Abstract
Acute ischemic stroke (AIS) is characterized by the abrupt onset of neurological dysfunction stemming from focal brain ischemia, confirmed through imaging evidence of infarction. In contrast, transient ischemic attack (TIA) manifests with neurological deficits in the absence of infarction, with imaging serving as the definitive diagnostic criterion. This study aims to assess the diagnostic value of combining non-high-density lipoprotein cholesterol (non-HDL-C) and blood pressure (BP) in differentiating AIS from TIA. We recruited 207 untreated AIS patients diagnosed within 72 h and 99 age- and gender-matched TIA patients. Upon admission, serum non-HDL-C levels, other lipid profiles, and BP measurements were obtained. Binary logistic regression was employed to identify risk factors, while receiver operator characteristic (ROC) curves were used to evaluate diagnostic performance. Furthermore, least absolute shrinkage and selection operator (LASSO) regression coupled with multivariate logistic regression was utilized to develop a nomogram model. The AIS group exhibited higher prevalence rates of hypertension, diabetes, family history of diabetes, and smoking (P < 0.05). Notably, non-HDL-C, systolic BP, diastolic BP, and other lipid markers were significantly elevated in the AIS group (P < 0.05). Multivariate analysis pinpointed non-HDL-C (OR [odds ratio] = 1.663, 95% CI [confidence interval]: 1.239–2.234, P < 0.01) and systolic BP (OR = 1.035, 95% CI: 1.012–1.057, P < 0.01) as independent risk factors. ROC analysis revealed that systolic BP alone achieved an AUC of 0.686 (sensitivity: 78.7%, specificity: 51.5%), whereas the combination of systolic BP and non-HDL-C enhanced diagnostic accuracy (AUC [area under the ROC curve] = 0.736, sensitivity: 75.4%, specificity: 64.6%). A nomogram incorporating low-density lipoprotein cholesterol (LDL-C), glucose (GLU), homocysteine, and smoking demonstrated high predictive accuracy, with training and validation AUCs of 0.769 and 0.704, respectively. Non-HDL-C and systolic BP emerge as independent risk factors for AIS, and their combined use augments diagnostic precision in differentiating AIS from TIA. A nomogram model presents a practical differentiation tool, particularly in settings with limited resources.
Similar content being viewed by others
Introduction
Acute Ischemic Stroke (AIS), also termed acute cerebral infarction, represents the most prevalent type of stroke, comprising more than 80% of strokes worldwide1. It is characterized by high morbidity, disability, mortality, recurrence rates, and significant economic impact2. A transient ischemic attack (TIA), often referred to as a “mini-stroke,” originates similarly to AIS and displays comparable symptoms3,4. However, it typically resolves within 24 h5. In clinical practice, even when advanced imaging modalities are unavailable, an effective distinction between AIS and TIA can frequently be accomplished through meticulous observation of the patient’s neurological symptoms6. The key distinguishing factor resides in the duration and persistence of neurological deficits, necessitating a thorough clinical assessment focused on the temporal evolution of symptoms6. However, this assessment hinges entirely on the clinician’s individual experience, and not all clinicians can accurately discern during diagnosis. Currently, the primary method for diagnosing AIS and TIA still relies on neuroimaging technologies7,8. Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) are considered the gold standard, however, they are costly and require extensive equipment and expert operation9. Moreover, in settings lacking CT or MRI capabilities, or when patients cannot be transported, these methods face significant limitations in the continuous monitoring of critically ill patients, making it challenging to differentiate early AIS from TIA and other related conditions9,10. Effective management of AIS aims to alleviate initial symptoms, prevent further brain damage, improve functional outcomes, and reduce the risk of recurrence11. In this context, peripheral blood tests offer a non-invasive diagnostic alternative for early differentiation between AIS and TIA.
