Abstract
Comparing the effects of 8 weeks of aerobic (AE) and resistance exercise (RE) on cardiovascular function in obese young adults. Exploring the mechanisms of resistance exercise on cardiovascular function. 44 subjects (22.4 ± 2.7 years, female/male: 26/18) were randomly divided into the AE and RE group. AE group performed platform running with 70–75% heart rate max. RE group performed deep squat, bench press, and hard pull training in sequence with 70–75% 1 repetition maximum. Body composition, cardio-vascular function indicators were measured before and after the interventions. After eight weeks, body fat percentage was reduced in both groups (AE: P < 0.0001, RE:P = 0.03). Hemodynamic indices (P < 0.05), endothelium-dependent diastolic function (AE:P = 0.01, RE:P = 0.024) and endothelial nitric oxide synthase (P < 0.0001) were increased. Both AE and RE exercise increased cardiac function (P < 0.05). The RE group (P = 0.002) had a significantly higher muscle mass. Brachial-ankle pulse wave conduction velocity (left: P = 0.006; right: P < 0.0001) was significantly lower in the AE group. Both AE and RE significantly reduced the body fat percentage, improved cardiac function, hemodynamic, and vascular endothelial function in obese young adults. AE has a stronger effect on reducing arterial stiffness, whereas resistance exercise has a stronger effect on building muscle, but does not change arterial stiffness.
Similar content being viewed by others
Introduction
Over the past few decades, cardiovascular disease has been a significant contributor to global mortality. Obesity is an independent predictor of cardiovascular disease, with the Global Burden of Disease Obesity Collaborators estimating that it was responsible for 4 million deaths in 2015, two-thirds of which were attributed to cardiovascular disease1. Obesity significantly alters the structure and function of the cardiovascular system, mainly characterized by ventricular hypertrophy2, impaired haemodynamic properties3, reduced nitric oxide (NO) availability, endothelial dysfunction and increased arterial stiffness4. On the other hand, a 40-year longitudinal study found increasing rates of obesity and cardiovascular disease among young adults, suggesting a global trend toward obesity and cardiovascular disease at a younger age5. This will increase the future health care burden of cardiovascular disease. Obesity-related cardiovascular risks can be prevented through lifestyle changes. Thus, attention should be paid to young obese adults and active measures should be taken to prevent the development of obesity-related cardiovascular disease.
Exercise is an important non-pharmacological strategy for weight loss and the prevention of cardiovascular disease. Many studies have demonstrated the benefits of aerobic exercise (AE) in improving cardiovascular function in young, old and obese individuals6,7. Although the beneficial effects of AE are well known, it remains controversial whether resistance exercise (RE) improves cardiovascular function. A study has shown that RE significantly improves vascular endothelial dysfunction in overweight or obese patients8. But other evidence suggests that RE is less effective than AE in improving arterial stiffness and altering cardiovascular health and that it may exacerbate arterial stiffness9. In addition, RE has become increasingly more popular among young people due to its muscle building and fat loss effects. Endothelial function and arterial stiffness represent different aspects of vascular health, and endothelial dysfunction occurs in early stages of atherosclerotic vascular injury. Previous studies on the effects of RE on cardiovascular function have focused on a single factor, and there has been a lack of studies that comprehensively examined the effects of AE and RE on cardiovascular function in young obese adults. Therefore, this study provides an 8week AE and RE intervention for obese young adults and comprehensively compares the different effects of AE and RE on cardiovascular function. Moreover, this study found that 8 weeks of 70–75% 1RM RE exercise not only reduces body fat and increases vascular endothelial function, but also does not change arterial stiffness. RE is a safe exercise option for young obese people to lose fat and promote cardiovascular health.
