Abstract
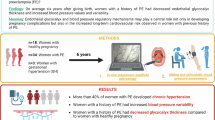
Genomic information from pregnant women and the paternal parent of their fetuses may provide effective biomarkers for preeclampsia (PE). This study investigated the association of parental polygenic risk scores (PRSs) for blood pressure (BP) and PE with PE onset and evaluated predictive performances of PRSs using clinical predictive variables. In the Tohoku Medical Megabank Project Birth and Three-Generation Cohort Study, 19,836 participants were genotyped using either Affymetrix Axiom Japonica Array v2 (further divided into two cohorts—the PRS training cohort and the internal-validation cohort—at a ratio of 1:2) or Japonica Array NEO (external-validation cohort). PRSs were calculated for systolic BP (SBP), diastolic BP (DBP), and PE and hyperparameters for PRS calculation were optimized in the training cohort. PE onset was associated with maternal SBP-, DBP-, and PE-PRSs and paternal SBP- and DBP-PRSs only in the external-validation cohort. Meta-analysis revealed overall associations with maternal PRSs but highlighted significant heterogeneity between cohorts. Maternal DBP-PRS calculated using “LDpred2” presented the most improvement in prediction models and provided additional predictive information on clinical predictive variables. Paternal DBP-PRS improved prediction models in the internal-validation cohort. In conclusion, Parental PRS, along with clinical predictive variables, is potentially useful for predicting PE.
Similar content being viewed by others
Introduction
Preeclampsia (PE) is a multisystem disorder characterized by de novo hypertension and proteinuria, affecting approximately 3.4% and 2.7% of pregnant women in the USA1 and Japan2, respectively. It causes approximately 45,900 maternal deaths worldwide each year3. In addition to effective interventions, such as moderate exercise4, aspirin administration5, and calcium supplementation6, increased vigilance throughout the high-risk pregnancy can aid in the early detection and treatment of PE, potentially reducing adverse pregnancy outcomes and leading to better outcomes7 for mothers and fetuses. Therefore, developing accurate PE prediction models is crucial for clinical practice.
More than a hundred prediction models for PE are reported8, with some using genomic information from pregnant women (here, “maternal genomic information”)9,10,11,12. As the heritability of PE was estimated to be 55% in a family study, with maternal and fetal contributions accounting for 35% and 20%, respectively13, maternal genomic information is expected to provide a good biomarker for PE prediction. Integrating maternal genomic information as a polygenic risk score (PRS) can potentially improve existing predictive models; however, relevant research remains insufficient. In previous studies9,10,11,12, PRS for blood pressure (BP) and PE were utilized but variables such as actual measured BP values and family history of PE, which are important clinical predictive variables, were not simultaneously incorporated into the prediction models. Actual measured BP values in early pregnancy are an effective PE biomarker and are included in many clinical prediction models8,14. Similarly, family medical history is a predictive variable for diseases and reflects the genetic load and shared environmental factors15,16. A recent study revealed that the PRS and family medical history provide complementary information on noncommunicable diseases17. As actual measured BP values and family history of hypertensive disorders of pregnancy (HDP) are more accessible than PRS, their combined clinical usefulness with genomic information should be explored. Although a previous study18 reported no improvement when PRS was incorporated into a machine learning-based prediction model utilizing clinical predictive variables, this study had a small number of cases (< 100) and was not externally validated.
Given that genomic information from the paternal parent of the fetus (here, “paternal genomic information”) is transmitted to both fetal and placental tissues, the paternal genomic information holds predictive potential for PE. As obtaining the fetal genome is difficult, combining the maternal and paternal genomes may capture a comprehensive genetic predisposition to PE. However, no previous studies have employed such information in PE prediction models.
Herein, we examined the relationship of maternal and parental genomic information with PE onset. Additionally, we investigated whether parental PRSs have predictive information in addition to clinical models, including actual measured BP values and family history of HDP.
Methods
Participants
The Tohoku Medical Megabank (TMM) Project Birth and Three-Generation Cohort Study (the BirThree Cohort Study)19,20 recruited pregnant women and their families between 2013 and 2017. More than 50 obstetric clinics and hospitals in the Miyagi Prefecture, Japan, participated, registering 23,406 pregnant women and 8,823 participants who self-reported as fathers of the fetuses.
