Abstract
Acanthopanax senticosus is a unique wild resource in Northeast China. Its main active ingredient is a polysaccharide, which has a prominent immunomodulatory effect. In this study, the purified polysaccharide component of Acanthopanax senticosus leaves(ASPS-A1) with strong immunomodulatory activity was isolated by column chromatography, and its structure and properties were characterized by HPGPC, and FT-IR. Results showed that ASPS-A1 is mainly composed of α- 1,4-D-GalA, α- 1,5-L-Ara, and β- 1,4-D-Gal. RT-qPCR experiments and RNA-seq analysis were used to study the immunoregulatory mechanism of ASPS-A1. The results showed that ASPS-A1 could significantly up-regulate the levels of cytokines iNOS, IL- 1β, IL- 6, and TNF-α activated macrophages through MAPK, NF-κB, and Toll-like receptor signaling pathways. Inhibitory experiments further confirmed that ASPS-A1 promotes the expression of iNOS, TNF-α, and IL- 6 via the TLR4 receptor, and TNF-α and IL- 1β via the TLR2 receptor. In order to identify the target of ASPS-A1, molecular docking experiments were conducted. The results demonstrated that ASPS-A1 could bind to both TLR4 and TLR2, forming stable complexes with the cavities on the protein surface through hydrogen bonding and hydrophobic interaction. The docking scores indicated that ASPS-A1 could regulate the immune response through TLR2 and TLR4 signaling pathways, with a particularly strong interaction with TLR4. In summary, this study screened and characterized the most immunoreactive components of Acanthopanax senticosus polysaccharide, disclosed the immunomodulatory mechanism of ASPS-A1, and furnished a research basis for its potential application as a natural immune enhancer.
Similar content being viewed by others
Introduction
The immune system is the body’s first line of defense and is essential for maintaining health1. Immunosuppression is a common disorder of the immune system, which is commonly seen in aging, sepsis, malnutrition, and other states, and then leads to infections, cancers, cardiovascular complications, and other diseases2,3. Therefore, maintaining normal immune function is essential for disease prevention. At present, commonly used immune enhancers such as levamisole and interferon are effective, but they are often accompanied by side effects, such as nausea and fever4. Based on this, the search for a natural immune enhancer with fewer side effects has become a research hotspot.
Polysaccharides have attracted more and more attention due to their significant immunostimulative impact on the immune system5,6. As an important innate immune cell, macrophages not only play the role of immune sentinel but also play an important role in immune defense and surveillance through phagocytosis, antigen production, and cytokine secretion7,8. Therefore, macrophages are often regarded as target cells for immunostimulatory effects. Through pattern recognition receptors (such as TLR2, TLR4, and Dectin- 1) on the surface of macrophages, polysaccharides can activate signaling pathways such as MAPK and NF-κB, and promote the secretion of NO, TNF-α, IL- 6 and other cytokines, thus playing an immunomodulatory role9,10. For example, Zhao et al. demonstrated that Peucedanum praeruptorum Dunn polysaccharides control macrophage inflammatory responses through the TLR2/TLR4-mediated MAPK and NF-κB pathways, boosting macrophage phagocytosis and the release of NO and cytokines11. Similarly, through the TLR4-MAPK/NF-κB signaling pathway, sulfated Chinese yam polysaccharide increases the immunomodulatory activity of RAW 264.7 cells12.
A. senticosus is an abundant medicinal and edible plant in Northeast China. It has anti-inflammatory, antibacterial, and hypotensive effects. Its roots and stems are often used for medicinal purposes, while the leaves can be used as edible wild vegetable resources. A. senticosus contains polysaccharides, which are one of its key active components, which has immunomodulatory, anti-tumor, and anti-oxidation functions, especially in immunomodulatory aspects13. Other plant polysaccharides, such as Astragalus, Panax ginseng, and Lonicera japonica, etc., have also demonstrated significant immunomodulatory effects, mainly through the activation of macrophages, enhancing their phagocytosis and cytokine secretion levels14,15. Although the core role of plant polysaccharides in immune regulation through the TLR/MAPK/NF-κB pathway has been widely recognized, studies such as those by Wu et al. have shown that clematis polysaccharides regulate immunity in RAW 264.7 cells via TLR4/MAPK/NF-κB16,and Liu et al. found that sulfated yam polysaccharides also exert immune-modulating effects on RAW 264.7 cells through the same pathway17. However, most current research focuses on the single receptor TLR4, and the immune regulatory mechanisms and targets of A. senticosus polysaccharides on RAW 264.7 cells have not been systematically elucidated. Previous studies have demonstrated that A. senticosus polysaccharides that extracted from root and stem could promote cytokine release, however, polysaccharide extracted from leaves are rarely reported, what is more, the immune regulatory mechanisms and targets of polysaccharides are remain underexplored. Here, a novel acid polysaccharide named ASPS-A1 which mainly composed of composed of RG-I pectin and HG pectin were isolated. Compared with similar studies18,19, through the fine separation and purification of A. senticosus leaf polysaccharides, combined with structural characterization and functional analysis, is the first to systematically reveal its multi-target mechanism of immune regulation through TLR2/TLR4 receptor engagement, demonstrating subsequent activation of the MAPK/NF-κB signaling pathway for immune response modulation. Additionally, as a food-herb hybrid, A. senticosus polysaccharides are safer and non-toxic compared to traditional immune enhancers, providing a theoretical basis for the development of functional foods and plant-based immune boosters.
This study aimed to isolate the immunomodulatory polysaccharide component ASPS-A1 from the leaves of A. senticosus. The immunomodulatory mechanism of ASPS-A1 was systematically studied by RNA-seq technique combined with RAW 264.7 macrophage model. RNA-seq, as a tool to detect differentially expressed genes, can further explore the molecular mechanism of polysaccharide activation of macrophages. In addition, specific inhibitors and molecular docking techniques were used to further elucidate the binding ability of ASPS-A1 to TLR2/TLR4 and reveal the molecular mechanism of its activation of macrophages through the TLR2/TLR4 signaling pathway, promoting the secretion of NO and TNF-α, IL- 1β, IL- 6, and other cytokines. This study not only elucidates the structural properties of ASPS-A1 and its immunomodulatory mechanism but also reveals the potential of A. senticosus leaf polysaccharide as a novel natural immune enhancer in immunosuppression. This provides a new theoretical basis and practical support for the development and utilization of A. senticosus and its application in immunostimulatory effects.
Methods
Materials and reagents
The leaves of A. senticosus were purchased from Changchun Vegetable Center Wholesale Market. RAW 264.7 cells were purchased from Wuhan Proximity Limited (Wuhan, China). DEAE-cellulose was purchased from Yuanye Biotechnology (Shanghai, China), and Superdex-G100 gel was purchased from BIO-RAD Corporation(USA). Dulbecco’s Modified Eagle’s Medium(DMEM) was obtained from Shenggong BioEngineering(Shanghai, China), and lipopolysaccharide(LPS) from Sigma-Aldrich(USA). NF-κB p65, p-NF-κB p65, I-κBα, p-I-κBα, ERK, p-ERK, p- 38, p-p38, JNK, p-JNK, and GAPDH were obtained from Sevier(Wuhan, China). The TLR2 inhibitor C29 was supplied by McLean(Shanghai, China), and the TLR4 inhibitor TAK- 242 was supplied by McLean(Shanghai, China). All other reagents were purchased from Yun Guan Science Center(Shanghai, China).
Preparation of samples
Fresh A. senticosus leaves were taken and extracted with ultrasonic-assisted extraction at a material-liquid ratio of 1:20, rotary evaporation, alcohol precipitation, and lyophilization to obtain A. senticosus leaves crude polysaccharide(ASPS). ASPS was then separated and purified using a DEAE- 52 cellulose column. Elution was first performed with 5 column volumes of dH2O at a flow rate of 1.5 mL/min to collect the neutral polysaccharide(ASPS-N). Subsequently, elution with 0.5 mol/L NaCl solution yielded the acidic polysaccharide(ASPS-A). Both ASPS-N and ASPS-A solutions were concentrated by rotary evaporation, dialyzed, and freeze-dried to obtain purified ASPS-N and ASPS-A. ASPS-A was further isolated and purified using a Superdex-G100 gel column and eluted by NaCl at a flow rate of 0.15 mL/min, collecting 3 mL fractions per tube for a total of 80 tubes. The phenol-sulfuric acid method was used to determine the sugar content. Based on the elution curve, the liquid was collected, dialyzed, and freeze-dried to obtain ASPS-A1, ASPS-A2, and ASPS-A3.
Structural characterization
Molecular weight
The molecular weight was determined by high-performance liquid gel permeation chromatography(HPGPC)20. ASPS-A1(1 mg) was mixed with 200 µL ultra-pure water, and the membrane was filtered and determined by the Shimadzu HPLC system. The mobile phase was 0.2 mol/L NaCl solution, and the isobaric elution was performed at the flow rate of 0.6 mL/min.
