Abstract
Polycyclic aromatic hydrocarbons (PAHs) pollution and hearing loss are important issues in the environment and public health. However, current research still lacks data on their association in real-world environments. This study included 658 American adults who participated in the National Health and Nutrition Examination Survey (NHANES) from 2015 to 2016. The correlation between single PAH exposure and hearing was estimated by the weighted logistic regression, the overall association between mixed PAHs and hearing was evaluated by Bayesian kernel machine regression and grouped weighted quantile sum regression, and the important chemicals were identified in this study. The results showed that the 1-Hydroxynaphthalene (1-OHNa) was positively correlated with the hearing condition, hearing level and hearing threshold (OR = 1.41, 95% CI 1.08–1.84, P < 0.01; OR = 1.41, 95% CI 1.04–1.87, P < 0.05; OR = 4.34, 95% CI 1.74–10.81, P < 0.01, respectively). And 1-Hydroxypyrene (1-OHP) was positively correlated with the hearing condition and hearing threshold (OR = 1.83, 95% CI 0.99–3.36, P < 0.05; OR = 5.83, 95% CI 1.31–26.03, P < 0.05, respectively) after using covariate correction. The results of BKMR and WQS indicated a positive correlation between overall PAHs exposure and decreased hearing, and the higher the overall exposure to PAHs, the greater the risk of hearing loss. Further analysis revealed that 1-OHP was an important chemical substance related to PAHs and hearing. This result was consistent with the results of the single PAH exposure model. These insights provide a critical new perspective on the association between PAHs and hearing, highlighting the urgent need for strategies to reduce environmental pollution and protect human health.
Similar content being viewed by others
Introduction
Hearing loss is one of the most common diseases in adults, with the main symptom being difficulty hearing especially in the place with noise1. Hearing loss might cause serious communication barriers and psychological problems, further leading to loss of social function and even suicide2,3. It is estimated that 257.3 million people suffer from hearing loss each year, and approximately 33.8 million new hearing loss patients occur each year around the world because of some preventable causes4. According to existing research reports, totally 5% of population in the world was affected by hearing loss5 and there were more than 16.8% of people in the USA were diagnosed as deaf (hearing threshold greater than or equal to 40 dB) which will double by 2050 and 20606,7 and the annual global cost caused by hearing loss is over 660 billion Euros8. People generally believe that noise exposure and aging are the most general causes of hearing loss9. However, adverse industrial and environmental chemicals may also damage the auditory system and cause hearing loss10, as aromatic compounds can poison hair cells, which can transmit sound information to the auditory nerve and are the key parts that convert sound into nerve impulses11,12, disrupt membrane structure, and then damage hearing13,14. Therefore, identifying the preventable risk factors that cause hearing loss has a great practical significance for avoiding hearing loss, and subsequent social and psychological problems.
The PAHs are persistent toxic substances, which contain two or more benzene rings. These are difficult to degrade and can migrate over long distances15. The PAHs mainly come from automotive exhaust, industrial emissions, incomplete combustion of solid fuels, smoking and cooking fumes16,17, and they can also be used as industrial raw materials in production18. The PAHs could enter human through inhalation, ingestion and skin contact, and are an undervalued threat to human health19. These chemicals can induce oxidative stress, interfere the mutagenic effects caused by cell membranes and enzyme systems, and further lead to diseases such as cardiovascular diseases and cancer20,21,22. The European Union and the United States Environmental Protection Agency have announced 16 types of PAHs as key pollutants for regulation, 7 types of which are classified as Class 1, 2 A/2B carcinogens23. Moreover, PAHs, which have a certain lipophilicity and can cross the blood-brain barrier, also possess a certain degree of neurotoxicity, but current research has mostly focused on neurobehavior and neglected its impact on the sensory nerves24,25.
The PAHs pollution and hearing loss are receiving increasing global attention, but few studies have plumbed the association between mixed PAHs and hearing. Therefore, based on NHANES data from the US, this study evaluated the levels of PAHs exposure in Americans from 2015 to 2016, plumbed the association between single or mixed PAHs exposure and hearing in real-world environments, evaluated the weight of different PAHs on hearing loss, and identified the important chemicals between overall PAHs exposure and hearing.