We excluded the following pregnant women from the study: those who withdrew consent, had multiple pregnancies, had stillbirth before 20 weeks of gestation, and missed the diagnosis of PE or delivery date. Herein, we included only the first-time participations of those who had multiple participations in the TMM BirThree Cohort Study.
Participants were genotyped using either the Affymetrix Axiom Japonica Array v2 (JPA v2) or Japonica Array NEO (JPA NEO), depending on the timing of their genotyping within the TMM Project21. Participants analyzed earlier in the genotyping process were assigned to JPA v2, while those analyzed later were assigned to JPA NEO. Therefore, differences in the proportions of recruitment regions, locations, and the rate of missing family data may exist between the two genotyping platforms. Those with missing genotyping data were excluded after a standard quality control procedure22. Those genotyped using JPA v2 were divided into two cohorts—the PRS training cohort and the maternal internal validation cohort—at a ratio of 1:2. Those genotyped using JPA NEO were defined as the maternal external validation cohort. The paternal parents of the fetuses were genotyped using the same genotyping array platform as the participants. Cohorts with paternal genotypes in the maternal internal and external validation cohorts were defined as the parental internal and external validation cohorts, respectively (Fig. 1). Ethical approval was obtained from the Ethics Committee of the Tohoku Medical Megabank Organization (2013-1-103-1 and 2023-4-025), and informed consent for research participation was obtained from all participants. This study was performed in accordance with the Declaration of Helsinki.
Predictive variables
Based on previous research on PE prediction models23,24,25,26,27, we selected the following predictive variables: maternal age at conception, pre-pregnancy body mass index (BMI), chronic hypertension (CH), systemic lupus erythematosus (SLE), type 1 and 2 diabetes mellitus (DM), maternal family (mother or sisters) history of HDP, conception via in vitro fertilization (IVF), parity (nulliparous, parous with or without previous HDP), gestational age at the previous delivery, the inter-birth interval, and BP at the first antenatal care during 10–13 weeks of gestation. We collected BP data at 10–13 weeks of gestation when the majority of the population underwent the first or second antenatal care. Mean arterial pressure (MAP) was calculated and converted to log10 transformed multiple of the median (log MoM) for the prediction model. The median was chosen as the scaling factor due to the positively skewed data distribution, aligning with prior research for consistency and comparability23,24,25,26,27. Paternal age and family history of HDP were obtained for parental analyses.
Polygenic risk score
Genotyping and PRS calculations are described in Supplementary Methods. In brief, PRSs for three phenotypes, systolic BP (SBP), diastolic BP (DBP), and PE, were calculated using two methods, genome-wide clumping and thresholding (C + T) and a Bayesian approach using LDpred228,29. Although LDpred2 is generally considered more accurate than C + T, C + T is easier to implement and interpret.27 The hyperparameters for maternal PRS were optimized in the PRS training cohort and the same parameters were applied to the paternal PRS for optimization. The number of variants included in each PRS is summarized in Supplementary Table S1.
Outcome measurement
PE was identified according to the guidelines of the American College of Obstetricians and Gynecologists30,31, using medical records at antenatal care. PE superimposed on the CH was included in the definition of PE. PE was automatically diagnosed using an algorithm based on medical record data and validated by a physician. Additionally, PE was classified as early or late onset based on if it occurred earlier than 34 weeks of gestation. The details are given elsewhere24,30.
Model development
Herein, the competing risk model32 was applied, which has been validated both internally and externally in Europe33 and yielded comparable results in the TMM BirThree Cohort Study23,24. This model assumes that all pregnant women are at risk of developing PE during pregnancy. However, some women can give birth before PE can develop. This case, where delivery occurs before the onset of PE, is considered a competing event. Such competing events can influence the prediction model’s accuracy by reducing the number of observed PE cases. A competing risk model is particularly suited for this scenario because it accounts for these competing events, providing a more accurate estimation of the probability of PE onset compared to traditional models that assume no such competition. A parametric survival model with a Gaussian distribution was applied32 and delivery without PE was considered censored. Missing predictive variables were imputed using k-nearest neighbor imputation with k = 140 (square root of the total study population34). To assess the model discrimination, Harrell’s C-statistic was calculated for the whole gestational age. To assess model calibration, the calibration slope was calculated by regressing observed survival outcomes on predicted gestational age at delivery with PE in the same parametric survival models35.
Statistical analysis
Herein, baseline characteristics were compared by PE status or internal/external validation cohorts. P-values were calculated using the t-test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. Pearson’s correlation coefficients were calculated for all combinations of parental PRSs using LDpred2 in the parental internal validation cohort.