Carbohydrate composition
Monosaccharide composition was determined by pre-column derivatization of 1-phenyl- 3-methyl- 5-pyrazolinone(PMP) and high-performance liquid chromatography(HPLC)21. ASPS-A1(1 mg)was added to 2.0 mol/L hydrochloric acid-anhydrous methanol solution, sealed with N2, and dried by an air pump. Add 2.0 mol/L trifluoroacetic acid(TFA)solution, hydrolyze in a metal bath at 120 ℃ for 1 h, and blow dry with an air pump. Then it was mixed with 0.3 mol/L NaOH and PMP-methanol, and then 0.3 mol/L HCl solution was added in a water bath at 70 ℃ for 0.5 h, thoroughly mixed, and then CH2Cl2 was added, vortexed for 90 s, centrifuged at 8000 rpm for 3 min, retaining the water layer, and extract for 3 times. Filter membrane filtration and HPLC detection were carried out. The column temperature was kept at a constant 35 ℃. Nine standard monosaccharides were used as controls, namely, mannose(Man), glucuronic acid(GlcA), rhamnose(Rha), galacturonic acid(GalA), glucose(Glc), galactose(Gal), xylose(Xyl), arabinose(Ara), and fucose(Fuc).
Infrared/ultraviolet spectroscopy
As mentioned previously22, Grind and press the dried ASPS-A1(1 mg) with KBr (180 mg). Using KBr as a blank, infrared spectra were collected using a 4000–500 cm−1 NICOLE TIS5 FT-IR spectrometer. ASPS-A1(1 mg/mL) was used for UV full-wavelength scanning analysis in the range of 200–400 nm to determine the purity of the sample.
Scanning electron microscopy (SEM)
The dry ASPS-A1 sample was formed into a thin uniform layer on double-sided tape, then sprayed with gold and placed in the SEM sample chamber. The micromorphology of ASPS-A1 was observed at 100, 500, and 2000 times by scanning at 10.0 kV.
Enzymatic digestion
The sample concentration was prepared to 5 mg/mL using a 50 mM acetate buffer. Endo-Polygalacturonase M2, endo- 1,4-β-Galacturonase, and endo- 1,5-α-L-Arabinanase were added at ratios of 1:4, 1:4, and 1:20(U/mg), respectively. The reaction was carried out at 37 ℃ for 24 h. After the reaction, the mixture was heat-inactivated at 98 ℃ for 10 min, followed by centrifugation at 12,000 rpm for 10 min to remove proteins. The sample was then analyzed by HPGPC. De-esterification of ASPS-A1: 5 mg of ASPS-A1 was dissolved in a small amount of ddH2O and stirred overnight at 4 ℃. Then, pre-cooled 0.2 M NaOH was added, and the mixture was incubated at 4 ℃ for 4 h. Afterward, the sample was dialyzed and freeze-dried to obtain the de-esterified ASPS-A1 sample.
Immunoactivity analysis
Cell culture
RAW 264.7 cells were grown at 37 ℃ in a 5% CO2 incubator with DEME complete medium, 10% fetal bovine serum, and 1% penicillin streptomycin.
Cell viability
RAW 264.7 cells were seeded into 96 well plates at a density of 2 × 10⁴ cells per well and divided into different treatment groups. Cells in the experimental groups were treated with varying concentrations of ASPS-A1(50 µg/mL, 100 µg/mL, 200 µg/mL, 400 µg/mL, 600 µg/mL, and 800 µg/mL) and incubated for 24 h. The control group was treated with high-glucose DMEM. After 24 h of incubation, the supernatants were discarded, and MTT solution was added to each well, followed by a 4 h incubation at 37 °C. Upon completion, the remaining MTT was removed, and 100 µL of dimethyl sulfoxide(DMSO) was added to each well to dissolve the purple formazan crystals formed. Finally, absorbance was measured at 570 nm to assess cell viability.
NO content
RAW 264.7 cells were seeded into 96-well plates at a density of 2 × 10⁴ cells per well. The experimental groups were treated with different concentrations of ASPS-A1 (50 µg/mL, 100 µg/mL, 200 µg/mL) and incubated for 24 h. The blank control group was treated with high-glucose DMEM, and the positive control group received 1 µg/mL LPS. After 24 h of incubation, the supernatants from each well were collected, followed by the addition of 50 µL of 1% sulfanilamide solution and 0.1% naphthylamine solution in sequence. The mixture was allowed to react at room temperature for 15 min. Absorbance was then measured at 540 nm, and nitric oxide(NO) levels were calculated based on a sodium nitrite standard curve to evaluate the effect of ASPS-A1 on NO production.
Cytokine expression
The expression levels of cytokines(TNF-α, iNOS, IL- 6, and IL- 1β) were detected by quantitative real-time reverse transcription PCR(RT-qPCR). The cells were inoculated on a 6-well plate at a density of 4 × 105 cells/well and treated with ASPS-A1 (200 µg/mL) for 24 h. The total RNA was then extracted with Trizol reagent, and its purity was determined using Nanodrop. RT-qPCR was performed on the ABQ3&5 real-time quantitative PCR instrument, and the cycle conditions were as follows: 95 ℃ for 30 s, 95 ℃ for 10 s, 65 ℃ for 10 s, 72 ℃ for 30 s, for a total of 40 cycles. GAPDH was used as the internal reference gene to normalize the expression of other RNAs, and the gene expression quantity was represented by 2−△△Ct. Primers for RT-qPCR are listed in Table S1 below.
RNA-seq analysis
The RNA-seq analysis was performed on RAW 264.7 cells according to the literature report23. RAW 264.7 cells were cultured in 6-well plates at a concentration of 2 × 105 cells/mL for 24 h, then the supernatant was discarded, and the medium containing ASPS-A1(200 µg/mL) was added. The cells were collected for 24 h, treated with 1 mL Trizol, and immediately placed in a refrigerator at − 80 ℃. The sequencing process was performed at Guanshu Biotechnology Services(Changchun, China). The screening criteria for differentially expressed genes(DEGs) were |log2(fold change)|> 1 and p < 0.05. Subsequently, the main biological functions(BP), molecular functions(MF), cell components(CC), and signal transduction pathways related to DEGs were identified by Gene Ontology(GO) and the Kyoto Encyclopedia of Genes and Genomes(KEGG). The specific analysis process included the determination of the list and number of related genes by the GO terminology or the DEGs annotated by the KEGG pathway, and the calculation of p-value using the hypergeometric distribution method(p < 0.05 was significant). Ultimately, GO terms or KEGG pathways that are significantly enriched in DEGs in the entire genomic context were identified. RNA-Seq results were validated by RT-qPCR.
Western blot
RAW 264.7 cells were inoculated into 6-well plates at a density of 2 × 105 cells/well and cultured for 24 h. After removing the medium, fresh DMEM containing ASPS-A1(50 µg/mL, 100 µg/mL, 200 µg/mL) was added to each well of the plate and continued cultured for an additional 24 h. The cells were washed with PBS and lysed in a lysis buffer. Centrifuge at 4 ℃ at 12,000×g for 30 min and remove the supernatant. The protein was quantified by the Coomassie Brilliant Blue method. 12% separation gel and 5% concentration gel were configured, and electrophoresis was conducted for 2 h. After electrophoresis, the proteins on the gel were transferred to the PVDF membrane by the wet transfer method. The PVDF membrane was placed in a transfer buffer and incubated in an ice box for 2 h. Cut out the corresponding position according to the marker, and incubate at 4 ℃ for the primary antibody overnight. On the second day, the film was washed in TBST 3 times, 15 min each time. Incubate the antibody at room temperature for 1 h, and continue to wash it with TBST 3 times for 15 min each time. Add the ECL developer and put it under the exposure machine for development.
Molecular docking
First, the structures of Gal, GalA, and Ara were obtained from the NCBI database(https://www.ncbi.nlm.nih.gov). The molecules were prepared using AutoDock Tools 1.5.7 by adding hydrogen atoms and then exported in PDBQT format. Next, the three-dimensional structures of TLR2 and TLR4 were retrieved from the PDB database(https://www.rcsb.org). Protein-energy optimization was performed using Chem3D 20.0. Water molecules were removed in AutoDock Tools 1.5.7, and the protein structures were converted to PDBQT format. Molecular docking was conducted using AutoDock 1.5.7, generating nine docking conformations for each monosaccharide. Finally, PyMOL 3.0 was used to analyze the binding modes, select the conformation with the lowest binding energy, and visualize receptor-ligand interactions, highlighting key features such as hydrogen bonds and hydrophobic contacts.
Statistical analysis
Three independent repeated tests were conducted for each group of samples, and statistical analysis was performed using GraphPad Prism 8.0, and the results were represented by mean ± standard deviation. A p-value less than 0.05 was considered statistically significant.