Methods
Study population
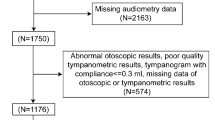
The project of the National Health and Nutrition Examination Survey (NHANES) in the United States, aimed at assessing the status of adults’ health and nutrition in the US. This project uses a complex, multi-stage, probability sampling design to recruit approximately 5000 volunteers from across the country each year. The database of NHANES interviews includes demographic, socioeconomic, and the issues of health-related. The examination data includes audiometry, blood pressure, physiological measurements, etc. The Laboratory data is collected by highly trained medical personnel, which includes the levels of common chemicals or elements detected from biological samples such as blood and urine. Strict quality control is implemented throughout all data collection processes26,27. The data files involved in this study are linked using unique survey participant identifiers (i.e. SEQN). This study included adults aged 20–69 who completed all measurements of Polycyclic Aromatic Hydrocarbons (PAHs) and audiometry. We excluded participants with missing covariate data and recent noise exposure. The cold could cause swelling of the mucous membranes in the nasal cavity and throat, leading to obstruction of the Eustachian tube and uneven pressure inside and outside the middle ear, which in turn affects hearing28. The sinus problem and earache could both cause otitis media, which are potential factors that affect hearing29,30,31. Moreover, the abnormalities in otoscopy, which include excessive cerumen, impacted cerumen, collapsing ear canals and others, could also affect the results of audiometry32,33,34. Therefore, we removed these possible factors when conducting the analysis. Finally, a total of 658 participants were included in the final analysis (Fig. 1).
The measurement of PAHs
Totally 7 kinds metabolites of PAH were measured in urine using isotope dilution high-performance liquid chromatography-tandem mass spectrometry (on-line SPE-HPLC-MS/MS) after the step of enzymatic hydrolysis and solid-phase extraction. In this study, we considered PAHs whose detection rate of > 50%, and the names and abbreviations of all substances are shown in Table 135. The value is divided by the square root of two if it is below the limit of detection36.
The measurement of audiometry and the definition of hearing
The examination of audiometry was conducted by trained examiners in the dedicated soundproof room of the Mobile Examination Center (MEC) for participants. Seven frequencies (500, 1000, 2000, 3000, 4000, 6000, and 8000 Hz) were chosen for hearing threshold tests on the ears of participants. Manual testing was performed if participants did not know how to operate or automatically encounter problems37. The hearing threshold was calculated by using the value of 500, 1,000, 2,000 and 4,000 Hz in the better ear, and the hearing level is based on the final result of the hearing threshold, and exceeding 20 dB is considered as “hearing loss” and the other is “normal hearing”38. The level of hearing condition is divided based on the results of the “General condition of hearing” in the NHANES database, where “Excellent” and “Good” are considered as “good hearing”, and “bad hearing” includes “A little trouble”, “Modify hearing trouble”, “A lot of trouble”, and “Deaf”39.
Covariates
We included age, gender, body mass index (BMI), waist circumference, alcohol drinks, smoking, race, education level and annual household income as covariates. The age, BMI and waist circumference were treated as continuous variables. And the gender (Male, Female), Alcohol drinks (Yes, No), Smoking (Yes, No), Race (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other Race), Education level (Less than 9th grade, 9-11th grade, High school graduate or equivalent, AA degree or equivalent, College graduate or above) and Annual household income ($ 0 to $ 19,999, $20,000 to $54,999, $55,000 and over) were treated as categorical variables.
Statistical analyses
Descriptive analyses were performed for all variables. The mean and standard deviation (SD) for continuous variables and the frequency of categorical variables were calculated in this study. The t-tests and chi-square tests were conducted to assess the differences in the distribution of “Age”, “Gender”, “Height”, “Weight”, “BMI”, “Waist Circulation”, “Symmetric pressure”, “Diagnostic pressure”, “Alcohol drinks”, “Smoking”, “Race”, “Education level”, and “Annual Household income” across different hearing conditions and levels. Urine PAH concentrations were divided by urine creatinine concentrations for subsequent analyses to control for concentration differences due to urine dilution. The concentration of PAHs does not meet the normality test, so it was converted to an approximately normal distribution by lg. The Student’s t-test was used to analyze the differences in PAHs between good hearing and bad hearing, as well as between normal hearing and hearing loss. We calculated the Spearman correlation coefficients between the lg conversion concentrations of PAHs chemicals. Logistic regression (LR) modeling was used to explore the association between the concentration of PAHs and hearing. To simulate real-world mixed exposure, Bayesian kernel machine regression (BKMR) and Weighted quantile sum (WQS) regression analyses were used to assess the association between mixed PAH exposure and the results of audiometry measurement. The direction of mixed exposure effects and the weights of single PAH were calculated. All statistical analyses were performed in R software (version: 4.3.1).