Association analyses were performed using SBP-PRS, DBP-PRS, and PE-PRS using LDpred2. In the maternal internal and external validation cohorts, the associations between maternal PRSs and PE onset were examined using logistic regression analysis adjusted for maternal age and four genetic principal components (PC), with parameters estimated using the Maximum Likelihood Estimation method. Two models were developed, one with PRSs as continuous values and the other as tertile values. In the parental internal and external validation cohorts, associations between both maternal and paternal PRSs and PE onset were examined using logistic regression analysis adjusted for parental age and four genetic PCs. Maternal and paternal PRSs for the same phenotype were simultaneously included in the model. The results of internal and external validation cohorts were merged using inverse-variance weighting for meta-analysis. Interactions between maternal and paternal PRSs were also examined. As subanalyses, participants with CH were excluded from the study population, and analyses were performed again. To further assess the robustness of the results, linear regression analyses were performed with PRSs as exposures and BP in early pregnancy as the outcome. Additionally, the early- and late-onset PE were analyzed as outcomes to determine if the association with PRS differed by PE pathology.
The prediction model was developed in two stages: identification of a suitable PRS for prediction models and development of clinical prediction models using parental PRSs. In the first stage, prediction models were developed using each maternal PRS, age, and four genetic PCs as continuous variables. Based on the C-statistics of prediction models, we selected either C + T or LDpred2 as the PRS calculation algorithm, and SBP-PRS, DBP-PRS, or PE-PRS as the PRS phenotype. Four prediction models were developed in the second stage: models with maternal age (the reference model), models with maternal age and maternal family history of HDP (the family-history model), models with variables available at pregnancy confirmation such as maternal age, pre-pregnancy BMI, CH, SLE, DM, maternal family history of HDP, conception via IVF, parous with or without previous PE, gestational age at the previous delivery, and the inter-birth interval (the at-pregnancy-confirmation model), models with variables available in early pregnancy such as variables included in the at-pregnancy-confirmation model and BP in early pregnancy (the in-early-pregnancy model). C-statistics and calibration slopes were calculated for each model. Model training and internal validation were conducted using the internal validation cohort. Apparent performance, which reflects the raw performance of the model trained on the internal validation cohort and may be overestimated, was also reported. External validation was conducted using the external validation cohort. The bootstrap method with 5,000 resamplings was used for two purposes: (1) to perform internal validation of the prediction models and (2) to calculate the 95% confidence intervals (CIs) for apparent and external validation results. This dual application ensures robustness in internal validation and accurate estimation of CIs across validation cohorts. P-values represent comparisons between models with and without PRSs. A simulation study was performed to confirm the reproducibility of the first step. The cohort genotyped with JPA v2 was divided into PRS training and internal validation cohorts, with split ratios of 1:2, 2:3, and 1:1, repeated with 100 random numbers, to determine the PRS with the best predictive ability.
All analyses were conducted using R (4.1.0) unless otherwise noted. We considered a two-tailed P-value of < 0.05 as significant.
Results
A total of 19,836 participants were eligible for the present study: 3,384 in the PRS training cohort, 6,768 in the maternal internal validation cohort, and 9,684 in the maternal external validation cohort (Fig. 1). In the maternal internal and external validation cohorts, 3,673 (54.3%) and 2,616 (27.0%) participants, respectively, had paternal genotyping data.
In total, 352 of 6,768 (5.2%) and 268 of 9,684 (2.8%) participants developed PE in the maternal internal and external validation cohorts, respectively (Table 1). Of the 3,673 and 2,616 participants in the parental internal and external validation cohorts, respectively, 216 (5.9%) and 29 (1.1%) participants developed PE (Supplementary Table S2). Participants with PE tended to have an earlier delivery; were older; had a higher BMI; had a history of CH, DM, and SLE; were nulliparous or parous with previous PE; had a shorter last delivery gestational age; conceived via IVF; and had a higher BP at 10–13 weeks of gestation, with slight variations among the maternal and parental cohorts (Table 1 and Supplementary Table S2). Notable differences in the characteristics between the internal and external validation cohorts were observed (Supplementary Tables S3 and S4). Strong correlations existed between maternal PRSs or among paternal PRSs, whereas weak correlations existed between maternal and paternal PRSs (Supplementary Figure S1).