Results
Polysaccharide preparation and active ingredient screening
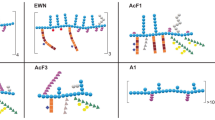
The flowchart of polysaccharide extraction, isolation and purification is shown in Fig. 1A. ASPS was obtained by ultrasonic-assisted water extraction. ASPS was purified in deionized water using a DEAE- 52 cellulose column. The process was followed by rotary evaporation and freeze-drying, yielding ASPS-N and ASPS-A with yields of 4.2% and 52.3%, respectively. Given the significantly higher yield of ASPS-A compared to ASPS-N, we selected ASPS-A for subsequent experiments. Then, ASPS-A was further separated by a Superdex-G100 gel column to obtain three components, ASPS-A1 at 75–105 mL, ASPS-A2 at 111–171 mL, and ASPS-A3 at 177–201 mL, ASPS-A1, ASPS-A2, and ASPS-A3, with yields of 41.7%, 36.7%, and 21.6%, respectively. The immunomodulatory effect of each component was evaluated by comparing the expression levels of cytokines, such as iNOS, TNF-α, IL- 1β, and IL- 6, in a RAW 264.7 cell model. As shown in Fig. 1B to E, ASPS-A1 exhibited expression levels of 2.44 ± 0.04 for iNOS, 2.70 ± 0.31 for TNF-α, 45.80 ± 1.16 for IL- 1β, and 279.77 ± 3.21 for IL- 6. In comparison, ASPS-A2 demonstrated expression levels of 2.13 ± 0.24 for iNOS, 2.49 ± 0.30 for TNF-α, 25.19 ± 0.80 for IL- 1β, and 229.50 ± 1.92 for IL- 6, while ASPS-A3 showed levels of 1.84 ± 0.26, 2.02 ± 0.09, 15.93 ± 1.48, and 134.55 ± 2.42 for the same cytokines. Among the three, ASPS-A1 showed a significantly stronger ability to up-regulate cytokines than that of ASPS-A2 and ASPS-A3, indicating that ASPS-A1 was a promising immunopotentiator.
ASPS extraction, isolation and purification process, and immunomodulatory activity. (A) Flow chart of ASPS extraction and isolation and purification; (B-E) Release of cytokines (iNOs, TNF-α, IL- 1β and IL- 6). The values are expressed as the means ± SD (n = 3). *p < 0.05, **p < 0.01,***p < 0.001 and ****p < 0.0001 vs. the CON group.
Polysaccharide structure characterization
Composition of monosaccharides and molecular weight
As shown in Fig. 2A, the molecule weight of ASPS-A1 presents unimodal symmetry and narrow peak shape, indicating that it has good homogeneity and belongs to a pure polysaccharide indicating that ASPS-A1 had good homogeneity and belonged to a pure polysaccharide. The molecular weight analysis showed that the molecular weight of ASPS-A1 was 147.8 kDa. The total sugar content of ASPS-A1 was about 86.72%, and the glucuronic acid content of ASPS-A1 was about 37.23%, ASPS-A1 contains almost no protein and has a higher purity. The monosaccharide composition analysis results were shown in Fig. 2B. The main components of ASPS-A1 are Gal(30.1%), GalA(24.5%), Ara(16.9%), and Rha(13.1%), with small amounts of Xyl(6.6%), Glc(4.2%), Man(2.4%), GlcA(1.5%), and Fuc(0.8%). These results indicated that ASPS-A1 was a typical acidic polysaccharide with a complex sugar composition distribution, which may be related to its biological activity. Based on the above analysis combined with related studies24,25,26, it can be inferred that ASPS-A1 may be mainly composed of RG-I pectin and HG pectin. This study provided a basis for further bioactivity studies.
Spectral analysis
The infrared spectral analysis results were shown in Fig. 2C. The absorption peak of ASPS-A1 at 3424 cm⁻¹ was attributed to the stretching vibration of the O-H bond, which indicated the existence of intermolecular or intramolecular hydrogen bonds of the polysaccharide. The absorption peak at 2918 cm⁻¹ corresponded to the stretching vibration of the C-H bond, and the absorption peaks at 3400 cm⁻¹ and 2900 cm⁻¹ were typical characteristic peaks of polysaccharide27, further confirming that ASPS-A1 had the characteristic absorption peak of polysaccharide. In addition, the absorption peaks that could reflect the types of monosaccharides, substituents, and their differential isomerism are concentrated in the range of 1800 to 700 cm⁻¹. The absorption peak near 1600 cm⁻¹ corresponded to C = O stretching vibration in free uronic acid, indicating that ASPS-A1 contained esterified polysaccharide components, such as galacturonic acid or glucuronic acid28. The absorption peak of 1720 cm⁻¹ was the characteristic peak of esterified glyuronic acid, while the symmetric stretching vibration peak of COO⁻ near 1410 cm⁻¹ further supports the existence of esterification modification29. The absorption peak at 1242.73 cm⁻¹ indicated that ASPS-A1 contains unprotonated carboxyl group vibration, which further proved that ASPS-A1 contains uronic acid and has partial esterification modification. The absorption peaks near 1072.29 cm⁻¹ and 760 cm⁻¹ were related to the stretching vibration of the pyranoid ring30. Combined with the monosaccharide composition analysis, it could be inferred that ASPS-A1 may be mainly composed of partially esterified galacturonic acid.
As shown in Fig. 2D, the UV spectrum analysis results showed that no obvious absorption peaks of nucleic acid and protein were detected at 260 nm and 280 nm for ASPS-A1, indicating that ASPS-A1 basically did not contain nucleic acid and protein, and had a high purity31. Consistent with the chemical composition as described in composition of monosaccharides and molecular weight.
SEM analysis
SEM is a commonly used analytical technique for directly observing and analyzing the particle morphology and surface characteristics of polysaccharides. As shown in Fig. 2E, ASPS-A1 primarily exhibits a rod-like structure at magnifications of 100×, 500×, and 2000×. At lower magnifications, ASPS-A1 appears as rod-shaped aggregates. As the magnification increases to 500×, small particles begin to emerge on the surface. At 2000×magnification, these particles are revealed to be ellipsoidal in shape, varying in size, and exhibiting a relatively smooth surface. In summary, ASPS-A1 consistently maintains a rod-like morphology across different magnifications, indicating a stable structural configuration.
Analysis of enzymatic hydrolysis
Based on the monosaccharide composition, enzymatic hydrolysis experiments were performed on ASPS-A1 using endo-Polygalacturonase M2, endo- 1,4-β-Galacturonase, and endo- 1,5-α-L-Arabinanase. The results shown in Fig. 2F, indicate that without prior treatment, the addition of these enzymes did not cause significant changes in the peak area or average molecular weight of ASPS-A1. This observation suggests that esterification modifications of the polysaccharide may interfere with enzymatic activity. To further investigate, ASPS-A1 was subjected to de-esterification treatment, which altered its spatial structure and enhanced its susceptibility to enzymatic action. As shown in Fig. 2G, the de-esterified ASPS-A1 exhibited varying degrees of peak shifts and changes in peak areas under the action of the three enzymes. These findings indicate that ASPS-A1 is primarily composed of α− 1,4-D-GalA, α− 1,5-L-Ara, and β− 1,4-D-Gal residues, with partial esterification. The results of endo-Polygalacturonase M2 confirmed that ASPS-A1 exhibits the structural characteristics of HG type pectin. Meanwhile, the results from endo- 1,4-β-Galacturonase and endo- 1,5-α-L-Arabinanase revealed the presence of AG type pectin domains in ASPS-A1. AG type pectin typically has an RG-I type pectin backbone, where the ratio of GalA to Rha acid is approximately 1:1. However, analysis of ASPS-A1’s monosaccharide composition showed a GalA to Rha ratio close to 2:1. This result suggests that ASPS-A1 contains not only HG type pectin but also RG-I type pectin with AG type pectin as its side chains.
Structural characterization of ASPS-A1. (A) Molecular weight diagram of ASPS-A1; (B) ASPS-A1 monosaccharide composition diagram; (C) Infrared spectrogram of ASPS-A1; (D) Ultraviolet spectrogram of ASPS-A1; (E) Electron microscope image of ASPS-A1; (F) HPGPC assay of ASPS-A1; (G) HPGPC assay of ASPS-A1 after deesterification.
Immunoactivity analysis
Evaluation of RAW 264.7 cell viability
To examine the toxicity of ASPS-A1, the MTT assay was employed to evaluate cell viability. Cells were treated with ASPS-A1 at concentrations of 50 µg/mL, 100 µg/mL, 200 µg/mL, 400 µg/mL, 600 µg/mL, and 800 µg/mL for 24 h. As shown in Fig. 3A, cell viability exceeded 80% at all concentrations, indicating that ASPS-A1 within this range does not exhibit significant cytotoxicity toward RAW 264.7 cells but rather promotes cell proliferation. The most notable enhancement of cell proliferation occurred in the 50–200 µg/mL range. Therefore, this concentration range was selected for further evaluation of ASPS-A1’s immunomodulatory effects.
