major cause of maternal and perinatal morbidity and mortality, and leading long-term damage to the cardiovascular system of both mater and fetus1,2. PE is characterized by the onset of hypertension and end-organ damage, including proteinuria, occurring after 20 weeks of gestation. During normal implantation, trophoblasts invade into the decidualized endometrium, resulting in spiral artery remodeling and obliteration of the tunica media of myometrial spiral arteries, and facilitating increased blood flow to the placenta3. However, in PE, trophoblasts are unable to acquire the phenotype of endothelial, resulting in compromised trophoblast invasion and insufficient remodeling of spiral arteries4. The etiology of PE is still unclear, while inflammation and oxidative stress are believed to contribute the development of PE5. Currently, the management strategy for PE primarily focuses on screening, prevention, monitoring, and timely intervention. Unfortunately, terminating pregnancy is the only treatment for this condition in diagnosed patients with PE. Increasing evidence suggests that various biological markers may serve as potential targets for predicting or treating PE6,7.
PE can be classified into early-onset PE (with delivery at < 34 weeks of gestation) and late-onset PE (with delivery at ≥ 34 weeks of gestation)8. Although the pathogenesis of early-onset PE and late-onset PE may differ, endothelial injury is implicated in both of them. There are various controversies surrounding the mechanism and extent of endothelial injury in these two types of PE. However, it is suggested that a higher degree of vascular endothelial injury and dysfunction was observed in early-onset PE compared to late-onset PE9. This study seeks to investigate the differentiation between early-onset PE and normal control, as well as to identify a potential biological marker for predicting or treating early-onset PE.
The high temperature requirement factors (Htras) family consists of four members, namely Htra1, Htra2, Htra3, and Htra4, is essential for cell growth, apoptosis, invasion and inflammation10,11. Aberrant expression of Htras has been linked to various diseases, such as cancer12, arthritis13, neurodegenerative disorders14, age-related macular degeneration15, and PE16. Previous research has shown the involvement of Htra1, Htra3, and Htra4 in pregnancy and their alteration during the early stages of PE17,18,19. Levels of Htra1 and Htra3 were utilized for early pregnancy prediction of PE onset, whereas Htra4 levels were employed for late pregnancy prediction18,19. Despite the association between elevated levels of Htra4 and early-onset PE, the underlying mechanisms remain unclear.
This study is grounded in clinical research, investigating the expression, localization and changes of Htra4 in blood, as well as its correlation with the clinical manifestations of early-onset PE. Furthermore, we also meaured the function of Htra4 in HUVECs cells to further explore the pathogenesis of early-onset PE and potentially provide a basis for prediction or prognosis.
Materials and methods
Baseline data and samples collection
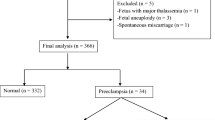
Please refer to the supplementary information for the collection, inclusion and exclusion criteria for early-onset PE patient and normal control baseline data, as well as the collection of peripheral blood and umbilical cord blood, and the tissue of decidua and placenta. This study was conducted in accordance with the Helsinki Declaration. This study has been approved by the Ethics Committee of the Second Hospital of Hebei Medical University in Shijiazhuang, China (Approval No. 2024-R581), and it has been confirmed that informed consent has been obtained from all subjects.
Western blot assay
Placental tissue (Approximately 100 g) or HUVECs was lysed using high-efficiency RIPA lysis buffer (Solarbio, Beijing, China) containing 1% PMSF at 4 °C for 30 min to extract total protein. The lysate was then centrifuged at 12,000 rpm for 15 min to collect the protein supernatant. Protein content was measured using the Pierce BCA protein assay kit (Thermo, Rockford, USA). Following electrophoresis on SDS-PAGE gels, protein samples were transferred onto PVDF membranes by electroblotting. Subsequently, the PVDF membranes were blocked with nonfat milk and then incubated with primary antibody at 4 oC overnight, followed by incubation with HRP-conjugated secondary antibodies at room temperature for 1 h. Finally, blots were developed using supper ECL. (CWBIO, Beijing, China). Primary antibodies used include ERK/p-ERK, JNK/p-JNK, P38/p-P38, NF-κB p65 (Santa Cruz), NRF2, HO-1, NQO-1, Htra4 (Bioss, Beijing, China), GAPDH, β-actin. HRP-conjugated secondary antibody (ABclonal, Wuhan, China).