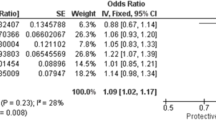
In association studies on the maternal cohorts (Table 2), maternal SBP-, DBP-, and PE-PRSs were significantly associated with PE onset in the meta-analysis; odds ratio (OR) and 95% CI per 1 standard deviation (SD): 1.14 (1.05 to 1.24), 1.20 (1.11 to 1.31), and 1.10 (1.01 to 1.19), respectively. These significant associations were primarily driven by the external validation cohort, as the internal validation cohort showed weaker and non-significant associations. Significant heterogeneity was observed (P for heterogeneity = 0.001, 0.003, and 0.030, respectively). In association studies for the parental cohorts (Supplementary Table S5), maternal and paternal PRSs were not related to PE onset in the meta-analysis. Only in the parental external validation cohort, paternal SBP-PRS and paternal DBP-PRS were associated with PE onset; OR and 95% CI per 1 SD: 1.93 (1.32 to 2.83) and 1.91 (1.29 to 2.84), respectively. Similar results were observed after excluding participants with CH. However, some results did not converge in the parental external validation cohort because of the small number of outcomes (Supplementary Tables S6 and S7). No interaction was noted between the maternal and paternal PRSs (data not shown). The associations of PRSs with BP during early pregnancy were similar to their associations with PE (Supplementary Table S8). When early- and late-onset PE were compared in relation to PRS, the effect sizes were generally comparable (Supplementary Table S9).
After parameter optimization in the PRS training cohort, the predictive performances of SBP-, DBP-, and PE-PRS calculated using the two methods, C + T and LDpred2, were compared in the maternal internal and external validation cohorts (Table 3). The DBP-PRS calculated using LDpred2 improved the discrimination performance the most in both the internal (0.570 in the model with PRS versus 0.567 in the model without PRS, P-value = 0.200) and external (0.592 in the model with PRS versus 0.551 in the model without PRS, P-value < 0.001) validation. The simulation study revealed that among the PRSs predicted by LDpred2, those for DBP were the most frequent with the highest predictive power in the external validation cohort (Supplementary Table S10).
Thereafter, clinical prediction models were developed with maternal and paternal DBP-PRSs calculated using LDpred2. In the maternal cohort, models including maternal PRS demonstrated potential superior predictive discrimination compared to models without maternal PRS in the reference and family-history models in internal validation, although these differences were not statistically significant. This was also observed in all four external validation models, namely the reference, family-history, at-pregnancy-confirmation, and in-early-pregnancy models (Table 4). However, the differences in predictive performance between models with and without maternal PRS were not statistically significant in all cases, indicating limited evidence of robust improvement in discrimination ability. For example, in external validation, the C-statistics of the at-pregnancy-confirmation model were 0.777 without PRS and 0.784 with maternal PRS (P-value = 0.242), indicating the possible utility of maternal PRS at pregnancy confirmation. Even in the in-early-pregnancy model, which included BP in early pregnancy, the C-statistics improved slightly by including maternal PRS (0.849 without PRS versus 0.851 with maternal PRS, P-value = 0.520), suggesting the possible utility of maternal PRS in the prediction of early pregnancy. In the parental internal validation cohort (Table 5), paternal PRS, but not maternal PRS, improved the reference, family-history, and at-pregnancy-confirmation models (P-value = 0.018, 0.059, and 0.022, respectively). In the parental external validation cohort, maternal, but not paternal, PRS improved the reference, family-history, and at-pregnancy-confirmation models (P-value = 0.568, 0.549, and 0.410, respectively), although C-statistics had a wide 95% CI and the results were not stable or statistically significant. Models with both maternal and paternal PRSs did not demonstrate better discrimination ability than those with either maternal or paternal PRS (Table 5).
Discussion
The maternal SBP-, DBP-, and PE-PRSs were associated with PE onset in the meta-analysis, with significant associations observed only in the external validation cohort, reflecting heterogeneity across cohorts. The paternal SBP- and DBP-PRSs were associated with PE onset only in the external validation cohort. Maternal DBP-PRS calculated using LDpred2 improved prediction models the most. Maternal DBP-PRS improved both at-pregnancy-confirmation and in-early-pregnancy models, indicating the clinical utility of maternal PRS. Paternal PRS improved prediction models in the parental internal validation cohort. These results suggest the potential for PRSs to improve predictive performance, but the lack of statistical significance in some cases highlights the need for cautious interpretation and further validation in larger, diverse cohorts.