Measurement of NO content
NO is a key signaling molecule that regulates the ability of activated macrophages to carry out host defense functions. To investigate the immunomodulatory activity of ASPS-A1, NO production in RAW 264.7 cells was measured. As shown in Fig. 3B, ASPS-A1 significantly promoted NO release in a dose-dependent manner at concentrations of 50–200 µg/mL, with effects similar to those of the positive control(LPS). These findings suggest that ASPS-A1 exhibits potent immunomodulatory activity.
Measurement of cytokine expression
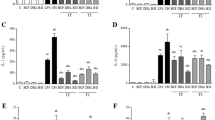
Extensive research has shown that macrophages are a major source of cytokines such as TNF-α, IL- 1β, IL- 6, and iNOS, playing a critical role as effectors in immune system activation and pathogen defense. The pro-inflammatory cytokines IL- 1β, TNF-α, and IL- 6, along with the phenotypic marker iNOS, are key indicators of M1 macrophage polarization, and their secretion levels are directly correlated with the intensity of immune stimulation32. Therefore, RT-qPCR was used to measure the expression of these cytokines in the culture supernatant. As shown in Fig. 3C-F, ASPS-A1 significantly enhanced the secretion of iNOS, TNF-α, IL- 1β, and IL- 6 by RAW 264.7 cells in a dose-dependent manner at concentrations ranging from 50 to 200 µg/mL. At a concentration of 200 µg/mL, the expression levels of iNOS, TNF-α, IL- 1β, and IL- 6 were 3.08 ± 0.04, 25.22 ± 1.33, 29.57 ± 1.96, and 2.61 ± 0.27, respectively. Our experimental results demonstrate that ASPS-A1 significantly enhances the secretion of iNOS, TNF-α, IL- 1β, and IL- 6 in RAW 264.7 cells in a dose-dependent manner. Additionally, the marked increase in NO release suggests that ASPS-A1 effectively promotes the polarization of RAW 264.7 macrophages toward the M1 phenotype, exerting a potent immunostimulatory effect. To further investigate the immunomodulatory properties of ASPS-A1, we examined the expression levels of the anti-inflammatory cytokine IL- 10 as shown in Fig. 5D, where the ASPS-A1 treatment group shows a upregulation of IL- 10 mRNA compared to the control group. The increase in IL- 10 helps maintain immune response balance, ensuring effective pathogen clearance while minimizing collateral damage caused by excessive immune activation33. In summary, ASPS-A1 not only promotes M1 polarization but also induces IL- 10 secretion to regulate immune homeostasis. This finding aligns with previous research on polysaccharides from Rubus chingii Hu34. We further validated the above experiments using primary peritoneal macrophages from mice. As shown in Fig.S2, ASPS-A1 exhibited higher cell viability within the concentration range of 50–200 µg/mL. Within this range, the expression levels of iNOS, TNF-α, IL- 1β, and IL- 6 in primary macrophages increased in a dose-dependent manner. At 200 µg/mL, the expression of pro-inflammatory cytokines peaked, consistent with the results observed in RAW 264.7 cells. In summary, ASPS-A1 effectively enhances the secretion of pro-inflammatory cytokines in both RAW 264.7 cells and primary mouse peritoneal macrophages, demonstrating strong immunostimulatory activity. Among the tested concentrations, 200 µg/mL had the most pronounced effect on cell proliferation, making it the concentration of choice for subsequent RNA-seq analysis.
Effects of different concentrations of ASPS-A1 on RAW 264.7 cells. (A) Cell viability; (B) NO production; (C) iNOS mRNA relative expression; (D) TNF-α mRNA relative expression; (E) IL- 1β mRNA relative expression; (F) IL- 6 mRNA relative expression. The values are expressed as the means ± SD(n = 3). ns: not significant, *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 vs. the CON group.
mRNA expression of RAW 264.7 cells induced by ASPS-A1. (A) Stack histogram of con and ASPS-A1 expression values; (B) Content Correction; Reference citation PCA relationship diagram between con and ASPS-A1; (C) Differential gene comparison map between ASPS-A1 and con; (D) Volcanic map of differential genes between ASPS-A1 and con.
Analysis of RNA-seq
In order to further explore the immunomodulatory mechanism of ASPS-A1 on RAW 264.7 cells, we treated RAW 264.7 cells with ASPS-A1(200 µg/mL), and used untreated cells as a blank control group to analyze the differential gene expression of the two groups of cells by RNA-seq technique. The expression levels of genes and transcripts were determined by Transcripts Per Million(TPM) as quantitative indicators. As shown in Fig. 4A, the expression levels of con and ASPS-A1 were close, and the data were uniform and reasonably distributed, which ensured the reliability of the experiment. As shown in Fig. 4B, principal component analysis(PCA) results revealed substantial variations in gene expression between the ASPS-A1 and con groups, suggesting that ASPS-A1 therapy significantly affected RAW 264.7 cell gene expression. DEGs were further screened. As shown in Fig. 4C, a total of 1234 genes were significantly differentially expressed between the ASPS-A1 group and control group (p< 0.05, |log2 FoldChange|> 1), of which 923 genes were up-regulated in ASPS-A1 group, 311 genes were down-regulated. The volcano map(Fig. 4D) visually showed the distribution of these differential genes, with green dots representing significantly up-regulated genes and blue dots representing significantly down-regulated genes. These results reveal the extensive effects of ASPS-A1 treatment on RAW 264.7 cells at the genetic level, laying the foundation for further elucidations of its immunomodulatory mechanisms.
ASPS-A1 biological information analysis. (A) GO enrichment analysis of immune-related differential genes in ASPS-A1 vs. con; (B) KEGG pathway enrichment analysis of immune-related differential genes in ASPS-A1 vs. con; (C) PPI network of differentially related genes in immune-related pathways; (D) RT-PCR validation of immune-related differential genes in ASPS-A1 vs. con. The values are expressed as the means ± SD ( n = 3). ** p < 0.01,*** p < 0.001 and **** p < 0.0001 vs. the CON group.
Bioinformatic analysis
In order to further explore the regulatory function of ASPS-A1 on RAW 264.7 cells, functional enrichment analysis of differentially expressed genes was conducted using the GO database, and explored their potential biological functions from three dimensions: BP, CC, and MF35. Figure 5A showed the top 30 GO entries with the highest degree of enrichment. The ordinate represents the proportion of differentially expressed genes, and different colors represent the enrichment results. The GO analysis identified a total of 11 BP entries, 12 MF entries, and 7 CC entries between the con and ASPS-A1 groups. In BP, RNA polymerase II-mediated positive regulation of transcription and translation was the most significant and abundant functional entries; In MF, protein binding and RNA binding were the most prominent and enriched functions; In CC, cytoplasm and nucleus were the most prominent functional items.
In order to further reveal the immunomodulatory mechanism of ASPS-A1, a KEGG enrichment analysis was conducted. KEGG is a reference database that combines genomic, chemical, and systems biology data36,37,38,39. By analyzing the changes in differentially expressed, we explored the signaling pathway that ASPS-A1 induces RAW 264.7 macrophages. Figure 5B showed the top 20 typical signaling pathways with the highest enrichment, which were involved in environmental information processing, cellular processes, biological systems, and metabolic regulation. Among them, the pathways closely related to immune function include the NF-κB signaling pathway, the Toll-like receptor signaling pathway, and the MAPK signaling pathway. These results provided a theoretical basis for further study of the multi-target role of ASPS-A1 in immunostimulatory effects.
In addition, to explore the protein-protein interaction(PPI) between ASPS-A1 and 200 potential targets, we used the STRING database for PPI network construction40. The cross-target data were shown in detail in Fig. 5C, and the PPI network is grouped by modular analysis. Further visual analysis revealed the core genes in this network, which were thought to be key targets of ASPS-A1 in regulating immune responses. Taken together, these findings suggested that ASPS-A1 may play an important role in regulating the body’s immune response by regulating the immune system through multiple targets.
RT-qPCR validation of key genes
The expression levels of Fcgr3, Ccl4, Ikbke, IL1β, Cxcl2, IL10, Ccl3, IL1α, Ccl2 and IL6 genes were detected by RT-qPCR, and the results were shown in Fig. 5D. The RNA expression level of Fcgr3 was lower than that of the blank group, while the RNA expression levels of Ccl4, Ikbke, IL1β, Cxcl2, IL10, Ccl3, IL1α, Ccl2, and IL6 were higher than that of the con group. This was consistent with transcriptomic data, which proved the reliability of the transcriptomic data.
ASPS-A1 activates the MAPK and NF-κB signaling pathway. (A) Blot bands of p-ERK, ERK, p-p38, p38, p-JNK, JNK; (B) The relative protein expression of p-JNK; (C) The relative protein expression of p-p38; (D) The relative protein expression of p-ERK. (E) Blot bands of p-p65, p65, p-IKB-α, IKB-α; (F) The relative protein expression of p-p65; (G) The relative protein expression of p-IKB-α. The values are expressed as the means±SD (n=3). ns: not significant, **p < 0.01,***p < 0.001 and ****p < 0.0001 vs the CON group.