Sensitivity analyses
The sensitivity analyses were performed in this study. Firstly, the continuous value of audiometry was further included in the analysis for main models. Moreover, the participants with abnormal hearing were excluded to evaluate whether the preliminary results had changed40.
Results
Participant characteristics
Totally 658 participants were included in this study. The demographic characteristics between groups of hearing condition and hearing level are shown in Table 2. The age of people with bad hearing or hearing loss was older than those with good hearing or normal hearing (both P < 0.001). Males might have better hearing condition and hearing levels compared to females (P < 0.05, P < 0.001, respectively). The people with bad hearing or hearing loss had a larger waist circumference and higher systolic pressure compared to those with good hearing or normal hearing (both P < 0.05). There were significant differences in the distribution of smoking, race and annual household income between the people with good or bad hearing (P < 0.01, P < 0.001, P < 0.01, respectively), which was consistent between people with normal hearing and hearing loss (P < 0.01, P < 0.001, P < 0.05, respectively). Moreover, there are significant differences in the distribution of height and education level between the people with normal hearing and hearing loss (both P < 0.05).
The concentrations of PAHs
The distribution and detection rate (DR) of PAHs are shown in Table 1. All PAHs had a relatively high detection rate (both DR > 60%) and were included in this study. The geometric means (GM) (P25-P75) for 2-OHNa, 1-OHNa, 2-OHFlu, 2 & 3-OHPh, 1-OHP, 1-OHPh and 3-OHFluwere 62.34 (33.31-117.88), 19.13 (6.28–50.59), 2.27 (1.08–4.08), 1.36 (0.82–2.04), 1.33 (0.78–2.15), 1.11 (0.71–1.68), 1.00 (0.44–2.07) ng/L, respectively. The difference of lg conversion concentration of PAHs between different hearing condition or hearing level were analyzed in this study (Fig. 2). The concentration of 1-OHNa in people with bad hearing was significantly lower than the people with good hearing (P < 0.05). Moreover, the correlation between the lg conversion concentrations of PAHs and hearing was analyzed by the Spearman’s rank correlation, and the results were shown in Fig. 3. There is a positive correlation between PAHs in whole people, people with good and bad hearing, as well as in people with normal hearing and hearing loss (0.329 < r < 0.943, both P < 0.001; 0.321 < r < 0.947, both P < 0.001; 0.235 < r < 0.952, both P < 0.001, respectively).
Associations of single PAH and hearing
The lg conversion concentration of PAHs was used in generalized linear model and logistic regression model, as the original concentration did not meet the normality test. The results of the association between single PAH and hearing were shown in Fig. 4. The exposure of 1-OHNa had a significant positive association with hearing condition, hearing level and hearing threshold (OR = 1.41, 95% CI 1.08–1.84, P < 0.01; OR = 1.41, 95% CI 1.04–1.87, P < 0.05; OR = 4.34, 95% CI 1.74–10.81, P < 0.01, respectively). However, the exposure of 1-OHP rather than 1-OHNa had a significant positive association with hearing condition and hearing threshold after using covariate correction (OR = 1.83, 95% CI 0.99–3.36, P < 0.05; OR = 5.83, 95% CI 1.31–26.03, P < 0.05, respectively). We found no significant differences between exposure to other PAHs and hearing.
Associations of mixed PAHs exposure and hearing
The BKMR was used to analyze the association between mixed PAHs exposure and hearing. Figure 5A showed that the concentrations of 1-OHPh and 1-OHP were positively correlated with hearing condition, hearing level, and hearing threshold. The concentration of 3-OHFlu was negatively correlated with hearing condition, hearing level, and hearing threshold. However, when the concentration of 1-OHNa was low, it was negatively correlated with hearing condition, hearing level, and hearing threshold, but when the concentration exceeded a certain concentration, the trend was reversed. The concentration of 2-OHNa was negatively correlated with hearing condition and hearing level. Although the concentration of 2-OHNa was overall negatively correlated with hearing threshold, the trend reversed at a certain concentration gradient. The concentration of 2-OHFlu was positively correlated with hearing condition, but the concentration of this was negatively correlated with hearing level and hearing threshold. The concentration of 2 & 3-OHPh was not significantly correlated with hearing condition, but was negatively correlated with hearing level and hearing threshold. As shown in Fig. 5B, in the analysis model of PAH exposure and hearing condition, the posterior inclusion probability (PIP) of all PAHs was below 0.5 except for the 1-OHP. However, the PIP of all PAHs in other models of PAHs exposure and hearing level, and PAHs exposure and hearing threshold exceeded 0.5. In addition, the mixed PAHs exposure was, on the whole, positively correlated with hearing condition, hearing level, and hearing threshold (Fig. 5C), and the majority of PAHs had no interaction with each other (Fig. S1-3). When the other PAHs concentrations were fixed at the 25th, 50th, and 75th percentiles, 1-OHP concentration showed a positive correlation with hearing condition, hearing level, and hearing threshold, although there was no significant difference in the results (Fig. 5D). The WQS was used to analyze the contribution of a single PAH in mixed exposure. The results showed that 1-OHP accounted for the main contribution in mixed exposure, which is consistent with the results of a single PAH exposure (Fig. 6).