qRT-PCR assay
Total RNA was isolated from placental tissue and HUVECs using RNAiso Plus (Takara, Beijing, China) and reverse transcribed into cDNA with PrimeScript™ IV 1st strand cDNA Synthesis Mix (Takara, Beijing, China). Gene expression was quantified using TB Green® Premix Ex Taq™ II FAST qPCR (CN830A) (Takara, Beijing, China). The primer sequences as follows: Htra4, F: 5’-TGAAGAGTGGAAGCGAGGAAGG-3’, R: 5’- AGAGGACGGGCACCAGGAG-3’, IL-6, F: 5’- GCGCTTGTGGAGAAGGAGT-3’, R: 5’-TGGAGATGTCTGAGGCTCATT-3’; IL-1β, F: 5’-TGGAGATGTCTGAGGCTCATT-3’, R: 5’- GACAAGCTGAGGAAGATGCT GG-3’; GAPDH, F: 5’-CTAAACAGATGAAGTGCTCC-3’, R: 5’-ACGACCAAATCCGTTG ACTC-3’.
Immunofluorescence staining
Tissue immunofluorescence staining: Paraffin sections were deparaffinized in xylene, followed by hydration in varying concentrations of ethanol. Permeabilization was achieved using a 0.1% Triton x-100 PBS solution at 4 °C for 1 h. Antigen retrieval was performed using a citrate buffer mixture. Subsequently, the sections were blocked in a 1% Tween-20 PBS solution at 4 °C for 10 min, followed by incubation with a 5% BSA PBS solution at room temperature for 1 h. The primary antibody was then diluted in the 5% BSA PBS solution and incubated the sections at 4 °C overnight. Next, the sections were then incubated with a fluorescein-conjugated secondary antibody, which was diluted in a 5% BSA PBS solution at room temperature for 1 h. Finally, DAPI was utilized to stain the nuclei at room temperature for 5 min prior to observation under a fluorescence microscope.
Cellular immunofluorescence staining: Following treatment with recombinant Htra4 protein, HUVECs were washed with cold PBS, and then fixed with 4% paraformaldehyde at room temperature for 15 min. Subsequently, the cells were permeabilized using a 0.1% Triton x-100 PBS solution at room temperature for 15 min, followed by blocking with a 5% BSA PBS solution at room temperature for 1 h. The cells were incubated overnight at 4 °C with the primary antibody, which was diluted in a 5% BSA PBS solution. Next, the cells were incubated with a fluorescein-conjugated secondary antibody, also diluted in the same solution, at room temperature for 1 h. Finally, DAPI was utilized to stain the nuclei at room temperature for 5 min prior to observation under a fluorescence microscope.
Hematoxylin and Eosin staining
Paraffin sections were deparaffinized in xylene and subsequently hydrated in ethanol of diverse concentrations, followed by washing with tap water for 5 min. Hematoxylin staining was conducted for 5 min, followed by a 5 min wash with tap water. The sections were treated with hydrochloric acid alcohol for 5 S and washed with tap water for 3 min. Subsequently, sections were treated with 1% ammonia water for 30 S and washed with tap water for 5 min. After that, the sections were incubated with 80% and 90% ethanol for 5 min respectively. Eosin staining was performed for 2 min. Next, 95%, 100% I, and 100% II alcohol were used to treat the paraffin sections for 5 min. Finally, after a 10 min treatment with xylene, the sections were mounted with neutral resin.
ROS detection
The levels of reactive oxygen species (ROS) in HUVECs were measured using the Reactive Oxygen Species Assay Kit (Yeasen, Shanghai, China). HUVEC cells with robust growth were seeded in a 96-well plate. After treatment with recombinant Htra4 protein for 12 h, the cells were incubated in serum-free DMEM medium containing 10 µM DCFH-DA for 30 min. Subsequently, the medium was removed, and the cells were washed three times with cold PBS before being observed and photographed under a fluorescence microscope.
MDA and GSH detection
The Malondialdehyde (MDA) Content Assay Kit (Solarbio, BC0025, Beijing, China) was utilized to measure MDA levels in HUVECs. HUVECs cells were seeded in a 6-well plate. Following treatment with recombinant Htra4 protein for 12 h, the cells were washed with cold PBS and harvested using 1 ml of extraction solution. Cell lysis was achieved using an ultrasonic processor, followed by centrifugation at 8,000 g for 10 min to collect the supernatant. Subsequently, reagents were added according to the manufacturer’s instructions and heated at 100 °C for 1 h. Absorbance was then measured at wavelengths of 532 nm and 600 nm using a microplate reader.