MAPK signaling pathway stimulated by ASPS-A1 in RAW 264.7 cells
ASPS-A1 could exert immunomodulatory effects through the MAPK signaling pathway. To further validate this finding, we performed Western blot analysis to assess protein phosphorylation levels of three key markers in the MAPK pathway, p-ERK1/2, p-p38, and p-JNK41. As shown in Fig. 6A-D, the ASPS-A1 treatment group showed significant differences in p-p38 in the concentration range of 50–200 µg/mL, with p-p38 expression at 200 µg/mL being approximately 1.86 times that of the control group. Meanwhile, p-ERK1/2 and p-JNK also showed significant differences at 100–200 µg/mL, with expression levels at 200 µg/mL approximately 1.49 and 1.63 times that of the control, respectively. At the treatment concentration of 200 µg/mL, the protein expression of p-ERK1/2, p-p38, and p-JNK reached the highest level. These results provide strong evidence that ASPS-A1 enhances immune function by activating the MAPK pathway.
Effect of TLR4/TLR2 inhibitors on ASPS-A1-induced activation of RAW 264.7 cells. (A,C, E, G) The levels of iNOS, TNF-α, IL-6, and IL-1β in the supernatant of RAW 264.7 cells were measured following treatment with C29; (B, D, F, H) The release of iNOS, TNF-α, IL-6, and IL-1β in the supernatant was assessed after treatment with TAK-242. The values are expressed as the means±SD (n=3). ns: not significant, *p < 0.05, **p < 0.01,***p < 0.001 and ****p < 0.0001.
NF-κB signaling pathway stimulated by ASPS-A1 in RAW 264.7 cells
To further verify the KEGG analysis results, a Western blot assay was performed to detect changes in the expression of key proteins in the NF-κB signaling pathway. In the NF-κB pathway, IκB-α and p65 were presented in the cytoplasm in an unstimulated state in non-phosphorylated form42. However, when the cells were stimulated by an external signal, IκB-α was phosphorylated and subsequently degraded, causing the NF-κB p65 subunit to be activated and translocated to function in the nucleus. Based on this mechanism, we extracted cytoplasmic protein and nuclear protein from RAW 264.7 cells, and analyzed the changes in ASPS-A1 treated with different concentrations. The results were shown in Fig. 6E. With the increase in ASPS-A1 concentration, the ratio of p-p65 to p65 in the nucleus significantly increased(Fig. 6F). At 200 µg/mL, the expression level of p-p65 was approximately 2.65 times that of the control group. In addition, we also observed a significant increase in the p-IκB-α/IκB-α ratio under 50–200 µg/mL ASPS-A1 treatment(Fig. 6G), At 200 µg/mL, the p-IκB-α expression level was about 2.61 times that of the control group, further indicating that the phosphorylation level of IκB-α increases with the increase in ASPS-A1 treatment concentration. These results suggested that ASPS-A1 could significantly activate the NF-κB signaling pathway in RAW 264.7 cells. In summary, the results of Western blot analysis and differential gene expression data from RNA-seq analysis corroborate each other, suggesting that ASPS-A1 exerts its immunomodulatory effect by activating the NF-κB pathway. This provided further evidence to support the mechanism study of ASPS-A1 in immune-enhancing function.
ASPS-A1 stimulates toll signaling pathway in RAW 264.7 cells
RNA-seq results suggested that ASPS-A1 may play an immunomodulatory role through the Toll-like receptor(TLR) signaling pathway. To verify this hypothesis, we performed related experiments using specific inhibitors of TLR2 and TLR4. The experimental results in Fig. 7 showed that after pretreatment with TAK- 242, a TLR4 inhibitor, the levels of iNOS, TNF-α, and IL- 6 in the cells of ASPS-A1 and the positive control LPS group significantly decreased, suggesting that ASPS-A1 could activate macrophages through the TLR4 receptor and promote their secretion of the above cytokines. In order to exert immune activity. These findings align with the study by Masayuki I et al.43., which demonstrated that TAK- 242 inhibits LPS induced production of NO, TNF-α, and IL- 6 in human peripheral blood mononuclear cells (PBMCs). Further experiments showed that the expression levels of TNF-α and IL- 1β by cells in the ASPS-A1 and LPS groups were reduced under preconditioning with the TLR2 inhibitor C29, suggesting that ASPS-A1 induces macrophages to secrete TNF-α and IL- 1β through the TLR2 receptors. This observation is consistent with findings by Pragnesh Mistry et al.44, showing that C29 significantly suppresses P3 C and P2 C induced IL- 1β gene expression in THP- 1 cells and reduces P3 C induced, but not P2 C induced, TNF-α mRNA and IL- 12 p40 protein expression in primary mouse macrophages. In summary, these results suggested that the immunomodulatory effects of IL- 1β may depend on the TLR2 signaling pathway, while iNOS, and IL- 6 exert their effects mainly through the TLR4 signaling pathway. In addition, TNF-α is involved in immunostimulatory effects through the TLR2 and TLR4 signaling pathways.
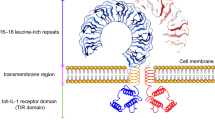
Theoretical binding model of major monosaccharides in ASPS-A1 and TLR2/TLR4. (A) Molecular docking of TLR4 with Gal; (B) Molecular docking of TLR2 with Gal; (C) Molecular docking of TLR4 with GalA; (D) Molecular docking of TLR2 with GalA; (E) Molecular docking of TLR4 with Ara; (F) Molecular docking of TLR2 with Ara; (G) Diagram of the immunomodulatory action of ASPS-A1.
In order to further explore the molecular mechanism of ASPS-A1 interacting with TLR2 and TLR4, we conducted a monosaccharide molecular docking simulation analysis. As shown in Fig. 8, the main monosaccharide components of ASPS-A1, Gal, GalA, and Ara could bind to TLR2 and TLR4 proteins, and formed stable complexes with the cavities on the protein surface through hydrogen bonding and hydrophobic interaction. In the docking of TLR2, the main components of ASPS-A1 formed hydrogen bonds through key residues such as AGR447, SER424, SER445, LYS422, HIS398, GLN383, and ASN397. In the docking of TLR4, the main component of ASPS-A1 forms hydrogen bonds with key residues such as LYS72, LYS89, LYS91, LYS433, GLY97, ARG106, ARG233, ASP99, and ASP- 100. The docking scores showed that the corresponding scores for TLR2 were − 5.1 kcal/mol, − 5.9 kcal/mol, and − 5.4 kcal/mol. The binding scores of TLR4 with Gal, GalA, and Ara were − 5.9 kcal/mol, − 6.3 kcal/mol, and − 5.6 kcal/mol, respectively. Therefore, the binding ability of TLR4 with ASPS-A1 was generally better than that of TLR2. In summary, ASPS-A1 can regulate the immune response through TLR2 and TLR4 signaling pathways, and the interaction with TLR4 is strong. The possible mechanism was shown in Fig. 8G.
Discussion
Screening for immune-enhancing agents that promote immune function is crucial for treating immune-related and tumor diseases. In this study, a novel structure polysaccharide named ASPS-A1 was isolated and purified from the leaves of A. senticosus, and it was shown to significantly enhance the viability of RAW 264.7 cells, indicating its low cytotoxicity. Studies have shown that activated macrophages release NO and various cytokines, including TNF-α, IL- 6, and IL- 1β45. NO, as a crucial intracellular signaling molecule, not only enhances macrophages’ ability to recognize target cells such as cancer cells but also facilitates their elimination. TNF-α, a key immunoregulatory factor, plays a vital role in triggering immune responses and regulating multiple immune cell functions, contributing to processes such as tumor suppression, coagulation, and hematopoiesis. IL- 6, primarily secreted by macrophages and T cells, is essential for the transition from innate to adaptive immunity. Meanwhile, IL- 1β plays a pivotal role in immune regulation by activating various immune cells46. Research has demonstrated that natural polysaccharides derived from plants, animals, and fungi can stimulate RAW 264.7 cells to release NO, TNF-α, IL- 6, and IL- 1β47. Consistently, our findings indicate that ASPS-A1 significantly enhances the secretion of NO and multiple cytokines, further confirming its potent immunostimulatory activity. Further, investigations of the immune-enhancement mechanism of ASPS-A1 were studied by RNA-seq and confirmed by western blot, RT-qPCR, and molecular docking experiments.