The BKMR model of PAHs exposure and hearing. (A) Univariate exposure-response functions for single PAH exposure. (B) The posterior inclusion probabilities (PIPs) of each PAH for hearing. (C) Overall effect of mixed PAHs exposure. (D) Effect of single PAH exposure. The model was adjusted for age, gender, body mass index (BMI), waist circumference, alcohol drinks, smoking, race, education level and annual household income.
Sensitivity analyses
By adjusting the people with hearing problems, in a single PAH exposure model, 1-OHP showed a positive correlation with hearing threshold after using covariate correction (Fig S4). In the mixed PAHs exposure model, the 1-OHP was the only PAH with a PIP exceeding 0.5 (Fig S5), and the mixed PAHs exposure was still positively correlated with hearing threshold and the majority of PAHs had no interaction with each other (Fig S6). Additionally, 1-OHP accounted for the main contribution in mixed PAHs exposure and hearing threshold (Fig S5). After further controlling for the people with hearing problems, the results of the relationships between PAHs exposure and hearing threshold were similar to the above analyses.
Discussion
Based on the data from 658 participants in the NHANES database 2015–2016, we explored the association between single or mixed PAHs and hearing. In a single PAH model, 1-OHNa had a significant positive correlation with decreased hearing, whether it is the hearing condition told by the participants, the hearing threshold obtained from audiometry, or the hearing level calculated based on the audiometry, and 1-OHP had a significant positive correlation with decreased hearing after adjusted by covariate. The mixed exposure results showed an overall positive correlation between mixed PAHs and decreased hearing, and there were different effects between different PAHs on hearing. Further analysis revealed that 1-OHP remained the main contributor on an overall level.
The PAHs pollution and hearing loss are currently important public health issues, but the studies focused on the relationship between PAHs and hearing loss are sporadic. Chou et al. included 1071 adults and analyzed the association between single PAH and hearing using a linear regression model41. It was found that exposure to single PAH harms hearing and as the level of single PAH increases, hearing gradually decreases. And Li et al. included 4200 adults and 1337 adolescents to analyze the association between single PAH exposure and hearing loss using a multiple logistic regression model42. The results showed that single PAH exposure, especially 3-OHFlu and 2-OHFlu were positively correlated with hearing loss in both adults and adolescents. Although the outcome variables of these two studies are different, both of these can indicate a positive correlation between single PAH and decreased hearing, which is consistent with the results of this study.
However, audiometry is influenced by many factors and these two studies only considered the absence of PAH and audiometry in the process of inclusion and exclusion, while ignoring the factors that affect the results of audiometry (such as cold, abnormal otoscopy and exposure to noise in 24 h, etc.)43,44. The studies above only considered the association between single PAH exposure and hearing, while ignoring the possible interaction between PAHs. Therefore, this study adopted stricter inclusion and exclusion criteria, excluding the factors that may affect the results of audiometry at the beginning of the study, and analyzed the subjective description results of the participants’ hearing condition told by themselves. Moreover, the hearing condition told by the participants, the hearing level calculated based on the audiometry and the hearing threshold obtained from audiometry were all considered in this study. All of the results demonstrated that there was a significant positive correlation between PAHs and decreased hearing which indicates a stable association between PAHs and decreased hearing. Moreover, we analyzed the population without hearing problems and the results showed that this association between PAHs and hearing still exists. Based on analyzing the relationship between single PAH exposure and hearing, this study used the BKMR and WQS models to analyze the interaction and intrinsic connection between PAHs.