The GSH levels in HUVECs were measured using the Reduced Glutathione (GSH) Content Assay Kit (Solarbio, BC0025, Beijing, China). Following treatment with recombinant Htra4 protein for 12 h, cells were washed with cold PBS and collected using 1 ml of extraction solution. Cell lysis was achieved using an ultrasonic processor, followed by centrifugation at 12,000 g for 10 min to collect the supernatant. Subsequently, reagents were added according to the manufacturer’s instructions and incubated at room temperature for 2 min before measuring the absorbance at a wavelength of 412 nm.
Statistical analysis
SPSS 26 and GraphPad Prism 10 were utilized for data analysis. The data were presented as mean ± SEM. Pairwise comparisons were conducted using the SNK (Student-Newman-Keuls) test. The interdependent relationship between two variables was examined through Pearson/Spearman linear correlation analysis, with a significance level of α = 0.05 employed for the test. Levels of *p < 0.05, **p < 0.01, ***p < 0.001 indicate statistically significant differences.
Results
Characteristics of baseline data in patients with early-onset PE
Table 1 results revealed that, in comparison to the normal control group, early-onset PE patients had no significant differences in baseline data such as gestational age, pre-pregnancy BMI, gestational weight gain, and parity (P > 0.05). However, early-onset PE patients demonstrated significantly higher levels of gestational weight gain (p < 0.05), systolic BP (p < 0.001), and diastolic BP (p < 0.001) compared to the normal control group, suggesting an increase in body weight and elevation of blood pressure in early-onset PE patients.
Characteristics of serum biomarkers in patients with early-onset PE
Compared to the normal control of pregnant women (Table 2), there were no statistically significant variances in creatine kinase (CK), glutamic pyruvic transaminase (ALT), and platelet (PLT) levels among early-onset PE patients (p > 0.05). However, levels of creatine kinase MB (CK-MB) isoenzyme (p < 0.001), aspartate transaminase (AST) (p = 0.002), and β2-microglobulin/creatinine/ uric acid (p < 0.001) in early-onset PE are significantly higher than those in normal control, indicating potential cardiac, hepatic, and renal damage in early-onset PE patients.
A comparative analysis of pregnancy outcomes between women with early-onset PE and normal control
The comparison of pregnancy outcomes (Table 3) revealed that early-onset PE patients had a significantly prolonged hospital stay (p = 0.029). Additionally, newborns of early-onset PE patients exhibited significantly lower birth weight (p = 0.005) and length (p = 0.004). Furthermore, placental weight (p = 0.012) and area (p < 0.001) were both reduced in early-onset PE patients, while no difference was observed in the ratio of newborn weight to placental weight (p = 0.098), possibly due to decreases in both newborn and placental weights. The Apgar scores at 1 min, 5 min, and 10 min (p > 0.05) showed no significant differences between the two groups. Overall, these findings suggest that early-onset PE is associated with adverse pregnancy outcomes, such as prolonged hospitalization for expectant mothers and impact on fetal development.
The alteration of Htra4 levels in maternal and umbilical cord blood
Peripheral blood samples were obtained from pregnant women prior to delivery, and umbilical cord blood samples were collected post-delivery for the assessment of Htra4 concentration (Table 4). The results revealed a significantly higher level of Htra4 in the peripheral blood of early-onset PE patients (p < 0.001). While the concentration of Htra4 in umbilical cord blood was also elevated in early-onset PE patients, the difference did not reach statistical significance (p = 0.10). Overall, these results suggest a potential correlation between alterations in Htra4 concentration and early-onset PE pathology.
Correlational analysis of Htra4 levels in the blood with clinical indicators of patients with early-onset PE
Our research findings demonstrate a correlation between early-onset PE with the concentration of Htra4 in blood. We will now proceed to investigate the correlation between the concentration of Htra4 in peripheral blood (Table 4) and significant variances in baseline data (Table 1), peripheral blood serum biomarkers (Table 2), and pregnancy outcomes (Table 3) among early-onset PE patients. The results indicated a significant positive correlation between the concentration of Htra4 in peripheral blood prior to delivery and systolic BP (p < 0.001), diastolic BP (p < 0.001), CK-MB (p = 0.002), AST (p = 0.001), Cr (p < 0.001), UA (p < 0.001), β2 microglobulin (p < 0.001), and urea (p < 0.001) (Table 5). Notably, the most significant correlation was found in relation to systolic and diastolic BP. Additionally, it was noted that the pre-delivery concentration of Htra4 in peripheral blood exhibited a significant negative correlation with neonatal weight (p = 0.002), neonatal length (p = 0.016) as well as placental weight (p = 0.012) and placental area (p < 0.001), neonatal length, placental weight, and placental area (Table 5). Conversely, there was no apparent correlation between umbilical cord blood Htra4 concentration and the aforementioned indicators (Table 5). These findings indicate a strong correlation between the level of Htra4 in the peripheral blood of early-onset PE patients and adverse pregnancy outcomes, as well as indicators of neonatal development. This suggests that elevated levels of Htra4 in peripheral blood may contribute to negative pregnancy outcomes for early-onset PE patients.