The structure of polysaccharides is closely linked to their biological activity. Given the complexity of polysaccharide structures, current research primarily focused on their primary structure, monosaccharide composition, and linkage patterns, foundational information for understanding polysaccharide bioactivity48. The monosaccharide composition analysis in this study indicates that ASPS-A1 primarily consists of Gal, GalA, and Ara, which are closely associated with immunological activity. Zhao et al. reported that a high proportion of Ara, Gal, and Xyl in acidic polysaccharides derived from Dryopteris crassirhizoma significantly enhanced their immunomodulatory effects49. Similarly, Sun et al. demonstrated that the high content of Gal, GalA, and Ara in the polysaccharide SPF3 - 1 from Lysimachia foenum-graecum could promote phagocytic activity and cytokine secretion in RAW 264.7 macrophages50. Additionally, we found that higher Gal and Ara content in polysaccharides was positively correlated with stronger prebiotic activity. Huang et al. observed that polysaccharides with elevated Gal and Ara content from longan pulp promoted the growth of probiotics such as Bifidobacterium, thereby modulating the intestinal immune microenvironment51. Structural analytic discovery that ASPS-A1 was composed predominantly of α− 1,4-D-GalA, α− 1,5-L-Ara, and β− 1,4-D-Gal residues, indicating that the main domains of ASPS-A1 were RG-I and HG. Studies have shown that polysaccharides containing both RG-I and HG pectin structures exhibited strong immunomodulatory activity52,53. Some evidence also indicated that polysaccharides with higher molecular weights and more complex structures tend to bind more membrane targets, thereby exhibiting stronger immunostimulatory activity54. Carrot rhamnogalacturonan-I(cRG-I) showed significant activity at a molecular weight of 100 kDa, while at 75 kDa, it was proven to be inactive55. In this study, ASPS-A1 was found to have a molecular weight of 147.8 kDa, which was considered a higher molecular weight component, indicating its strong immune chance effect.
RNA-Seq is a well-established transcriptomic technology widely used to explore the mechanisms of polysaccharide activity56. To clarify the immune-regulatory mechanism of ASPS-A1 in RAW 264.7 cells, we applied RNA-seq for gene expression analysis. The results showed that RAW 264.7 cells treated with ASPS-A1 led to 1,234 differentially expressed genes, with 923 genes upregulated and 311 downregulated. Through bioinformatics analysis, the top 20 pathways were enriched out, among them, MAPK, NF-κB, and Toll signaling pathways closely related to immunostimulatory effects and were selected for further investigating.
The MAPK signaling pathway plays a crucial role in innate immunity by regulating cell activation, proliferation, and differentiation. Key components of this pathway, ERK1/2, JNK, and p38, are involved in controlling the production of inflammatory factors and gene expression41. Research by Zhang et al. has demonstrated that polysaccharides from Anemarrhena asphodeloidesactivate immune mechanisms through both the MAPK and NF-κB pathways57. Our findings indicate that ASPS-A1 enhances the phosphorylation levels of these three proteins in a dose-dependent manner, thus activating the MAPK pathway.
During immune responses, the NF-κB signaling pathway is activated through phosphorylation, leading to its translocation into the nucleus, where it regulates the transcription of chemokine, cytokine, and inflammatory mediator genes58. Studies have shown that certain plant polysaccharides can enhance immune activity by promoting NF-κB activation59. Our study confirmed that ASPS-A1 stimulates the phosphorylation of IκB-α and promotes the nuclear translocation of p65, thereby activating the NF-κB pathway. Results also confirmed that ASPS-A1 could up-regulate the expression level of IL- 1β, TNF-α, iNOS, and IL- 6, which were considered the Immune regulatory cytokines.
In immunostimulatory effects, polysaccharides, due to their large molecular weight, cannot directly penetrate the cell membrane. Instead, they exert their effects by binding to receptors on the cell surface60. TLR2 and TLR4 are key immune receptors for polysaccharides, belonging to the PRR family of Toll-like receptors61. When TLR2 binds with polysaccharides, it forms heterodimers with TLR1 or TLR6, whereas TLR4 binds with MD- 2 to activate the MyD88 dependent signaling pathway. This, in turn, initiates IRAK activation and triggers MAPK and NF-κB signaling pathways62,63. Ultimately, these pathways stimulate the production of immune cytokines, including TNF-α, and IL- 6. The findings indicate that ASPS-A1 activates macrophages through the TLR4 receptor, enhancing their secretion of TNF-α, iNOS, and IL- 6. Treatment with a TLR4 inhibitor significantly reduced these secretions, suggesting that ASPS-A1’s immune activity relies on the TLR4 pathway. Additionally, in the presence of a TLR2 inhibitor, ASPS-A1 reduced the secretion of TNF-α and IL- 1β. Molecular docking experiments confirmed that ASPS-A1 binds to both TLR4 and TLR2, showing a stronger affinity for TLR4, thereby effectively activating immune responses through TLR4/TLR2 pathways. This study confirms that TLR4/TLR2 are the primary receptors for ASPS-A1; however, the mechanism of polysaccharide action involves a more intricate receptor network, including Toll-like receptors(TLRs), dendritic cell-associated C-type lectin- 1(CLRs), the mannose receptor(MR), and scavenger receptors(SR). Among these, C-type lectin receptors CLRs have gained increasing attention in recent years. Deng et al. reported that polysaccharides from Phallus indusiate can activate macrophages by upregulating the expression of Dectin- 1 and TLR receptors, thereby enhancing phagocytic activity and significantly promoting the secretion of IL- 1β and TNF-α, ultimately boosting immunostimulatory effects64.
LPS consists of three main components: lipid A, a core oligosaccharide, and an O-side chain. Among these, lipid A is the key structural determinant of LPS immunoactivity. However, excessive immune activation by LPS can lead to inflammatory diseases. To mitigate its toxicity while enhancing its potential as an immunoadjuvant, some studies have explored structural modifications of lipid A65. In contrast, the food-derived polysaccharide ASPS-A1 lacks lipid A, granting it the advantages of low toxicity and high safety. These properties make ASPS-A1 a promising candidate for applications in immune modulation.
This study systematically analyzed the immunomodulatory mechanism of ASPS-A1 and found that ASPS-A1 activates the NF-κB and MAPK pathways through TLR4/TLR2, significantly promoting the secretion of key inflammatory mediators such as TNF-α, IL- 6, and IL- 1β in RAW 264.7 cells. The production of IL- 1β may be closely associated with the activation of the NLRP3 inflammasome. The secretion of IL- 1β is regulated by two critical signaling pathways: one linked to TLR4-mediated immune signaling and the other to NLRP3 inflammasome activation. During immune recognition, pathogen-associated molecular patterns(PAMPs) are detected by pattern recognition receptors(PRRs), such as TLR4, triggering NF-κB signaling and inducing the transcription and synthesis of IL- 1β precursors. Subsequently, the assembly of the inflammasome complex drives the autocleavage of Caspase- 1, ultimately facilitating the maturation of IL- 1β into its bioactive form66,67.
This study demonstrated that ASPS-A1 exerts its immunostimulatory effects through the TLR/MAPK/NF-κB signaling pathway, clarifying its precise action targets and providing important theoretical support for natural polysaccharide-based immunotherapy strategies. Although preliminary progress has been made in elucidating the action mechanism of ASPS-A1 in this study, the conclusions are still mainly based on in vitro experimental models, which have certain limitations. Therefore, in future research, we plan to use in-vivo models to further validate the binding of ASPS-A1 to receptors and explore the potential involvement of other receptors, such as CLRs and the NLRP3 inflammasome pathway, in its immunoregulatory effects. This will enable a more systematic and comprehensive elucidation of the immune response mechanisms mediated by polysaccharides.
Conclusion
This study systematically revealed the mechanism of ASPS-A1 in immunostimulatory effects. Through in vitro experiments and RNA-seq bioinformatics analysis, we demonstrated that ASPS-A1 could significantly activate macrophages and promote the RNA expression level of key cytokines such as NO, iNOS, TNF-α, IL- 1β, and IL- 6. In addition, combined with the results of KEGG and GO analysis, we found that the immunomodulatory effect of ASPS-A1 was closely related to the MAPK and NF-κB signaling pathways, especially through the immune signal transduction mediated by targeting TLR4 and TLR2 receptors. Specifically, ASPS-A1 activated the expression of iNOS, and IL- 6 through the TLR4 receptor, and induced the expression of IL- 1β dependent on the TLR2 signaling pathway, while TNF-α is synergistically regulated through the TLR2 and TLR4 dual pathways. These findings not only enrich our understanding of the immunomodulatory function of ASPS-A1 but also provide a scientific basis for its use as a potential immune enhancer.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request. The datasets generated during the current study are available in the Gene Expression Omnibus repository. The corresponding GSE number for this data is GSE287586.
References
S, O. B., Julie, M. S. O., A, A. E. & B. & Sustained delivery approaches to improving adaptive immune responses advanced drug delivery reviews. Adv. Drug Del. Rev. 187, 114401–114401. https://doi.org/10.1016/j.addr.2022.114401 (2022).
S, T. K., Neha, S. & Javier, C. Secondary immunodeficiencies. An overview. Annals of allergy, asthma & immunology: official publication of the American college of allergy. Asthma Immunol. 127, 617–626. https://doi.org/10.2500/aap.2024.45.240063 (2021).
Chin, J. L., Chan, L. C., Yeaman, M. R. & Meyer, A. S. Tensor-based insights into systems immunity and infectious disease. Trends Immunol, 44(5), 329–332. https://doi.org/10.1016/j.it.2023.03.003 (2023).