Inconsistent with previous studies, this study found that the 1-OHP had a significant positive correlation with decreased hearing after covariate adjustment and had a major contribution in mixed PAHs exposure and hearing. The level of 1-OHP in urine can not only serve as an important biomarker for exposure to PAHs, but also have a potential impact on health45. Freitas et al. collected the data of 1-OHP, oxidative stress biomarkers, hematological and biochemical parameters, and inflammatory biomarkers from 58 post-infarction patients and 41 control groups to evaluate the potential association between 1-OHP and inflammatory biomarkers or oxidative stress in patients with acute myocardial infarction (AMI). It was found that 1-OHP has a certain correlation with inflammatory biomarkers or oxidative stress46,47. Choi et al. found that oxidative stress in the body increases in proportion to the level of 1-OHP, which is consistent with the study above48. And there are some research had also found a significant correlation between oxidative stress/inflammation and 1-OHP49,50, which had been considered as the principal mechanism of damage in the auditory system51,52. However, the other PAHs only had a weak correlation compared to 1-OHP.
Although oxidative stress and inflammation might act as an intermediary during the process of PAHs damage to hearing, the specific mechanisms were still unclear. Studies have shown that exposure to PAHs can cause structural changes in high-density lipoprotein cholesterol (HDL-C), increase vascular constriction, induce degeneration and edema surrounding the stria vascularis, lower antioxidant capacity, and ultimately affect hearing53,54,55. In addition, PAHs can also interfere with glutamate signaling, activate glial cells, and reduce neural plasticity, while glial cell activation and dysregulation of gap junction protein expression can cause auditory system damage56,57. The BKMR model was used to analyze the potential association between mixed PAHs and hearing. The results showed that the dose-response pattern between most PAHs and hearing was linear, and the overall trend between PAHs and hearing was positive, indicating that hearing would decrease with increasing exposure levels of mixed PAHs. This trend is consistent with the trends in the studies for the association between PAHs and cardiovascular, and PAHs and neurological diseases58,59,60. PAHs might affect the auditory system by inducing changes in oxidative stress or inflammatory indicators, affecting the nervous or cardiovascular system, and changing the auditory sensing or the blood supply of cochlear cells61, which provides a basis for our previous inference. However, the specific mechanisms were needed to verify by further research.
In this study, we considered the coexistence of multiple PAHs in the real world, evaluated the results under the model of single exposure and mixed exposure, and identified the key substance 1-OHP with stable and consistent results among multiple models, drew a conclusion that 1-OHP exposure had a clear and stable association with hearing. This study has the several clear strengths. Firstly, this study adopted stricter inclusion and exclusion criteria, taking into account factors that affect audiometry results to get more realistic and reliable results. Secondly, this study analyzed the relationship between single PAH and hearing from multiple dimensions. The subjective description of hearing condition by participants, specific hearing threshold calculated by audiometry, and the definition of hearing loss based on hearing threshold were all considered in this study. Finally, the BKMR and WQS regression models not only avoid the highly complex collinearity and interaction of homologous pollutants, but also calculate the weights between different PAHs to reflect their impact on hearing loss. Nevertheless, there are also some shortcomings in this study. This study only analyzed urine samples from 2015 to 2016 cannot reflect long-term dynamic levels of PAHs exposure, and did not explore the potential mechanisms between PAH and hearing. In addition, this study did not include occupational exposure in the analysis and was unable to quantify the noise exposure of the study population. In the future, more samples at different time points and more details about noise exposure need to be included to dynamically monitor the levels of PAHs in the population, and further evaluate the impact of multi-time point exposure to PAHs on hearing loss. And we look forward to more research focusing on potential mechanisms in the future.
Conclusions
This study comprehensively evaluated the relationship between single or mixed PAHs and hearing. The results showed that the higher the overall exposure to PAHs, the greater the risk of hearing loss. The 1-OHP was an important chemical substance associated with hearing loss under both single and mixed exposure models. We believe that these findings would provide additional evidence for the impact of PAHs on hearing, and controlling PAHs in the environment may reduce the risk of hearing loss. However, further research is still needed to verify the potential mechanisms during the process of PAHs damage to hearing, and provide a scientific basis for the prevention of hearing loss.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Blazer, D. G. & Tucci, D. L. Hearing loss and psychiatric disorders: a review. Psychol. Med. 49 (6), 891–897 (2019).