Expression and localization of Htra4 in placenta and decidua tissue
We have confirmed the elevated concentration of Htra4 in the peripheral blood of patients with early-onset PE. Subsequently, we will verify the expression and localization of Htra4 in the placenta and decidua. Western blot analysis demonstrated increased levels of Htra4 in the placenta (Fig. 1A), as well as higher Htra4 mRNA levels in early-onset PE patients (Fig. 1B). H&E staining results indicated that the placenta tissue of normal control pregnant women had mature intermediate villi, with two layers of cells covering the surface. The inner layer consisted of cytotrophoblasts and the outer layer was composed of syncytiotrophoblasts, with syncytial knots rarely occurring. However, in patients with early-onset PE, the villi were slender and displayed signs of excessive maturity, along with an increase in syncytial knots (Fig. 1C). Tissue immunofluorescence further validated these findings that early-onset PE have a highly content of Htra4, and revealing predominant expression of Htra4 in syncytiotrophoblast cells of the placenta (Fig. 1D, E). Fluorescence staining intensity for Htra4 was significantly higher in trophoblast cells from early-onset PE patients, indicating an upregulation of Htra4 expression in placental tissues (Fig. 1F, G). Additionally, a higher expression of Htra4 was observed in decidual tissue from patients with early-onset PE, along with abundant staining within interstitial spaces (Fig. 1F, G). In decidual tissues of early-onset PE, features such as narrow spiral artery lumens and irregularly remodeled vessel walls were observed. Additionally, non-reshaped or incompletely reshaped vessels were also noted (Fig. 1F, H). CK7 staining indicated that there were fewer trophoblast cells, suggesting a decreased invasion of trophoblast cells in the decidua of early-onset PE (Fig. 1F, I).
Expression and localization of Htra4 in placenta and decidua tissue. (A) Western blot was used to analyze the expression of Htra4 in placental tissue; (B) qRT-PCR was employed to analyze the levels of Htra4 RNA in placental tissue; (C) H&E staining of placenta tissue, the red arrow points to the mature villi, and the black arrow indicates the syncytial knots, image amplification 5× (scale bar 100 μm) and 10× (scale bar 100 μm) (n = 4); (D) Tissue immunofluorescence was used to analyze the expression and localization of Htra4 in the placenta, image amplification 20× (scale bar 100 μm) and 40× (scale bar 50 μm) (n = 4); (E) The scanning statistics regarding Htra4 level in placental tissues (n = 4); (F) Tissue immunofluorescence was used to analyze the expression and localization of Htra4 in the decidua, image amplification 10× (scale bar 200 μm) and 20× (scale bar 100 μm) (n = 4). (G-I) The scanning statistics regarding Htra4/CD31/CK7 levels in decidua (n = 4). Means ± SEM, * p < 0.05, ** p < 0.01, and *** p < 0.001.
Htra4 facilitates the initiation of oxidative stress in HUVECs
Strong Htra4 immunostaining was observed in both the placenta and decidua tissues of early-onset PE patients, suggesting a potential correlation between Htra4 and impaired remodeling of the spiral arteries in the decidua. Subsequently, we supplemented recombinant Htra4 protein to stimulate HUVEC cells in order to investigate the adverse effects of elevated levels of Htra4 on blood vessels, mimicking the impact of increased Htra4 in peripheral blood of early-onset PE patients on vascular endothelium. We found that Htra4 significantly elevated the levels of MDA (Fig. 2A) while significantly decreasing the levels of GSH in HUVECs (Fig. 2B). Furthermore, our findings indicated that supplementation with Htra4 in the cell culture medium of HUVECs significantly increased the production of ROS (Fig. 2C), suggesting that Htra4 may induce oxidative stress in umbilical vein endothelial cells. Oxidative stress is the result of an imbalance between oxidation and antioxidant processes at the cellular level, caused by free radicals in the body. When cells are exposed to external stimuli that generate ROS and experience oxidative stress, they have the ability to restore internal equilibrium through activation of the antioxidant system, which includes the crucial intracellular antioxidant protein NRF2. The results of cellular immunofluorescence demonstrated that supplementation with Htra4 significantly enhanced the nuclear translocation of NRF2, and the fluorescence intensity of NRF2 in the nucleus was higher than that in the cytoplasm (Fig. 2D). Furthermore, Htra4 increased the expression of NRF2/HO-1/NQO-1 (Fig. 2E, F). These findings indicate that the supplementation of Htra4 does in fact result in oxidative stress in HUVECs.