Maternal PRS
The present study shows that maternal PRSs for BP were associated with PE onset in the external validation cohorts, consistent with previous studies in the European population9,10, indicating cross-ethnicity generalizability. However, the internal-validation cohort showed weaker and non-significant associations. This discrepancy may be partly explained by differences in baseline characteristics (Supplementary Table S3) and genotyping platform, as discussed later. Regarding the onset time, the association between PRS and PE was not considerably affected, suggesting that PRSs are not pathology-specific biomarkers and broadly capture the genetic predisposition to hypertension. Maternal PRS improved the prediction model beyond the family history of HDP. While family history provides plentiful information on genetic predisposition and environment15, the amount of information is determined by family structure and relationships, as well as by disease prevalence. Owing to the demographic trend toward smaller families36 and the need to obtain data retrospectively because of the transient nature of the condition during pregnancy, obtaining a comprehensive family history of HDP is difficult. Herein, < 2% of the participants and < 1% of the paternal parents of their fetuses reported a family history of HDP. Moreover, reliance only on the binary categorization of family history may result in overlooking the intricate genetic predisposition. Therefore, incorporating PRSs into risk prediction models in addition to family history is crucial for precision medicine. Maternal PRS improved the at-pregnancy-confirmation model and slightly improved the in-early-pregnancy model. This indicates that the maternal PRS is useful in clinical practice at pregnancy confirmation and even in early pregnancy, although its predictive significance is diminished by the fact that some genetic predisposition for high BP may manifest itself as the actual measured BP values in early pregnancy. In contrast, PE-PRS was significantly associated with the development of PE in the meta-analysis, though the association was relatively modest compared to SBP- and DBP-PRSs. The genome-wide association study (GWAS) for PE37 used in our study had a relatively small number of cases, and the number of East Asian populations in the GWAS was limited (123 in the BioBank Japan, 1,031 in the UK Biobank, and 1,324 in the FinnGen), which might result in failure to reflect genetic PE predisposition.
Paternal PRS
Furthermore, in this study, paternal PRSs for BP were associated with PE onset in the external validation cohort. The paternal genetic contribution to PE onset has been predicted in family13 and genetic studies38. However, as of date, the association between paternal PRS and PE onset has not been investigated. Our results were inconsistent between the internal and external validation cohorts. One possible reason for this is the biological differences between the parental internal and external validation cohorts. Placental and maternal factors are involved in the development of PE39. The baseline characteristics of the parental internal and external cohorts were quite different; the prevalence of CH in participants with PE was much higher in the external validation cohort than in the internal validation cohort (79.3% versus 25.0%). This may have led to differences between the two cohorts in terms of the ratio of placental to maternal contributions, resulting in different associations between paternal PRSs and PE. In addition to these biological differences, differences in genotyping platforms between the internal (JPA v2) and external (JPA NEO) validation cohorts may also have contributed to the observed discrepancies21. Both JPA v2 and JPA NEO arrays share common SNPs after imputation, ensuring consistency in PRS calculations. However, the genotyping and imputation quality metrics differ due to advances in the design of JPA NEO. While JPA v2 used mutual information for tag SNP selection, JPA NEO adopted a more standardized protocol using pairwise r² for selecting tag SNPs21. JPA NEO showed a greater number of variants with INFO scores > 0.8 compared to JPA v2 highlighting improved imputation accuracy driven by advancements in its design and reference panel21. These advancements in JPA NEO likely contributed to the observed differences in predictive performance between the cohorts. Paternal PRS improved the prediction models in the parental internal validation cohort, despite no significant association in the association analyses. The prediction models developed in the parental internal validation cohort did not have predictive power in the parental external validation cohort possibly, because of cohort differences, as mentioned previously. This suggests that the contribution of paternal PRS to PE onset may differ across populations and should be considered when developing predictive models. We also observed significant decreases in the C-statistics when including paternal PRS in the external validation cohort. One possible reason is that adding a variable with limited predictive value can sometimes introduce noise rather than improving accuracy, ultimately destabilizing the model. Another possibility is that, in the external validation cohort, the association observed in the internal validation cohort may not have held, causing paternal PRS to introduce misleading information and reduce predictive performance. Furthermore, the near-perfect prediction observed in the at-pregnancy-confirmation and in-early-pregnancy models in the parental external validation cohort is likely driven by the high prevalence of CH. CH is the strongest risk factor for PE, and its overrepresentation simplifies prediction. Additionally, the small number of PE cases in the parental external validation cohort (29 cases) may lead to unstable estimate.