Methods
Study participants
A power analysis was performed by G*Power (Version 3.1) to justify the sample size. Setting power of 0.8 at α = 0.05 and an effect size of 0.25, a sample size of 17 participants for each group was necessary. Considering a dropout rate of ~ 20%, a total sample size of 41 would be expected. Forty-four (18 male and 26 female) obese young adults were randomly assigned to an AE group (n = 21) and an RE group (n = 23). The inclusion criteria were (1) an age of 17–35 years (young adults); (2) BF% of > 25% for male and > 30% for female10; (3) a sedentary lifestyle (no participation in systematic/supervised exercise programs in the previous six months); (4) no history of cardiovascular disease, diabetes, hypertension, or other related diseases. Before the study, all subjects were fully informed about the exercise intervention procedures and purpose of the experiment and voluntarily signed informed consent forms. This study was approved by the Ethics Committee of Beijing Sport University. The ethical clearance form number is (NO.):2022191 H, and all procedures conformed to the Declaration of Helsinki.
Exercise intervention
Aerobic exercise
Subjects in the AE group performed AE on a running platform for eight weeks, with a researcher supervising and guiding the entire sessions. Each session consisted of a 5-min low-intensity (50–60% of HRmax) warm-up, 50 min of formal training at 70–75% HRmax, and 5 min of relaxation at 50–60% HRmax three times a week on alternate days6. The exercise sessions were scheduled between 14:00 and 18:00 p.m. HRmax was calculated as HRmax = (207 − 0.7 × age). During each session, a heart rate monitor (Team2; Polar Electro, Finland) was used to ensure that the heart rate was maintained within the target range.
Resistance exercise. Subjects in the RE group were tested for 1RM using the American Society of Exercise Physiologists recommendations. Warmed up at 20–30% 1RM intensity for 5 min before each formal training session, then complete the squat, bench press and hard pull in sequence, with a load of 70–75% 1RM for each movement in 3 sets, 12 repetitions per set, 2-min intervals between them, and a final 5-min static stretch11. Each session lasted 60 min, 3 times/week, for 8 weeks. A researcher supervised the participants throughout the sessions. The 1RM was remeasured at the end of week 4 12. Exercise during women’s menstrual cycle is based on self-reported physical condition, and in the absence of menstrual cramps, subjects could choose another time during the week to complete 3 sessions of exercise.
Body composition
Weight, BF%, and fat-free mass (FFM) of subjects were measured using a digital dual-energy radiometer (iDXA; GE Healthcare, USA) one day before and early in the morning 48 h after the end of the interventions in a fasting state. Before the test, subjects were reminded not to wear metal-containing clothing and jewelry.
Cardiac ultrasound
Cardiac function indices were measured before and after the interventions using color doppler ultrasound (Vivid7; GE Healthcare, USA). Each subject was placed in the supine position, breathing calmly, and a M4S (2.5-MHz) probe was placed on the left edge of the sternum to obtain an M-shaped image of the left ventricular short-axis mitral tendon level under two-dimensional image guidance. The cardiac structure and function parameters measured included left ventricular end-diastolic volume (LV-EDV), left ventricular end-systolic volume (LV-ESV), heart rate (HR), stroke volume (SV), eject fraction (EF), and cardiac output (CO).
Haemodynamics
Hemodynamic parameters were measured using color doppler ultrasound (Vivid7; GE Healthcare, USA), with the subjects lying quietly on their backs with their heads elevated to expose their necks. A 10-MHz linear probe was used to scan longitudinally along the outer edge of the sternocleidomastoid muscle from the beginning of the common carotid artery to the bifurcation. The long- and transverse-axis views of the common carotid artery were examined, and peak systolic velocity (PSV) and internal vessel diameter were measured in the R wave of electrocardiograms. Wall shear stress (WSS) was calculated as follows13. The shear rate (SR) was calculated based on the data obtained from the flow-mediated dilation FMD measurements as follows.
Where τ is the WSS (dyn·cm− 2), η is blood viscosity (mPa·s− 1), V is maximum carotid flow velocity (cm·s− 1), and D is the diastolic internal vessel diameter (cm).
FMD
Brachial artery FMD was assessed a using a 10-MHz linear array transducer ultrasound probe (UNEX; EF, Japan), with each subject resting in a quiet room at a comfortable temperature for 10 min before recording the initial resting vessel diameter and arterial pressure. The basal diameter of the brachial artery of the subject’s right arm was located with a H-ultrasound probe under quiet conditions and recorded with the instrument’s automated edge detection software. Subsequently, the cuff was then inflated to 50 mm-Hg above systolic pressure to induce congestion, monitored in real time for 5 min, and then released, followed by continuous post-deflation diameter acquisition, with the peak diameter recorded 45–60 s after deflation. The percentage change in diameter (compared to baseline values) at maximum dilation during reactive congestion was used to estimate the FMD as follows. The shear rate (SR) was calculated based on the data obtained from the FMD.