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet (London England). 396 (10248), 413–446 (2020).
Su, B. M. & Chan, D. K. Prevalence of hearing loss in US children and adolescents: findings from NHANES 1988–2010. JAMA otolaryngology– Head Neck Surg. 143 (9), 920–927 (2017).
Prasad, K. et al. Priorities for hearing loss prevention and estimates of global cause-specific burdens of hearing loss: a systematic rapid review. Lancet Global Health. 12 (2), e217–e225 (2024).
Sheffield, A. M. & Smith, R. J. H. The epidemiology of deafness. Cold Spring Harbor Perspect. Med. 9(9). (2019).
Varma, R. et al. Visual impairment and blindness in adults in the united States: demographic and geographic variations from 2015 to 2050. JAMA Ophthalmol. 134 (7), 802–809 (2016).
Goman, A. M., Reed, N. S. & Lin, F. R. Addressing estimated hearing loss in adults in 2060. JAMA otolaryngology– Head Neck Surg. 143 (7), 733–734 (2017).
Uy, J. & Forciea, M. A. In the clinic. Hearing loss. Ann. Intern. Med. 158 (7), ITC4–ITC1 (2013). quiz ITC4-16.
Lieu, J. E. C., Kenna, M., Anne, S. & Davidson, L. Hearing loss in children: A review. Jama 324 (21), 2195–2205 (2020).
Nieman, C. L. & Oh, E. S. Hearing loss. Ann. Intern. Med. 173 (11), Itc81–itc96 (2020).
Beurg, M. & Fettiplace, R. PIEZO2 as the anomalous mechanotransducer channel in auditory hair cells. J. Physiol. 595 (23), 7039–7048 (2017).
Dallos, P. Cochlear physiology. Ann. Rev. Psychol. 32, 153–190 (1981).
Wang, S. et al. Association between blood volatile organic aromatic compound concentrations and hearing loss in US adults. BMC Public. Health. 24 (1), 623 (2024).
Campo, P., Morata, T. C. & Hong, O. Chemical exposure and hearing loss. Dis. Mon. 59 (4), 119–138 (2013).
Wang, M., Jia, S., Lee, S. H., Chow, A. & Fang, M. Polycyclic aromatic hydrocarbons (PAHs) in indoor environments are still imposing carcinogenic risk. J. Hazard. Mater. 409, 124531 (2021).
Ma, Y. & Harrad, S. Spatiotemporal analysis and human exposure assessment on polycyclic aromatic hydrocarbons in indoor air, settled house dust, and diet: A review. Environ. Int. 84, 7–16 (2015).
Ohura, T., Amagai, T., Fusaya, M. & Matsushita, H. Polycyclic aromatic hydrocarbons in indoor and outdoor environments and factors affecting their concentrations. Environ. Sci. Technol. 38 (1), 77–83 (2004).
Farzan, S. F., Chen, Y., Trachtman, H. & Trasande, L. Urinary polycyclic aromatic hydrocarbons and measures of oxidative stress, inflammation and renal function in adolescents: NHANES 2003–2008. Environ. Res. 144 (Pt A), 149–157 (2016).
Kim, K. H., Jahan, S. A. & Kabir, E. A review on human health perspective of air pollution with respect to allergies and asthma. Environ. Int. 59, 41–52 (2013).
Kim, K. H., Jahan, S. A., Kabir, E. & Brown, R. J. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 60, 71–80 (2013).
Idowu, O. et al. Beyond the Obvious: environmental health implications of Polar polycyclic aromatic hydrocarbons. Environ. Int. 123, 543–557 (2019).
Best, E. A., Juarez-Colunga, E., James, K., LeBlanc, W. G. & Serdar, B. Biomarkers of exposure to polycyclic aromatic hydrocarbons and cognitive function among elderly in the united States (National health and nutrition examination survey: 2001–2002). PloS One. 11 (2), e0147632 (2016).
Santos, J. L., Aparicio, I. & Alonso, E. A new method for the routine analysis of LAS and PAH in sewage sludge by simultaneous sonication-assisted extraction prior to liquid chromatographic determination. Anal. Chim. Acta. 605 (1), 102–109 (2007).
Mortamais, M. et al. Effect of exposure to polycyclic aromatic hydrocarbons on basal ganglia and attention-deficit hyperactivity disorder symptoms in primary school children. Environ. Int. 105, 12–19 (2017).