Clinical and research implications
Risk prediction is crucial in clinical practice because it can lead to preventive interventions, allowing for intensive perinatal management, which facilitates early detection and treatment. Genomic information remains unchanged and can be used throughout life. This is a crucial difference from other biomarkers26, which are only reflective of temporary conditions. In addition, PRS is useful for predicting diseases other than those in the perinatal period40,41. Given the unchangeability of genomic information, our study suggests that genomic information should be obtained during relatively healthy childbearing age rather than during middle or old age to avoid poor outcomes in pregnant women due to inadequate prediction of PE. We showed that maternal PRS can improve clinical PE prediction models and suggest its application in other perinatal diseases. In addition, we showed that the utility of paternal genomic information in predicting PE is inconsistent across cohorts; therefore, further studies should aim at identifying populations in which paternal PRS has predictive information for PE.
Strengths and limitations
Our study is the first to investigate the utility of maternal and paternal PRS for PE prediction. We applied a well-validated prediction model, and while the observed improvements in predictive performance were modest, our findings provide a preliminary step toward clinical application. Two cohorts with genomic data from different genotype array platforms and baseline characteristics enabled us to examine the generalizability of the results.
Despite these strengths, our study had four limitations. First, our model did not include effective biomarkers specific to PE, such as the uterine artery pulsatility index and placental growth factor26, which may have improved the performance. However, given the widespread use of genomic information beyond perinatal diseases, prediction models using PRS may be more clinically applicable than those using disease-specific biomarkers. Second, there were several differences between the internal and external validation cohorts. The external validation cohort included more participants with CH, which was the strongest predictive variable, than the internal validation cohort, resulting in calibration slopes of > 1 in the external validation. Moreover, in the external cohort, a family history of HDP was not associated with PE, limiting the purpose of this study to confirm the predictive value of the PRS in addition to a family history of HDP. The differences between the two cohorts occurred possibly because of differences in the source populations, and although the study does not provide sufficient replication, it does provide some insight into the type of population for which the predictive model is useful. Third, because there is no paternal GWAS on PE, we had no choice but to apply the maternal GWAS to the fathers. Fourth, owing to the small number of parental cohorts, the effectiveness of paternal PRS in predicting PE may have been overlooked. Optimization was conducted only for maternal PRS, which may have resulted in an underestimation of the ability of paternal PRS to predict PE.
Conclusions
Parental PRS, along with clinical predictive variables, is potentially useful for predicting PE.
TMM BirThree Cohort Study flow for three study cohorts. After excluding ineligible participants, the cohort was divided into three cohorts, namely PRS training, maternal internal validation, and maternal external validation. The cohorts with paternal genotypes in the maternal internal and external validation cohorts were the parental internal and external validation cohorts, respectively. The numbers of PE cases and total participants in each cohort are indicated in the format “PE cases, total participants”. TMM BirThree Cohort Study: The Tohoku Medical Megabank Project Birth and Three-Generation Cohort Study; JPA v2: Affymetrix Axiom Japonica Array v2; JPA NEO: Affymetrix Axiom Japonica Array NEO; PRS, polygenic risk score; and HDP, hypertensive disorders of pregnancy.
Data availability
The datasets used during the current study available from the corresponding author on reasonable request.
References
Ananth, C. V., Keyes, K. M. & Wapner, R. J. Pre-eclampsia rates in the united States, 1980–2010: Age-period-cohort analysis. BMJ 347, f6564 (2013).
Shiozaki, A., Matsuda, Y., Satoh, S. & Saito, S. Comparison of risk factors for gestational hypertension and preeclampsia in Japanese Singleton pregnancies. J. Obstet. Gynaecol. Res. 39, 492–499 (2013).
GBD 2015 Maternal Mortality Collaborators. Global, regional, and National levels of maternal mortality, 1990–2015: A systematic analysis for the global burden of disease study 2015. Lancet 388, 1775–1812 (2016).
Davenport, M. H. et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: A systematic review and meta-analysis. Br. J. Sports Med. 52, 1367–1375 (2018).
Rolnik, D. L. et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl. J. Med. 377, 613–622 (2017).
Woo Kinshella, M. L. et al. Calcium for pre-eclampsia prevention: A systematic review and network meta-analysis to guide personalised antenatal care. BJOG 129, 1833–1843 (2022).