Where PSV is the peak systolic flow velocity of the brachial artery (cm·s− 1), and D is the diastolic internal vessel diameter (cm).
PWV
Arterial stiffness was measured using an atherosclerosis detector (BP-203 RPE II; Omron, Japan). Each subject was seated for 10 min, lying flat on an examination bed, with limb cuffs tied to the upper arm and ankle, leaving a finger-wide pace between them. The upper arm cuff balloon marker was aligned with the brachial artery, and the lower edge of the cuff was 2–3 cm from the transverse elbow fossa. The lower limb cuff balloon marker was located on the medial side of the lower limb, and the lower edge of the cuff was 1–2 cm from the medial ankle. The heart sound acquisition device was placed in the precordial region, and the left and right wrists were clamped to the cardiac acquisition device. The test was performed with automatic pressure, and baPWV was recorded from the left and right sides and calculated as follows.
where L1 = (0.5643 × H − 18.381) + (0.2486 × H + 30.709), L2 = 0.2195 × H − 2.0734, ΔT is the time difference between the humeral and ankle waveforms (s), and H is height (cm).
Blood collection and analyses
Venous blood (5 mL) was taken from the elbow of each subject in a fasting state at 8–10 a.m. 48 h before exercise and after the interventions by the same operator. The samples were centrifuged at 4000 rpm for 5 min, and the serum was separated and stored at − 4 °C. Serum nitric oxide (NO) levels were calculated indirectly by measuring nitric oxide metabolites (NOx), nitrite (NO2-), and nitrate (NO3-) using an enzyme-linked immunosorbent assay kit (A012; Nanjing Jiancheng Institute of Biological Engineering, China) according to the manufacturer’s instructions. An ELISA kit (JH-00224; Jinhai Keyu, Beijing, China) was used to detect eNOS levels according to the manufacturer’s instructions for assessing the ability of vascular endothelial cells to produce NO.
Statistical analyses
All indicators were tested individually by a professional tester who was specialized in testing the results of three tests with a margin of error of less than 5% after training. Each tester was responsible for extracting and organizing the test data into Excel form and uniformly sending it to a statistician for blind analysis. All data were processed by IBM SPSS Statistics 26 software. Continuous variables were tested for normal distribution and chi-squared test using the Shapiro–Wilk test and Levene’s test and expressed as means ± standard deviations. Differences in baseline indicators between the two exercise groups were assessed using independent-samples t-tests. Two-factor repeated-measures ANOVA was used to assess time–group interaction effects. For significant main effects or interactions, post hoc multiple pre- and post- intervention comparisons between and within the groups were performed using bonferroni methods. Values of P < 0.05 were considered statistically significant.
Results
Subjects characteristics
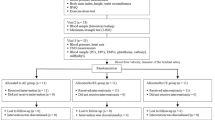
At the start of the program, 55 young obese adults were eligible. Reasons for exclusion and withdrawal of subjects throughout the intervention are shown in Fig. 1. In total, 44 obese subjects completed the study (AE: n = 21, RE: n = 23). At baseline, age, BF%, FFM and HR were comparable between groups (Table 1). There were no statistically significant differences between the AE and RE groups in terms of age, BF%, FFM and HR.
Body composition
In Table 2, FFM (P = 0.042) had statistically significant time × group interaction effects after the 8-week exercise intervention. BF% was significantly lower in both the AE (P < 0.001) and RE (P = 0.03) groups compared to pre-exercise and did not differ significantly between groups (P = 0.94). FFM was significantly increased in the RE group (P = 0.002) compared to pre-exercise.