Htra4 facilitates the initiation of oxidative stress in HUVECs. (A, B) The impact of recombinant Htra4 protein on MDA and GSH production in HUVECs (n = 6); (C) The impact of recombinant Htra4 protein on ROS production in HUVECs (n = 6), and the scanning statistics regarding the fluorescence intensity of ROS; (D) Cellular immunofluorescence analysis of NRF2 nuclear translocation in recombinant Htra4 protein-induced HUVECs (n = 6), and the ratio of the fluorescence intensity of the nucleus to that of the cytoplasm; (E,F) Western blot analysis of NRF2/HO-1/NQO-1 expression in recombinant Htra4 protein induced-HUVECs (n = 3). Means ± SEM, ** p < 0.01, and *** p < 0.001.
Htra4 facilitates the inflammatory response in HUVECs
This systemic inflammatory response is considered a crucial determinant of the vascular endothelial damage observed in early-onset PE patients, and supplementation with recombinant Htra4 protein enhances the expression of pro-inflammatory cytokines IL-6 and IL-1β. Therefore, we proceeded to investigate the impact of recombinant Htra4 protein on the inflammatory response of HUVECs cells. Our findings indicated that recombinant Htra4 protein upregulates the expression of pro-inflammatory cytokines IL-6 and IL-1β (Fig. 3A, B). Htra4 facilitated the phosphorylation of ERK/JNK/P38 signal pathway. Moreover, Htra4 enhanced the nuclear translocation of NF-κB p65 (Fig. 3C), and the fluorescence intensity of NF-κB p65 in the nucleus was higher than that in the cytoplasm (Fig. 3D). ERK/JNK/P38 and NF-κB p65 are key components of the classical inflammatory signaling pathway, suggesting that recombinant Htra4 protein may contribute to the inflammatory response in HUVECs.
Htra4 facilitates the inflammatory response in HUVECs. (A, B) qRT-PCR was used to identify the impacts of recombinant Htra4 protein on pro-inflammatory cytokines IL-6 and IL-1β in HUVECs (n = 3); (C Western blot analysis of ERK/JNK/P38 phosphorylation in recombinant Htra4 protein induced-HUVECs (n = 3). (D) Cellular immunofluorescence analysis of NF-κB p65 nuclear translocation in recombinant Htra4 protein-induced HUVECs (n = 3), and the ratio of the fluorescence intensity of the nucleus to that of the cytoplasm. Means ± SEM, * p < 0.05, and *** p < 0.001.
Discussion
The placenta serves as a crucial connection between the mother and the fetus, facilitating normal fetal development by means of hormone production, nutrient absorption, and regulation of gas exchange20. Early-onset PE is a severe pregnancy-related hypertensive disorder, which can exert significant influences on both maternal and fetal health due to its early occurrence and severity. Hence, identifying early predictive indicators for early-onset PE is of the greatest importance. Research has demonstrated that Htra4 is primarily produced by the placenta, and its secretion rises with gestational age until 24–25 weeks of pregnancy, remaining stable in the middle to late stages of pregnancy21. Htra4 is essential for placental implantation and embryonic development processes and may exert an influence on trophoblast fusion and differentiation22. The elevation of Htra4 in the blood of PE patients during the mid-to-late stages of pregnancy, suggesting its association with the onset of PE, especially for early-onset cases23,24. However, the influence of Htra4 on PE remains unclear, and whether Htra4 can potentially act as an early predictive indicator for adverse pregnancy outcomes in early-onset PE has not been recorded.
In this study, it was observed that the early-onset PE patients gained significantly more weight during pregnancy. Moreover, more pronounced organ damage was evident in these patients, as indicated by elevated indicators of the liver, kidney, heart, and blood, including significant increases in CKMB, AST, Cr, UA, and β2 microglobulin compared to normal pregnant women. The findings suggest that patients with early-onset PE suffer from serious maternal injuries which is consistent with previous research25. Furthermore, the levels of Htra4 were significantly increased in patients with early-onset PE compared to control pregnant women; however, no significant difference was observed in the levels of Htra4 in umbilical cord blood, suggesting that Htra4 may be more relevant to adverse maternal pregnancy outcomes. Additionally, there exists a strong correlation between the levels of maternal blood Htra4 and vascular-related indicators, such as systolic pressure and diastolic pressure as well as a significant association with neonatal weight, placental area, and the outcome of maternal organ damage. Therefore, it can be deduced that the levels of maternal blood Htra4 might serve as an early predictive marker for the outcome of pregnancies complicated by early-onset PE patients and could be related to vascular dysfunction that affects maternal organ damage.