Chu, Q. et al. Apios Americana medik flowers polysaccharide (AFP) alleviate Cyclophosphamide-induced immunosuppression in ICR mice. Int. J. Biol. Macromol. 144, 829–836. https://doi.org/10.1016/j.ijbiomac.2019.10.035 (2019).
Luan, F. et al. Structural diversity and bioactivity of polysaccharides from medicinal mushroom Phellinus spp. A. Rev. Food Chem. 397, 133731–133731. https://doi.org/10.1016/j.foodchem.2022.133731 (2022).
Luan, F. et al. An update review of extraction, purification, structural features, bioactivities, and applications of schisandra chinensis polysaccharides. Int. J. Biol. Macromol. 262, 130030–130030. https://doi.org/10.1016/j.ijbiomac.2024.130030 (2024).
M, S. K. & Alexander, H. Functional hallmarks of healthy macrophage responses: their regulatory basis and disease relevance. Annu. Rev. Immunol. 40, 295–321. https://doi.org/10.1146/annurev-immunol-101320-031555 (2022).
Lars, F., Marzena, S. & Christian, K. Macrophages: sentinels and regulators of the immune system. Cell. Microbiol. 18, 475–487. https://doi.org/10.1111/cmi.12580 (2016).
Lv, Y. et al. Structural characterization and Immunomodulatory activity of a water-soluble polysaccharide from Poria cocos. Int. J. Biol. Macromol. 261, 129878–129878. https://doi.org/10.1016/j.ijbiomac.2024.129878 (2024).
Jiang, P. et al. Structural characteristics, antioxidant properties and antiaging activities of Galactan produced by mentha Haplocalyx Briq. Carbohyd Polym. 234, 115936. https://doi.org/10.1016/j.carbpol.2020.115936 (2020).
Ming, Z. et al. Peucedanum praeruptorum Dunn polysaccharides regulate macrophage inflammatory response through TLR2/TLR4-mediated MAPK and NF-κB pathways. Biomed. Pharmacother. 152, 113258–113258. https://doi.org/10.1016/j.biopha.2022.113258 (2022).
Xuan, L., Xianxiang, C., Liuming, X., Jianhua, X. & Mingyue, S. Sulfated Chinese Yam polysaccharide enhances the Immunomodulatory activity of RAW 264.7 cells via the TLR4-MAPK/NF-κB signaling pathway. Food Funct. 13. https://doi.org/10.1039/d1fo03630k (2022).
Zhang, X. et al. A review of the extraction and purification methods, biological activities, and applications of active compounds in acanthopanax senticosus. Front. Nutr. 11, 1391601–1391601. https://doi.org/10.3389/fnut.2024.1391601 (2024).
Huihai, Y., Lulu, W. & Jing, Z. Leukocyte modulation by natural products from herbal medicines and potential as cancer immunotherapy. J. Leukoc. Biol. 112, 185–200. https://doi.org/10.1002/jlb.3ru0222-087rrr (2022).
Choudhary, S. et al. Immunomodulatory effect of phytoactive compounds on human health: A narrative review integrated with bioinformatics approach. Curr. Top. Med. Chem. https://doi.org/10.2174/0115680266274272240321065039 (2024).
Jiayuan, W. et al. Immunoregulatory effects of tetrastigma Hemsleyanum polysaccharide via TLR4-mediated NF-κB and MAPK signaling pathways in Raw264.7 macrophages. Biomed. Pharmacother. 161, 114471–114471. https://doi.org/10.1016/j.biopha.2023.114471 (2023).
Xuan, L., Xianxiang, C., Liuming, X., Jianhua, X. & Mingyue, S. Sulfated Chinese Yam polysaccharide enhances the Immunomodulatory activity of RAW 264.7 cells via the TLR4-MAPK/NF-κB signaling pathway. Food Funct. 13 https://doi.org/10.1039/d1fo03630k (2022).
Shubao, Y. et al. Immunomodulatory effect of acanthopanax senticosus polysaccharide on immunosuppressed chickens. Poult. Sci. 100, 623–630. https://doi.org/10.1016/j.psj.2020.11.059 (2020).
Fan, C. et al. Modulation of hypoxia-inducible factor-1 α /cyclo-oxygenase-2 pathway associated with Attenuation of intestinal mucosa inflammatory damage by acanthopanax senticosus polysaccharides in lipopolysaccharide-challenged piglets. Br. J. Nutr. 122, 666–675. https://doi.org/10.1017/s0007114519001363 (2019).
Li, K., Li, S., Du, Y. & Qin, X. Screening and structure study of active components of astragalus polysaccharide for injection based on different molecular weights. J. Chromatogr. B. 1152, 122255–122255. https://doi.org/10.1016/j.jchromb.2020.122255 (2020).
Li, X. et al. Total fractionation and characterization of the water-soluble polysaccharides isolated from enteromorpha intestinalis. Int. J. Biol. Macromol. 111, 319–325. https://doi.org/10.1016/j.ijbiomac.2018.01.018 (2018).
Zhang, W. & Huang, G. Preparation, characteristics and antioxidant activity of mung bean Peel polysaccharides. Sci. Rep. 14, 22161–22161. https://doi.org/10.1038/s41598-024-73068-6 (2024).
Jialuo, S. et al. Unveiling the Immunomodulatory mechanism of polysaccharides from polygonum Cyrtonema based on RNA-seq. Food Res. Int. 175, 113755. https://doi.org/10.1016/j.foodres.2023.113755 (2024).
Denman, L. J. & Morris, G. A. An experimental design approach to the chemical characterisation of pectin polysaccharides extracted from cucumis Melo Inodorus. Carbohyd Polym. 117, 364–369. https://doi.org/10.1016/j.carbpol.2014.09.081 (2015).
Houben, K., Jolie, R. P., Fraeye, I., Loey, A. M. V. & Hendrickx, M. E. Comparative study of the cell wall composition of broccoli, Carrot, and tomato: structural characterization of the extractable pectins and hemicelluloses. Carbohydr. Res. 346, 1105–1111. https://doi.org/10.1016/j.procbio.2019.01.007 (2011).
York, W. S., Darvill, A. G., Mcneil, M. & Albersheim, P. 3-Desoxy-D-manno-2-octulosonic acid (KDO) is a component of rhamnogalacturonan II, a pectic polysaccharide in the primary cell walls of plants. Carbohydr. Res. 138, 109–126. https://doi.org/10.1016/0008-6215(85)85228-9 (1985).
Shanglong, Y. et al. Degradation of codium cylindricum polysaccharides by H2O2-Vc-ultrasonic and H2O2-Fe2+-ultrasonic treatment: structural characterization and antioxidant activity. Int. J. Biol. Macromol. 182, 129–135. https://doi.org/10.1016/j.ijbiomac.2021.03.193 (2021).
Farhadi, N. Structural Elucidation of a water-soluble polysaccharide isolated from Balangu Shirazi (Lallemantia royleana) seeds. Food Hydrocoll. 72, 263–270. https://doi.org/10.1016/j.foodhyd.2017.05.028 (2017).
Feng, S. et al. Antioxidant and anti-aging activities and structural Elucidation of polysaccharides from Panax Notoginseng root. Process. Biochem. 78, 189–199. https://doi.org/10.1016/j.procbio.2019.01.007 (2019).
Chen, Y., Jiang, X., Xie, H., Li, X. & Shi, L. Structural characterization and antitumor activity of a polysaccharide from ramulus Mori. Carbohyd Polym. 190, 232–239. https://doi.org/10.1016/j.carbpol.2018.02.036 (2018).
Chen, X., Song, W., Zhao, J., Zhang, Z. & Zhang, Y. Some Physical Properties of Protein Moiety of Alkali-Extracted Tea Polysaccharide Conjugates Were Shielded by Its Polysaccharide. Molecules. 22, 914–914. https://doi.org/10.3390/molecules22060914 (2017).
Mantovani, A. & Locati, M. Antonio Sica Macrophage Polarization Comes of Age. Immunity. 23, 344–346. https://doi.org/10.1016/j.immuni.2005.10.001 (2005).
Yang, W. et al. Structural characteristics and immune-enhancing activity of fractionated polysaccharides from athyrium multidentatum (Doll.) Ching. Int. J. Biol. Macromol. 205, 76–89. https://doi.org/10.1016/j.ijbiomac.2022.02.037 (2022).
Wei, X. et al. Molecular mechanisms underlying macrophage Immunomodulatory activity of rubus Chingii Hu polysaccharides. Int. J. Biol. Macromol. 185, 907–916. https://doi.org/10.1016/j.ijbiomac.2021.07.024 (2021).
Juntong, Y. et al. The alternate consumption of Quercetin and alliin in the traditional Asian diet reshaped microbiota and altered gene expression of colonic epithelial cells in rats. J. Food Sci. Technol. 84, 678–686. https://doi.org/10.1111/1750-3841.14473 (2019).