Cardiac function
As shown in Table 3, there were statistically significant time × group interaction effects for SV (P = 0.011) LV-EDV (P < 0.001) and LV-ESV (P = 0.038) after the eight-week interventions. LV-EDV (AE: P < 0.0001, RE: P = 0.003), CO (AE: P < 0.0001, RE: P = 0.001) and SV (AE: P < 0.0001, RE: P = 0.007) were significantly higher in the AE and RE groups compared to the pre-exercise. Moreover, the AE group had significantly greater LV-EDV (P = 0.002), SV (P < 0.0001), and CO (P < 0.0001) than the RE group after the interventions. In the AE group, LV-ESV (P = 0.011) was significantly higher than before the exercise. Other indicators did not differ significantly within the group pre- and post-intervention.
Haemodynamics
In Table 4, time × group interaction effects were seen for PSV (P = 0.01) and SR (P = 0.03). Both AE and RE resulted in significantly greater in diameter PSV (AE: P < 0.01, RE: P = 0.01) (P < 0.0001), WSS (P < 0.0001), and SR (P < 0.01), with no statistically significant differences between the two groups.
Vascular function
FMD, eNOS, NO and PWV responses across conditions are presented in Fig. 2. There are statistically significant time × group interaction effects were observed for LbaPWV and RbaPWV (P <0.01). Further post-hoc multiple comparisons revealed that FMD (AE: P < 0.0001, RE: P = 0.024), eNOS (P < 0.0001) and NO (P < 0.0001) were significantly higher in both the AE and RE groups after 8 weeks of exercise compared to pre-exercise, with a greater increase in NO in the RE group compared to the AE group (P = 0.025). Both LbaPWV (P = 0.006) and RbaPWV (P < 0.0001) in the AE group showed a significant decrease after 8 weeks of intervention, while PWV (LbaPWV: P = 0.142, RbaPWV: P = 0.053) in the RE group was not statistically significant. At week 8, both LbaPWV and RbaPWV were significantly higher in the RE group than in the AE group (P = 0.002).
Comparison of the changes in NO (A), eNOS (B), FMD (C), LbaPWV (D) and RbaPWV (E) in response to the aerobic and resistance exercise interventions. The data are expressed as means ± standard deviations. *p < 0.05, **p < 0.01 versus pre-intervention within group, † p < 0.05 versus AE at the same time point.
Discussion
This study analyzed and compared the effects of eight-week AE and RE interventions on cardiovascular function in obese young adults and found that RE could be a good option for obese young adults to improve cardiovascular function. The details of the results showed that: (1) AE and RE had similar effects on reducing BF%, and RE significantly increased FFM; (2) Both AE and RE enhanced cardiac function, with AE having a more significant effect; (3) AE and RE not only increase WSS, PSV, and SR but also improved endothelial function; and (4) AE significantly reduced vascular stiffness, while RE does not change arterial stiffness.
Exercise is one of the most important measures for weight loss among obese people. In this study, 8 weeks of moderate AE and RE reduced the BF%, with no statistically significant differences between the two groups. Moreover, RE was effective in increasing FFM. Exercise is an effective, low-cost, and safe strategy for improving cardiac structure and function. However, different exercise modalities have different effects. RE can increase peripheral resistance, or afterload, which leads to myocardial centripetal hypertrophy, whereas AE increases venous return and blood volume, or preload, promoting myocardial centrifugal hypertrophy. In a previous study, overweight and obese people undergoing a 30-min AE intervention with an intensity of 75% HRmax for eight weeks showed significant increases in LV-EDV (5.5%), LV-ESV (15.3%), LV-SV (5.9%)14. These results are similar to our study. The increases in EDV, ESV, and CO after AE interventions may be the result of enhanced cardiac filling due to LV volume overload hypertrophy, increased myocardial contractile function (e.g., β-adrenergic stimulation enhancing contractile responses), reduced afterload, and increased blood volume. In addition, 8 weeks of resistance exercise also significantly increased EDV, SV, and CO in obese young adults. RE may lead to increased contractility of trained cardiomyocytes by enhancing myosin ATPase activity and inward Ca2+ flow, thus maintaining adequate output per beat in the face of increased afterload15. Although there was a significant increase in EDV, SV, and CO in the AE and RE groups alone, there was no change in EF or HR, which may be due to the fact that the combined increase in EDV and SV did not change the EF, and the shorter duration of the intervention did not result in an exercise-induced decrease in heart rate. Exploring the reasons for the superior improvements observed with AE, a previous study found that endurance exercise resulted in higher ventricular compliance, which may be related to the fact that different exercise modalities cause different hemodynamic changes, leading to corresponding changes in ventricular wall stress16.