During the formation of the decidua, invasive trophoblast cells erode the walls of the spiral arteries in the middle layer, forming trophoblastic plugs and remodeling the smooth muscle of the blood vessels. The endothelial cells are replaced by extravillous trophoblasts, resulting in an open lumen that is unaffected by changes in maternal blood pressure and ensuring adequate perfusion of maternal blood to the placenta. This physiological transformation of uteroplacental spiral arteries is of crucial significance for successful implantation and normal placental function. In a normal pregnancy, there is a remarkable increase in the diameter of spiral artery lumens, with trophoblast cells replacing vascular endothelial cells, which is known as arterial remodeling26. Conversely, the persistence of smooth muscle in vessel walls can lead to insufficient perfusion from maternal blood vessels to the placenta. Studies have shown that the placentas of patients with PE might present severe defects in arterial remodeling due to impaired endothelial function, causing inadequate vascular remodeling and abnormal placental development27,28. Our findings revealed an accelerated villous maturation of placental tissue in the early-onset PE patients, which was characterized by an increased syncytiotrophoblast knots, a reduction in the invasion of trophoblast cells into decidua, and unsuccessful remodeling of the maternal uterine artery, and there was a narrowing and irregular shaping of the decidua spiral arterial lumen in PE patients. Additionally, Htra4 was found to be localized in villous cytotrophoblasts and syncytiotrophoblasts, decidual stroma and extravillous trophoblasts, which is consistent with previous research findings22. Strong Htra4 immunostaining was observed in both placental and decidual tissues of early-onset PE patients, suggesting a potential association between Htra4 levels and the impaired remodeling of spiral arteries in decidua.
Next, in vitro experiments were employed to investigate the influence of the increased levels of Htra4 in peripheral blood on vascular endothelial cells. Our results indicated that recombinant Htra4 protein promotes the generation of ROS and MDA, as well as a reduction in GSH levels. When cells are exposed to external stimuli that generate ROS and undergo oxidative stress, they can restore the internal balance through the antioxidant system, including the key intracellular antioxidant protein NRF2. In this study, we found that recombinant Htra4 protein promotes the nuclear translocation of NRF2, and the expression of NRF2/HO-1/NQO-1, suggesting that high levels of Htra4 in peripheral blood might induce the onset of oxidative stress in vascular endothelial tissue. Furthermore, the recombinant Htra4 protein promotes the expression of IL-6 and IL-1β mRNA, as well as the nuclear translocation of NF-κB p65, and the phosphorylation of ERK/JNK/p38, suggesting that high levels of Htra4 in peripheral blood trigger the inflammatory response in vascular endothelial tissue. The receptor of Htra4 remains unclear. Nevertheless, Htra4 is capable of cleaving the main receptor of VEGFA, namely the kinase domain receptor (KDR), thereby inhibiting VEGFA signaling. Subsequently, it disrupts endothelial cell function by inhibiting the phosphorylation of the Akt signaling pathway29. Moreover, supplementation with htra4 disturbs endothelial cell tube formation and permeability in a dose-dependent manner30, alters the expression of senescence genes in endothelial cells31, induces inflammation32. All the evidence suggests that Htra4 truly cause vascular endothelial dysfunction, which precisely coincides with the result of our experiment.
Conclusion
In this study, we observed significant organ damage in patients with early-onset PE, and there is a remarkable association between high levels of Htra4 and adverse pregnancy outcomes, especially regarding systolic and diastolic blood pressure. The vascular lumen remodeling of the placenta in early-onset PE patients was narrower and more irregular than that in normal control pregnant women, and intense Htra4 immunostaining was detected in both placental and decidual tissues in early-onset PE patients. The results demonstrating that the alterations of vascular function in the maternal body might be associated with oxidative stress and inflammatory responses of endothelial cells. In conclusion, our findings provide a new insight for early diagnosis of patients with early-onset PE and potential targets for treatment.
Data availability
Data are contained within the manuscript, that available from the corresponding author upon reasonable request.