Magee, L. A., Nicolaides, K. H., von Dadelszen, P. & Preeclampsia N Engl. J. Med. 386, 1817–1832 (2022).
De Kat, A. C., Hirst, J., Woodward, M., Kennedy, S. & Peters, S. A. Prediction models for preeclampsia: A systematic review. Pregnancy Hypertens. 16, 48–66 (2019).
Kivioja, A. et al. Increased risk of preeclampsia in women with a genetic predisposition to elevated blood pressure. Hypertension 79, 2008–2015 (2022).
Nurkkala, J. et al. Associations of polygenic risk scores for preeclampsia and blood pressure with hypertensive disorders of pregnancy. J. Hypertens. 41, 380–387 (2023).
Honigberg, M. C. et al. Polygenic prediction of preeclampsia and gestational hypertension. Nat. Med. 29, 1540–1549 (2023).
Tyrmi, J. S. et al. Genetic risk factors associated with preeclampsia and hypertensive disorders of pregnancy. JAMA Cardiol. 8, 674–683 (2023).
Cnattingius, S., Reilly, M., Pawitan, Y. & Lichtenstein, P. Maternal and fetal genetic factors account for most of Familial aggregation of preeclampsia: A population-based Swedish cohort study. Am. J. Med. Genet. A. 130A, 365–371 (2004).
Wright, A., Wright, D., Ispas, C. A., Poon, L. C. & Nicolaides, K. H. Mean arterial pressure in the three trimesters of pregnancy: effects of maternal characteristics and medical history. Ultrasound Obstet. Gynecol. 45, 698–706 (2015).
Valdez, R., Yoon, P. W., Qureshi, N., Green, R. F. & Khoury, M. J. Family history in public health practice: A genomic tool for disease prevention and health promotion. Annu. Rev. Public. Health. 31, 69–87 (2010).
Bennett, R. L. The family medical history. Prim. Care. 31, 479–495 (2004).
Mars, N. et al. Systematic comparison of family history and polygenic risk across 24 common diseases. Am. J. Hum. Genet. 109, 2152–2162 (2022).
Kovacheva, V. P. et al. Preeclampsia prediction using machine learning and polygenic risk scores from clinical and genetic risk factors in early and late pregnancies. Hypertension 81, 264–272 (2024).
Kuriyama, S. et al. The Tohoku medical megabank project: design and mission. J. Epidemiol. 26, 493–511 (2016).
Kuriyama, S. et al. Cohort profile: Tohoku medical megabank project birth and three-generation cohort study (tmm birthree cohort study): rationale, progress and perspective. Int. J. Epidemiol. 49, 18–19m (2020).
Sakurai-Yageta, M. et al. Japonica array Neo with increased genome-wide coverage and abundant disease risk Snps. J. Biochem. 170, 399–410 (2021).
Yamada, M. et al. Genetic loci for lung function in Japanese adults with adjustment for exhaled nitric oxide levels as airway inflammation indicator. Commun. Biol. 4, 1288 (2021).
Ohseto, H. et al. Preeclampsia prediction model using the dipstick test for proteinuria during early gestation. Hypertens. Res. Pregnancy. 10, 88–96 (2022).
Ohseto, H. et al. Dietary calcium intake was related to the onset of pre-eclampsia: the Tmm birthree cohort study. J. Clin. Hypertens. (Greenwich). 25, 61–70 (2023).
Wright, D., Syngelaki, A., Akolekar, R., Poon, L. C. & Nicolaides, K. H. Competing risks model in screening for preeclampsia by maternal characteristics and medical history. Am J Obstet Gynecol. 213, 62 e61-62 e10 (2015).
O’Gorman, N. et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11–13 weeks gestation. Am. J. Obstet. Gynecol. 214, 103e101–103e112 (2016).
Bartsch, E., Medcalf, K. E., Park, A. L. & Ray, J. G. High risk of Pre-eclampsia identification, G. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ 353, i1753 (2016).
Choi, S. W., Mak, T. S., O’Reilly, P. F. & Tutorial A guide to performing polygenic risk score analyses. Nat. Protoc. 15, 2759–2772 (2020).
Prive, F., Arbel, J. & Vilhjalmsson, B. J. Ldpred2: better, faster, stronger. Bioinformatics 36, 5424–5431 (2021).
Mizuno, S. et al. Development of phenotyping algorithms for hypertensive disorders of pregnancy (hdp) and their application in more than 22,000 pregnant women. Sci. Rep. 14, 6292 (2024).