Exercise has a direct impact on the vasculature through hemodynamics, leading to functional and structural adaptations. Montalvo et al. used three different intensities of AE and RE in healthy young adults and found that WSS increased in an intensity-dependent manner regardless of the exercise modality17. Similarly, our results suggested that eight weeks of AE and RE significantly increase prograde WSS in obese young adults. It is believed that exercise alters the body’s cardiac output and blood pressure and that the arteries adapt to these changes by enhancing segmental arterial compliance and pressure wave interactions, ultimately resulting in greater shear stress18.
The vascular endothelium is the first line of defense against cardiovascular disease. In a previous study, obese subjects undergoing AE intervention showed an increase in FMD of 3.21 ± 3.10%6. In our study, there was also a 3.6 ± 0.9% increase in FMD, confirming the effectiveness of AE in reversing vascular endothelial dysfunction caused by obesity. Unlike endurance training, little is known about the effects of RE on endothelial function in obese populations. In this study, FMD increased by 1.6 ± 1.2% in the RE group and did not differ significantly from the results obtained from AE. Exercise may inhibit the sympathetic nervous and renin–angiotensin systems, increasing shear stress. Intravascular blood flow increases, increasing the mechanical load on vessel walls, which in turn deforms the mechanosensitive channels of the endothelium and triggers molecular cascade reactions that secrete such mediators as NO, prostacyclin, prostaglandins, thromboxane, angiotensin II, endothelin-1, reactive oxygen species, and other compounds that mediate vascular function19. Interestingly, our findings also revealed that NO levels were significantly higher in both the AE and RE groups at the end of the 8-week intervention, and higher in the RE group than in the AE, and we suggest that resistance exercise involves more valsalvaal maneuvers, which increase cardiac afterload and reflexively increase the rate of blood flow to the organism, and this mechanical stimulation increases the production of more NO by the vascular endothelium20,21,22. In conclusion, AE and RE are similarly effective in improving vascular endothelial function in obese young adults.
AE significantly reduced PWV. Kobayashi et al.23 showed that regular AE reduced arterial stiffness in healthy adults, regardless of its intensity or duration. In our study, LbaPWV and RbaPWV decreased after eight weeks. The mechanism by which AE reduces PWV may be directly related to a reduction in elastin breakdown and collagen deposition in the arterial layer or indirectly related to reduced oxidative stress, inflammation, and sympathetic nerve activity24. In contrast, the effects of RE on atherosclerosis and PWV remain highly controversial. One study reported that RE reduced arterial stiffness in healthy young adults25, whereas another found that RE exacerbated it26. In our study, 8 weeks of resistance exercise did not significantly change baPWV. The mechanism by which RE physiologically regulates PWV has not been fully elucidated. It has been suggested that the activation of the sympathetic nervous system may affect arterial stiffness by regulating the complex interaction between smooth muscle tone and elevated blood pressure in large arteries and that high-intensity RE increases blood pressure and vagal activity, which in turn exacerbates arterial stiffness27. Although both FMD and PWV are commonly used to evaluate structural and functional vascular injury, studies on the relationship between the two indicators are scarce, and their findings are inconsistent. PWV and FMD represent different aspects of vascular injury and that early-stage endothelial function do not necessarily reflect the later arterial stiffness28. What’s more, baPWV reflected central elastic arterial stiffness and peripheral muscular arterial stiffness, whereas peripheral arteries did not seem to change significantly with age and disease state and thus had lesser clinical significance.
Conclusions
This study shows that both AE and RE interventions for eight weeks effectively reduced the BF% in obese young adults, improving cardiac function, hemodynamic indices and vascular endothelial function. AE was more effective in reducing arterial stiffness, while RE was more effective in muscle building and does not change arterial stiffness. This shows that both AE and RE can enhance cardiovascular function in obese young adults. As a conclusion, 75% 1RM RE is an effective choice for fat loss and improvement of cardiovascular function in young obese adults.