References
Manoharan, M. M. et al. Metabolic theory of preeclampsia: implications for maternal cardiovascular health. American journal of physiology. Heart and circulatory physiology 327, H582-H597, (2024). https://doi.org/10.1152/ajpheart.00170.2024
Ali, M. et al. A comprehensive review. Clin. Chim. Acta. 563 https://doi.org/10.1016/j.cca.2024.119922 (2024). Preeclampsia.
Sircar, M., Thadhani, R. & Karumanchi, S. A. Pathogenesis of preeclampsia. Curr. Opin. Nephrol. Hypertens. 24, 131–138. https://doi.org/10.1097/MNH.0000000000000105 (2015).
El-Sayed, A. A. F. & Preeclampsia A review of the pathogenesis and possible management strategies based on its pathophysiological derangements. Taiwan. J. Obstet. Gynecol. 56, 593–598. https://doi.org/10.1016/j.tjog.2017.08.004 (2017).
Roberts, J. M. & Bell, M. J. If we know so much about preeclampsia, why haven’t we cured the disease? J. Reprod. Immunol. 99, 1–9. https://doi.org/10.1016/j.jri.2013.05.003 (2013).
Jaatinen, N., Ekholm, E., Laivuori, F. H. & Jaaskelainen, T. Impact of physical activity on preeclampsia and angiogenic markers in the Finnish genetics of Pre-eclampsia consortium (FINNPEC) cohort. Ann. Med. 56, 2325480. https://doi.org/10.1080/07853890.2024.2325480 (2024).
Espinoza, J. et al. Plasma soluble fms-like tyrosine kinase 1 to placental growth factor ratio of 11.5 multiples of median predicts preeclampsia with severe features within 2 weeks of testing. Am. J. Obstet. Gynecol. 231 (363 e361-363 e311). https://doi.org/10.1016/j.ajog.2024.05.050 (2024).
Poon, L. C. et al. The international federation of gynecology and obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. 145 (Suppl 1), 1–33. https://doi.org/10.1002/ijgo.12802 (2019).
Wojtowicz, A. et al. Early- and Late-Onset preeclampsia: A comprehensive cohort study of laboratory and clinical findings according to the new ISHHP criteria. Int. J. Hypertens. 2019 (4108271). https://doi.org/10.1155/2019/4108271 (2019).
Faccio, L. et al. Characterization of a novel human Serine protease that has extensive homology to bacterial heat shock endoprotease HtrA and is regulated by kidney ischemia. J. Biol. Chem. 275, 2581–2588. https://doi.org/10.1074/jbc.275.4.2581 (2000).
Wang, L. J., Cheong, M. L., Lee, Y. S., Lee, M. T. & Chen, H. High-temperature requirement protein A4 (HtrA4) suppresses the fusogenic activity of syncytin-1 and promotes trophoblast invasion. Mol. Cell. Biol. 32, 3707–3717. https://doi.org/10.1128/MCB.00223-12 (2012).
Beleford, D., Rattan, R., Chien, J. & Shridhar, V. High temperature requirement A3 (HtrA3) promotes etoposide- and cisplatin-induced cytotoxicity in lung cancer cell lines. J. Biol. Chem. 285, 12011–12027. https://doi.org/10.1074/jbc.M109.097790 (2010).
Milner, J. M., Patel, A. & Rowan, A. D. Emerging roles of Serine proteinases in tissue turnover in arthritis. Arthritis Rheum. 58, 3644–3656. https://doi.org/10.1002/art.24046 (2008).
Grau, S. et al. Implications of the Serine protease HtrA1 in amyloid precursor protein processing. Proc. Natl. Acad. Sci. U.S.A. 102, 6021–6026. https://doi.org/10.1073/pnas.0501823102 (2005).
Coleman, H. R., Chan, C. C., Ferris, F. L., Chew, E. & 3rd & Y. Age-related macular degeneration. Lancet 372, 1835–1845. https://doi.org/10.1016/S0140-6736(08)61759-6 (2008).
Li, Y. et al. Placental HtrA3 is regulated by oxygen tension and serum levels are altered during early pregnancy in women destined to develop preeclampsia. J. Clin. Endocrinol. Metab. 96, 403–411. https://doi.org/10.1210/jc.2010-1405 (2011).
Liu, C. et al. Elevated HTRA1 and HTRA4 in severe preeclampsia and their roles in trophoblast functions. Mol. Med. Rep. 18, 2937–2944. https://doi.org/10.3892/mmr.2018.9289 (2018).