Hypertension in pregnancy. Report of the American college of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstet. Gynecol. 122, 1122–1131 (2013).
Wright, D., Wright, A. & Nicolaides, K. H. The competing risk approach for prediction of preeclampsia. Am. J. Obstet. Gynecol. 223, 12–23e17 (2020).
Wright, D. et al. Predictive performance of the competing risk model in screening for preeclampsia. Am. J. Obstet. Gynecol. 220, 199e191–199e113 (2019).
Zhang, Z. Introduction to machine learning: K-nearest neighbors. Ann. Transl Med. 4, 218 (2016).
Rahman, M. S., Ambler, G., Choodari-Oskooei, B. & Omar, R. Z. Review and evaluation of performance measures for survival prediction models in external validation settings. BMC Med. Res. Methodol. 17, 60 (2017).
United Nations Department of Economic and Social Affairs Population Division. Patterns and Trends in Household Size and Composition (Evidence from a united nations dataset ST/ESA/SER.A/433, 2019).
Sakaue, S. et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 53, 1415–1424 (2021).
Galaviz-Hernandez, C. et al. The paternal polymorphism rs5370 in the edn1 gene decreases the risk of preeclampsia. Pregnancy Hypertens. 6, 327–332 (2016).
Burton, G. J., Redman, C. W., Roberts, J. M. & Moffett, A. Pre-eclampsia: pathophysiology and clinical implications. BMJ 366, l2381 (2019).
Briggs, S. E. W. et al. Integrating genome-wide polygenic risk scores and non-genetic risk to predict colorectal cancer diagnosis using Uk biobank data: population based cohort study. BMJ 379, e071707 (2022).
Wedekind, L. E. et al. The utility of a type 2 diabetes polygenic score in addition to clinical variables for prediction of type 2 diabetes incidence in birth, youth and adult cohorts in an Indigenous study population. Diabetologia 66, 847–860 (2023).
Delaneau, O., Zagury, J. F. & Marchini, J. Improved whole-chromosome phasing for disease and population genetic studies. Nat. Methods. 10, 5–6 (2013).
O’connell, J. et al. A general approach for haplotype phasing across the full spectrum of relatedness. PLoS Genet. 10, e1004234 (2014).
Bycroft, C. et al. The UK biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Tadaka, S. et al. 3.5KJPNv2: an allele frequency panel of 3552 Japanese individuals including the X chromosome. Hum. Genome Var. 6, 28 (2019).
1000 Genomes Project Consortium. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Howie, B. N., Donnelly, P. & Marchini, J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 5, e1000529 (2009).
Acknowledgements
Acknowledgments: The authors would like to thank all the participants who consented to participate in this study and all the staff at the Tohoku Medical Megabank Organization, Tohoku University, Iwate Tohoku Medical Megabank Organization, and Iwate Medical University. A full list of the members of the Tohoku Medical Megabank Organization is available at https://www.megabank.tohoku.ac.jp/english/a240901/.
Funding
This work was supported by the Japan Agency for Medical Research and Development, Japan (Grant Nos. JP19gk0110039, JP17km0105001, JP21tm0124005, and JP21tm0424601) and Japan Society for the Promotion of Science KAKENHI (Grant No. JP21K10438).
Author information
Authors and Affiliations
Contributions
Conceptualization: H.O., M.I., and T.O. Methodology: H.O. and M.I. Visualization: H.O.Supervision: S.K.Writing – original draft: H.O. and M.I.Writing – review & editing: All the authors.
Corresponding author
Ethics declarations
Competing interests
K.M. is an employee of the Ministry of Education, Culture, Sports, Science and Technology, Japan. The other authors have no conflict of interests.
Consent for publication
This manuscript has not been published in any journal or presented at any conference in part or in entirety and is not under consideration by another journal. It has been made publicly available as a preprint (DOI: https://doi.org/10.1101/2024.02.07.24302476).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ohseto, H., Ishikuro, M., Obara, T. et al. Preeclampsia prediction with maternal and paternal polygenic risk scores: the TMM BirThree Cohort Study. Sci Rep 15, 13743 (2025). https://doi.org/10.1038/s41598-025-97291-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97291-x
Keywords
This article is cited by
-
Early pregnancy triglycerides and blood pressure: a combined predictor for preeclampsia
BMC Pregnancy and Childbirth (2025)