Chepelev, N. L., Moffat, I. D., Bowers, W. J. & Yauk, C. L. Neurotoxicity May be an overlooked consequence of benzo[a]pyrene exposure that is relevant to human health risk assessment. Mutat. Res. Reviews Mutat. Res. 764, 64–89 (2015).
Johnson, C. L., Dohrmann, S. M., Burt, V. L. & Mohadjer, L. K. National health and nutrition examination survey: sample design, 2011–2014. Vital Health Stat. Ser. 2 Data Evaluation Methods Res. 2014(162):1–33 .
Chen, T. C., Clark, J., Riddles, M. K., Mohadjer, L. K. & Fakhouri, T. H. I. National health and nutrition examination survey, 2015–2018: sample design and Estimation procedures. Vital Health Stat. Ser. 2 Data Evaluation Methods Res. 2020(184):1–35 .
Winther, B. et al. Viral respiratory infection in schoolchildren: effects on middle ear pressure. Pediatrics 109 (5), 826–832 (2002).
Benjamin, T., Gillard, D., Abouzari, M., Djalilian, H. R. & Sharon, J. D. Vestibular and auditory manifestations of migraine. Curr. Opin. Neurol. 35 (1), 84–89 (2022).
Müller, S. J., Durisin, M. & Behme, D. Sudden hearing loss and vertigo after sinus occlusion. JAMA Otolaryngol. Head Neck Surg. 150 (10), 925–926 (2024).
Alyono, J. C., Corrales, C. E. & Oghalai, J. S. Otalgia, facial nerve paralysis, and hearing loss. JAMA otolaryngology– Head Neck Surg. 140 (6), 575–576 (2014).
Crummer, R. W. & Hassan, G. A. Diagnostic approach to tinnitus. Am. Family Phys. 69 (1), 120–126 (2004).
Subha, S. T. & Raman, R. Role of impacted cerumen in hearing loss. Ear Nose Throat J. 85 (10), 650 (2006).
Mahoney, C. F. & Luxon, L. M. Misdiagnosis of hearing loss due to ear Canal collapse: a report of two cases. J. Laryngol. Otol. 110 (6), 561–566 (1996).
Luo, K. et al. Exposure to organophosphate esters and metabolic syndrome in adults. Environ. Int. 143, 105941 (2020).
Polycyclic Aromatic Hydrocarbons (PAH) - Urine (PAH_I).
Audiometry (AUX_I).
Masterson, E. A., Bushnell, P. T., Themann, C. L. & Morata, T. C. Hearing impairment among Noise-Exposed Workers - United States, 2003–2012. MMWR Morbidity Mortal. Wkly. Rep. 65 (15), 389–394 (2016).
Audiometry (AUQ_I).
Wang, X. et al. Trimester-specific effects of maternal exposure to single and mixed metals on cord serum inflammatory cytokines levels: A prospective birth cohort study. Sci. Total Environ. 895, 165086 (2023).
Chou, C. W. et al. Urinary biomarkers of polycyclic aromatic hydrocarbons and the association with hearing threshold shifts in the united States adults. Environ. Sci. Pollut. Res. Int. 27 (1), 562–570 (2020).
Li, W. et al. Association of polycyclic aromatic hydrocarbons exposure, systemic inflammation with hearing loss among adults and adolescents. Environ. Pollut. (Barking, Essex: 1987) 296, 118772 (2022).
Skarzynski, P. H. et al. Pilot hearing screening of School-age children in Lagos, Nigeria. J. Health Care Poor Underserved. 32 (3), 1444–1460 (2021).
Xu, K. et al. Intrinsic mechanism and Pharmacologic treatments of noise-induced hearing loss. Theranostics 13 (11), 3524–3549 (2023).
Hisamuddin, N. H. & Jalaludin, J. Children’s exposure to polycyclic aromatic hydrocarbon (PAHs): a review on urinary 1-hydroxypyrene and associated health effects. Rev. Environ. Health. 38 (1), 151–168 (2023).
Kho, Y. et al. 1-Hydroxypyrene and oxidative stress marker levels among painting workers and office workers at shipyard. Int. Arch. Occup. Environ. Health. 88 (3), 297–303 (2015).
Freitas, F. et al. Urinary 1-hydroxypyrene is associated with oxidative stress and inflammatory biomarkers in acute myocardial infarction. Int. J. Environ. Res. Public Health. 11 (9), 9024–9037 (2014).