Data availability
The data sets generated and analyzed during the study are available from the corresponding author upon reasonable request.
References
Collaborators, G. O. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl.. J. Med. 377, 13–27. https://doi.org/10.1056/NEJMoa1614362 (2017).
Savji, N. et al. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail. 6, 701–709. https://doi.org/10.1016/j.jchf.2018.05.018 (2018).
Shaalan, W., El Emam, A., Lotfy, H. & Naga, A. Clinical and hemodynamic outcome of morbidly obese patients with severe chronic venous insufficiency with and without bariatric surgery. J. Vasc. Surg. Venous Lymphat. Disord. 9, 1248–1256e1242. https://doi.org/10.1016/j.jvsv.2021.01.005 (2021).
Ryder, J. R. et al. Accelerated early vascular aging among adolescents with obesity and/or type 2 diabetes mellitus. J. Am. Heart Assoc. 9, e014891. https://doi.org/10.1161/jaha.119.014891 (2020).
Andersson, C. & Vasan, R. S. Epidemiology of cardiovascular disease in young individuals. Nat. Rev. Cardiol. 15, 230–240. https://doi.org/10.1038/nrcardio.2017.154 (2018).
Sawyer, B. J. et al. Effects of high-intensity interval training and moderate-intensity continuous training on endothelial function and cardiometabolic risk markers in obese adults. J. Appl. Physiol. 121, 279–288. https://doi.org/10.1152/japplphysiol.00024.2016 (2016).
Verboven, M. et al. Effect of exercise intervention on cardiac function in type 2 diabetes mellitus: A systematic review. Sports Med. 49, 255–268. https://doi.org/10.1007/s40279-018-1003-4 (2019).
Hasegawa, N. et al. Effects of different exercise modes on arterial stiffness and nitric oxide synthesis. Med. Sci. Sports Exerc. 50, 1177–1185. https://doi.org/10.1249/mss.0000000000001567 (2018).
Tabaie, E. A. et al. The relationship between arterial stiffness and resistance training. Cureus 13, e20213. https://doi.org/10.7759/cureus.20213 (2021).
Zhu, W., Tian, H., Zhang, R. & Zhang, D. Body mass index body fat percentage waist-to-hip ratio evaluates differences in obesity among college students. China School Health. 40, 1703–1705. https://doi.org/10.16835/j.cnki.1000-9817.2019.11.029 (2019).
Wilk, M., Zajac, A. & Tufano, J. J. The influence of movement tempo during resistance training on muscular strength and hypertrophy responses: A review. Sports Med. 51, 1629–1650. https://doi.org/10.1007/s40279-021-01465-2 (2021).
Legaard, G. E. et al. Effects of different doses of exercise and diet-induced weight loss on beta-cell function in type 2 diabetes (DOSE-EX): a randomized clinical trial. Nat. Metab. 5, 880–895. https://doi.org/10.1038/s42255-023-00799-7 (2023).
He, H. et al. The effects of HIIT compared to MICT on endothelial function and hemodynamics in postmenopausal females. J. Sci. Med. Sport. 25, 364–371. https://doi.org/10.1016/j.jsams.2022.01.007 (2022).
Vogelsang, T. W. et al. Effect of eight weeks of endurance exercise training on right and left ventricular volume and mass in untrained obese subjects: a longitudinal MRI study. Scand. J. Med. Sci. Sports. 18, 354–359. https://doi.org/10.1111/j.1600-0838.2007.00706.x (2008).
de Pinter, C. C. E., Padilha, R., de Oliveira, A. S. & Vassallo, E. M. De Fúcio Lizardo, J. H. Cardiovascular adaptive responses in rats submitted to moderate resistance training. Eur. J. Appl. Physiol. 103, 605–613. https://doi.org/10.1007/s00421-008-0761-3 (2008).
Levine, B. D., Lane, L. D., Buckey, J. C., Friedman, D. B. & Blomqvist, C. G. Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation 84, 1016–1023. https://doi.org/10.1161/01.cir.84.3.1016 (1991).