Fantone, S., Giannubilo, S. R., Marzioni, D. & Tossetta, G. HTRA family proteins in pregnancy outcome. Tissue Cell. 72, 101549. https://doi.org/10.1016/j.tice.2021.101549 (2021).
Tossetta, G. et al. HTRA1 in placental cell models: A possible role in preeclampsia. Curr. Issues. Mol. Biol. 45, 3815–3828. https://doi.org/10.3390/cimb45050246 (2023).
Apicella, C., Ruano, C. S. M., Mehats, C., Miralles, F. & Vaiman, D. The role of epigenetics in placental development and the etiology of preeclampsia. Int. J. Mol. Sci. 20 https://doi.org/10.3390/ijms20112837 (2019).
Inagaki, A. et al. Upregulation of HtrA4 in the placentas of patients with severe pre-eclampsia. Placenta 33, 919–926. https://doi.org/10.1016/j.placenta.2012.08.003 (2012).
Wang, Y., Li, Y. & Nie, G. HtrA4 is well conserved only in higher primates and functionally important for EVT differentiation. Placenta 152, 53–64. https://doi.org/10.1016/j.placenta.2024.05.132 (2024).
Wang, Y., Lim, R. & Nie, G. HtrA4 May play a major role in inhibiting endothelial repair in pregnancy complication preeclampsia. Sci. Rep. 9, 2728. https://doi.org/10.1038/s41598-019-39565-9 (2019).
Tseng, E., Yee Teoh, S. S., Wang, Y. & Nie, G. Elevated protease HtrA4 in the maternal circulation of preeclampsia May contribute to endothelial barrier disruption by cleaving key junctional protein VE-cadherin. Placenta 76, 51–53. https://doi.org/10.1016/j.placenta.2019.01.001 (2019).
Dimitriadis, E. et al. Pre-eclampsia. Nat. Reviews Disease Primers. 9, 8. https://doi.org/10.1038/s41572-023-00417-6 (2023).
de Suarez, G., de Paco Matallana, T., Plasencia, W. & C. & Spiral, uterine artery doppler and placental ultrasound in relation to preeclampsia. Best Pract. Res. Clin. Obstet. Gynecol. 92, 102426. https://doi.org/10.1016/j.bpobgyn.2023.102426 (2024).
Lyall, F., Robson, S. C. & Bulmer, J. N. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension 62, 1046–1054. https://doi.org/10.1161/HYPERTENSIONAHA.113.01892 (2013).
Wallace, A. E., Fraser, R. & Cartwright, J. E. Extravillous trophoblast and decidual natural killer cells: a remodelling partnership. Hum. Reprod. Update. 18, 458–471. https://doi.org/10.1093/humupd/dms015 (2012).
Wang, Y., La, M., Pham, T., Lovrecz, G. O. & Nie, G. High levels of HtrA4 detected in preeclamptic circulation May disrupt endothelial cell function by cleaving the main VEGFA receptor KDR. FASEB Journal: Official Publication Federation Am. Soc. Experimental Biology. 33, 5058–5066. https://doi.org/10.1096/fj.201802151RR (2019).
Singh, H. et al. Human HtrA4 expression is restricted to the placenta, is significantly up-regulated in early-onset preeclampsia, and high levels of HtrA4 cause endothelial dysfunction. J. Clin. Endocrinol. Metab. 100, E936–945. https://doi.org/10.1210/jc.2014-3969 (2015).
Wang, Y., Lim, R. & Nie, G. Elevated circulating HtrA4 in preeclampsia May alter endothelial expression of senescence genes. Placenta 90, 71–81. https://doi.org/10.1016/j.placenta.2019.12.012 (2020).
Wang, Y. & Nie, G. High levels of HtrA4 observed in preeclamptic circulation drastically alter endothelial gene expression and induce inflammation in human umbilical vein endothelial cells. Placenta 47, 46–55. https://doi.org/10.1016/j.placenta.2016.09.003 (2016).
Funding
This research was funded by the "S&T Program of Hebei, grant number 21377707D" and the "Medical Science Research Project of Hebei, grant number 20250045.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. HF Kong: Manuscript writing, Project development; XY Ma: Project development; F Wang: Data analysis; Y Su: Data collection; YQ Chang: Data collection; H Xin: Manuscript writing, Protocol, Editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kong, H., Ma, X., Wang, F. et al. Htra4 promotes vascular endothelial cell injury and is associated with the early-onset of preeclampsia. Sci Rep 15, 11752 (2025). https://doi.org/10.1038/s41598-025-96819-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-96819-5