Choi, S. H., Ochirpurev, B., Toriba, A., Won, J. U. & Kim, H. Exposure to Benzo[a]pyrene and 1-Nitropyrene in particulate matter increases oxidative stress in the human body. Toxics 11(9). (2023).
Barth, A. et al. Association between inflammation processes, DNA damage, and exposure to environmental pollutants. Environ. Sci. Pollut. Res. Int. 24 (1), 353–362 (2017).
Li, W., Chen, D., Peng, Y., Lu, Z. & Wang, D. Association of polycyclic aromatic hydrocarbons with systemic inflammation and metabolic syndrome and its components. Obes. (Silver Spring Md). 31 (5), 1392–1401 (2023).
Paciello, F. et al. Oxidative stress and inflammation cause auditory system damage via glial cell activation and dysregulated expression of gap junction proteins in an experimental model of styrene-induced oto/neurotoxicity. J. Neuroinflamm. 21 (1), 4 (2024).
Pushpan, C. K. et al. Repurposing AZD5438 and Dabrafenib for Cisplatin-Induced AKI. J. Am. Soc. Nephrology: JASN. 35 (1), 22–40 (2024).
Patel, C. J., Cullen, M. R., Ioannidis, J. P. & Butte, A. J. Systematic evaluation of environmental factors: persistent pollutants and nutrients correlated with serum lipid levels. Int. J. Epidemiol. 41 (3), 828–843 (2012).
Rosenson, R. S. et al. Translation of high-density lipoprotein function into clinical practice: current prospects and future challenges. Circulation 128 (11), 1256–1267 (2013).
Sun, Y. S. et al. Components of metabolic syndrome as risk factors for hearing threshold shifts. PloS One. 10 (8), e0134388 (2015).
Zhen, H. et al. Association of polycyclic aromatic hydrocarbons exposure with child neurodevelopment and adult emotional disorders: A meta-analysis study. Ecotoxicol. Environ. Saf. 255, 114770 (2023).
Dutta, K., Ghosh, D., Nazmi, A., Kumawat, K. L. & Basu, A. A common carcinogen benzo[a]pyrene causes neuronal death in mouse via microglial activation. PloS One. 5 (4), e9984 (2010).
Lu, L. & Ni, R. Association between polycyclic aromatic hydrocarbon exposure and hypertension among the U.S. Adults in the NHANES 2003–2016: A cross-sectional study. Environ. Res. 217, 114907 (2023).
Cheng, Y. et al. The association between polycyclic aromatic hydrocarbons exposure and neuropsychiatric manifestations in perimenopausal women: A cross-sectional study. J. Affect. Disord. 344, 554–562 (2024).
Luo, L. et al. Single and joint associations of exposure to polycyclic aromatic hydrocarbons with blood coagulation function during pregnancy: A cross-sectional study. Sci. Total Environ. 885, 163949 (2023).
Kim, J. H., Moon, N., Heo, S. J., Jeong, Y. W. & Kang, D. R. Repeated measurements and mixture effects of urinary bisphenols, parabens, polycyclic aromatic hydrocarbons, and other chemicals on biomarkers of oxidative stress in pre- and postpartum women. Environ. Pollut. (Barking, Essex: 1987) 342, 123057 (2024).
Acknowledgements
We appreciate the people who contributed to the NHANES data that we used in this study.
Funding
This work was supported by the three-year action plan for the construction of Shanghai’s public health system, key discipline construction project (Grant NO. GWV-10.1-XK12) and the Public Project of Jinshan District Health and Family Planning Commission (Grant NO. JSKJ-KTGW-2023-01).
Author information
Authors and Affiliations
Contributions
Xiuju Li: Conceptualization, Methodology, Formal analysis, Project administration, Visualization, Writing original draft, Writing-review & editing. Zhaofeng Liu: Conceptualization, Formal analysis. Xiaohong Ding: Data curation, Formal analysis. Yuanling Zhou: Conceptualization, Methodology. Tingting Yu: Conceptualization, Formal analysis, Funding acquisition, Supervision, Writing original draft. Jin Jiang: Conceptualization, Methodology, Conceptualization, Funding acquisition, Project administration, Writing-review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to publish
The authors declare that they agree with the publication of this paper in this journal.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, X., Liu, Z., Ding, X. et al. Associations of exposure to polycyclic aromatic hydrocarbons with hearing in U.S. Adults. Sci Rep 15, 13987 (2025). https://doi.org/10.1038/s41598-025-97315-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97315-6