Montalvo, S. et al. Differences in blood flow patterns and endothelial shear stress at the carotid artery using different exercise modalities and intensities. Front. Physiol. 13, 857816. https://doi.org/10.3389/fphys.2022.857816 (2022).
Green, D. J., Hopman, M. T., Padilla, J., Laughlin, M. H. & Thijssen, D. H. Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol. Rev. 97, 495–528. https://doi.org/10.1152/physrev.00014.2016 (2017).
Ratajczak, M. et al. Effects of endurance and endurance-Strength training on endothelial function in women with obesity: A randomized trial. Int. J. Environ. Res. Public Health. 16 https://doi.org/10.3390/ijerph16214291 (2019).
Pedralli, M. L. et al. Different exercise training modalities produce similar endothelial function improvements in individuals with prehypertension or hypertension: a randomized clinical trial exercise, endothelium and blood pressure. Sci. Rep. 10, 7628. https://doi.org/10.1038/s41598-020-64365-x (2020).
Morishima, T., Tsuchiya, Y., Iemitsu, M. & Ochi, E. High-intensity resistance exercise with low repetitions maintains endothelial function. Am. J. Physiol. Heart Circ. Physiol. 315, H681–h686. https://doi.org/10.1152/ajpheart.00281.2018 (2018).
Boeno, F. P. et al. Effect of aerobic and resistance exercise training on inflammation, endothelial function and ambulatory blood pressure in middle-aged hypertensive patients. J. Hypertens. 38, 2501–2509. https://doi.org/10.1097/hjh.0000000000002581 (2020).
Kobayashi, R. et al. Effects of different intensities and durations of aerobic exercise training on arterial stiffness. J. Phys. Ther. Sci. 32, 104–109. https://doi.org/10.1589/jpts.32.104 (2020).
Montero, D., Roberts, C. K. & Vinet, A. Effect of aerobic exercise training on arterial stiffness in obese populations: a systematic review and meta-analysis. Sports Med. 44, 833–843. https://doi.org/10.1007/s40279-014-0165-y (2014).
Okamoto, T., Masuhara, M. & Ikuta, K. Effect of low-intensity resistance training on arterial function. Eur. J. Appl. Physiol. 111, 743–748. https://doi.org/10.1007/s00421-010-1702-5 (2011).
Wilkinson, I. B., Franklin, S. S. & Cockcroft, J. R. Nitric oxide and the regulation of large artery stiffness: from physiology to Pharmacology. Hypertension 44, 112–116. https://doi.org/10.1161/01.Hyp.0000138068.03893.40 (2004).
Zhang, Y., Zhang, Y. J., Ye, W. & Korivi, M. Low-to-Moderate-Intensity resistance exercise effectively improves arterial stiffness in adults: evidence from systematic review, Meta-Analysis, and Meta-Regression analysis. Front. Cardiovasc. Med. 8, 738489. https://doi.org/10.3389/fcvm.2021.738489 (2021).
Koivistoinen, T. et al. Arterial pulse wave velocity in relation to carotid intima-media thickness, brachial flow-mediated dilation and carotid artery distensibility: the cardiovascular risk in young Finns study and the health 2000 survey. Atherosclerosis 220, 387–393. https://doi.org/10.1016/j.atherosclerosis.2011.08.007 (2012).
Acknowledgements
The authors thank the volunteers for their time and all the community staff for their support and the Key Laboratory of the Ministry of Education for Sports and Physical Fitness for supporting this study. The authors report no conflicts of interest.
Funding
The project was supported by the special fund for basic scientific research operating fees of the central university (2019PT012).
Author information
Authors and Affiliations
Contributions
Wang Tianyang and Zhou Huayi were involved in designing the exercise program, taking measurements, collecting and analyzing data, and writing the first draft and subsequent drafts of the thesis. Tang Chi was involved in designing the exercise program, supervising the training process, and helping to measure and collect data. He Hui was a co-investigator, proposed the study idea, guided the study design, monitored data collection and analysis, and read and approved the final draft of the thesis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tianyang, W., Huayi, Z., Hui, H. et al. Effects of different training modes on hemodynamics and vascular endothelial function in young obese adults. Sci Rep 15, 12608 (2025). https://doi.org/10.1038/s41598-025-97085-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97085-1