Xiang, Q. D. et al. Immunomodulatory activity of ganoderma atrum polysaccharide on purified T lymphocytes through Ca2+/CaN and Mitogen-Activated protein kinase pathway based on RNA sequencing. J. Agric. Environ. Ethics. https://doi.org/10.1021/acs.jafc.7b01763 (2017).
Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Watanabe, M. I. KEGG: biological systems database as a model of the real world. Nucleic Acids Res. https://doi.org/10.1093/nar/gkae909 (2024).
Institute for Chemical, Research, K. U. & Uji Kyoto, Japan. Toward Understanding the origin and evolution of cellular organisms. Protein Science: Publication Protein Soc. 28, 1947–1951. https://doi.org/10.1002/pro.3715 (2019).
M, K. & S, G. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Wang, Y. et al. Network Pharmacology and molecular Docking approach to investigate the mechanism of a Chinese herbal formulation Yougui pills against steroid-related osteonecrosis of the femoral head. Arab. J. Chem. 17, 105609. https://doi.org/10.1016/j.arabjc.2024.105609 (2024).
Yang, Y. et al. Hydroxypropyltrimethyl ammonium chloride Chitosan activates RAW 264.7 macrophages through the MAPK and JAK-STAT signaling pathways. Carbohyd Polym. 205, 401–409 (2019).
Bastian, H. & A, S. J. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer Res. 12, 86. https://doi.org/10.1186/1476-4598-12-86 (2013).
Masayuki, I. et al. A novel cyclohexene derivative, Ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol. Pharmacol. 69, 1288–1295. https://doi.org/10.1124/mol.105.019695 (2006).
Pragnesh, M. et al. Inhibition of TLR2 signaling by small molecule inhibitors targeting a pocket within the TLR2 TIR domain. Proc. Natl. Acad. Sci. U S A. 112, 5455–5460. https://doi.org/10.1073/pnas.1422576112 (2015).
Cavaillon, M. J. Cytokines and macrophages. Biomed. Pharmacother. 48, 445–453. https://doi.org/10.1016/0753-3322(94)90005-1 (1994).
Mauricio, G. J., Moura, S. H. & Guang, Y. J, L. J. P2X7 receptor inhibition ameliorates dendritic spine pathology and social behavioral deficits in Rett syndrome mice. Nature communications. 11, 1784. https://doi.org/10.1038/s41467-020-15590-5 (2020).
Long, J. et al. Harnessing chemical functionality of Xylan hemicellulose towards carbohydrate polymer-based pH/magnetic dual-responsive nanocomposite hydrogel for drug delivery. Carbohyd Polym. 343, 122461–122461. https://doi.org/10.1016/j.carbpol.2024.122461 (2024).
Wang, Q. C., Zhao, X., Pu, J. H. & Luan, X. H. Influences of acidic reaction and hydrolytic conditions on monosaccharide composition analysis of acidic, neutral and basic polysaccharides. Carbohyd Polym. 143, 296–300. https://doi.org/10.1016/j.carbpol.2016.02.023 (2016).
Zhao, Y. et al. Antioxidant and Immunomodulatory activities of polysaccharides from the rhizome of dryopteris crassirhizoma Nakai. Int. J. Biol. Macromol. 130, 238–244. https://doi.org/10.1016/j.ijbiomac.2019.02.119 (2019).
Sun, S., Li, K., Xiao, L., Lei, Z. & Zhang, Z. Characterization of polysaccharide from helicteres angustifolia L. and its Immunomodulatory activities on macrophages RAW264.7. Biomed. Pharmacother. 109, 262–270. https://doi.org/10.1016/j.biopha.2018.10.039 (2019).
Xiaoqin, Z. et al. Physicochemical properties and prebiotic activities of polysaccharides from Zizyphus jujube based on different extraction techniques. Int. J. Biol. Macromol. 223, 663–672. https://doi.org/10.1016/j.ijbiomac.2022.11.057 (2022).
Seung-U, S., Hye, C. E. & Kwang-Soon, S. Effects of rhamnogalacturonan-I type polysaccharide purified from Curcuma longa on immunostimulatory and intracellular signaling pathway mechanisms of macrophages. Food Bioscience. 53. https://doi.org/10.1016/j.fbio.2023.102589 (2023).
T, M. S. et al. Biological activity and Pharmacological application of pectic polysaccharides: A review. Polym. Polym. Compos. 10, 1407–1407. https://doi.org/10.3390/polym10121407 (2018).
Du, B., Meenu, M., Liu, H. & Xu, B. A concise review on the molecular structure and function relationship of β-glucan. Int. J. Mol. Sci. 20, 4032. https://doi.org/10.3390/ijms20164032 (2019).
Desai, K. et al. Associating structural characteristics to Immunomodulating properties of Carrot rhamnogalacturonan-I fractions. Carbohyd Polym. 347, 122730–122730. https://doi.org/10.1016/j.carbpol.2024.122730 (2025).
Houbo, L., Junhua, S., Mingkai, W. & Delin, X. The application and future prospect of RNA-Seq technology in Chinese medicinal plants. J. Appl. Res. Med. Aromatic Plants. 24 https://doi.org/10.1016/j.jarmap.2021.100318 (2021).
Shaojie, Z. et al. A zinc-modified anemarrhena asphodeloides polysaccharide complex enhances immune activity via the NF-κB and MAPK signaling pathways. Int. J. Biol. Macromol. 249, 126017–126017. https://doi.org/10.1016/j.ijbiomac.2023.126017 (2023).
Hui, Y., Liangbin, L., Zhiqiang, Z., Huiyuan, Z. & Hongbo, H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal. Transduct. Target. Therapy. 5, 209–209. https://doi.org/10.1038/s41392-020-00312-6 (2020).
Xie, Y. et al. Polysaccharide from alfalfa activated RAW 264.7 macrophages through MAPK and NF-κB signaling pathways. Int. J. Biol. Macromol. 126, 960–968. https://doi.org/10.1016/j.ijbiomac.2018.12.227 (2018).
Miao, Y., Ying, Z. & Hua, L. Advances in research on immunoregulation of macrophages by plant polysaccharides. Front. Immunol. 10, 145. https://doi.org/10.3389/fimmu.2019.00145 (2019).
Liu, W. et al. An antitumor Arabinan from glehnia littoralis activates immunity and inhibits angiogenesis. Int. J. Biol. Macromol. 263, 130242–130242. https://doi.org/10.1016/j.ijbiomac.2024.130242 (2024).
Ting, S. et al. Polysaccharide from wheat Bran induces cytokine expression via the toll-like receptor 4-mediated p38 MAPK signaling pathway and prevents cyclophosphamide-induced immunosuppression in mice. Food Nutr. Res. 61, 1344523. https://doi.org/10.1080/16546628.2017.1344523 (2017).
Yetty, M., Rita, A. D., Sari, P. Y., Sari, H. N. & Gaurav, P. Physical activity maintain immune response through TLR-2/TLR-4 gene expression in Type-2 diabetes mellitus patient at Medan City. Med. Archives. 77, 276–280. https://doi.org/10.5455/medarh.2023.77.276-280 (2023).
Deng, C., Fu, H., Shang, J., Chen, J. & Xu, X. Dectin-1 mediates the immunoenhancement effect of the polysaccharide from dictyophora indusiata. Int. J. Biol. Macromol. 109, 369–374. https://doi.org/10.1016/j.ijbiomac.2017.12.113 (2018).
Wang, X., Quinn, P. J. & Lipopolysaccharide Biosynthetic pathway and structure modification. Prog Lipid Res. 49, 97–107. https://doi.org/10.1016/j.plipres.2009.06.002 (2009).
Ho, S. C., Chang, Y. H. & Chang, K. S. Structural moieties required for Cinnamaldehyde-Related compounds to inhibit canonical IL-1β secretion. Molecules 23 https://doi.org/10.3390/molecules23123241 (2018).
Tőzsér, J. & Benkő, S. Natural Compounds as Regulators of NLRP3 Inflammasome-Mediated IL-1β Production. Mediators Inflamm. 1–16, (2016). https://doi.org/10.1155/2016/5460302 (2016).
Acknowledgements
We express our gratitude for the financial support provided by the Jilin Provincial Department of Science and Technology(YDZJ202101ZYTS077), and Changchun University Climbing Program(ZPK202123). We also acknowledge the financial support from Changchun University and their technical assistance in the laboratory. Thank you for your support.
Author information
Authors and Affiliations
Contributions
D. G.:funding acquisition, resources, writing-review and editing. Y. W.:conceptualization, writing-original draft preparation. L.W., H.Z and P. R:formal analysis and investigation. X. C., F. H., J. L. and R. Z.: methodology. J.Z.:supervision. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
was not required for this research.
Plant materials statement
The plant collection and use were in accordance with all the relevant guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Y., Wang, L., Zhang, H. et al. Immunostimulatory effects mechanism of polysaccharide extracted from Acanthopanax senticosus on RAW 264.7 cells through activating the TLR/MAPK/NF-κB signaling pathway. Sci Rep 15, 13440 (2025). https://doi.org/10.1038/s41598-025-97423-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97423-3