Non-HDL-C encompasses all blood cholesterol excluding high-density lipoprotein cholesterol (HDL-C), including low-density lipoprotein cholesterol (LDL-C), very LDL-C (VLDL-C), intermediate-density lipoprotein cholesterol (IDL-C), and chylomicron remnant cholesterol (CMR-C)12,13. Leading guidelines internationally recommend non-HDL-C as the primary indicator for managing blood lipids14. Unlike triglyceride (TG), total cholesterol (TC), and LDL-C, non-HDL-C levels provide a more accurate prediction of cardiovascular and cerebrovascular events, serving as a novel clinical marker13,15.
Blood pressure (BP) significantly influences vascular function and organ perfusion16, and its dysregulation can critically impact cerebral circulation, which is particularly vulnerable to such disturbances17. Ischemic stroke often stems from an array of pathologies, commonly associated with systemic cardiovascular (CV) conditions or direct cerebrovascular abnormalities18, many of which are related to or directly caused by hypertension19,20. Thus, chronic hypertension is frequently a precursor to AIS21,22. During the acute phase of AIS, characterized as an acute hypertensive response (AHR), elevated BP levels are recorded in roughly 75% of patients6,23. This AHR typically peaks within the initial hours post-stroke, subsequently diminishing and generally normalizing within the first 7 to 10 days following the onset6,23. Therefore, the critical examination of BP’s clinical significance in the context of ischemic stroke, particularly concerning AIS and TIA, warrants thorough investigation.
AIS and TIA both originate from cerebral blood flow interruption and exhibit similar pathophysiological mechanisms, like atherosclerosis and thrombosis, leading to potential overlaps in serological markers and blood pressure changes4,6. Consequently, there are currently no specific serological markers exclusively for AIS and TIA. Existing indicators are largely general markers reflecting systemic inflammation, coagulation function, or neuronal damage, posing a challenge in accurately differentiating AIS and TIA. Consequently, clinical indicators related to AIS exhibit relatively low accuracy, sensitivity, and specificity, and lack robust predictive capability24,25. Furthermore, comparisons are typically made between AIS and healthy groups, with limited analysis between AIS and TIA4. Clinical tools, such as the ABCD² score, which evaluates factors such as age, blood pressure, clinical features, duration of symptoms, and diabetes, can assist in assessing the risk of stroke following a TIA26. However, the ABCD² score primarily serves to predict the risk of AIS occurring shortly after a TIA (e.g., within 2 or 7 days), rather than directly differentiating between AIS and TIA27. Furthermore, the ABCD² score relies solely on clinical features and excludes neuroimaging results (like CT or MRI), which constitute the gold standard for distinguishing AIS from TIA (AIS patients typically exhibit infarct lesions on imaging, whereas TIA patients do not show structural changes)28,29,30. Additionally, the ABCD² score is primarily intended for early assessment following a TIA episode and has limited diagnostic value for patients who have already progressed to AIS31,32.
This study assessed serum levels of TG, TC, HDL-C, LDL-C, glucose (GLU), ischemia modified albumin (IMA), and Hcy in both AIS and TIA groups, calculated non-HDL-C levels, and measured systolic and diastolic pressures at admission to evaluate their diagnostic utility in differentiating AIS from TIA. Significantly, a nomogram diagnostic scoring model was developed using LASSO regression analysis. Additionally, a training set and a validation set were established to further evaluate the diagnostic effectiveness of the nomogram in distinguishing between AIS and TIA. This study aims to address the challenges faced by primary healthcare units, particularly in cases involving inexperienced clinicians, atypical patients, or the absence of CT/MRI facilities. The primary objective of this study is to develop a streamlined and efficient diagnostic model utilizing readily available serum biomarkers and clinical parameters. This model aims to enhance the rapid and accurate diagnosis and management of TIA and AIS in primary care settings. By identifying straightforward and practical indicators, we seek to provide robust medical evidence that can help toy improve clinical decision-making and patient outcomes in primary care settings.
Materials and methods
Clinical data, inclusion, and exclusion criteria
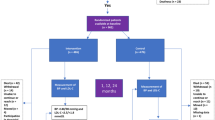
A total of 207 patients diagnosed with AIS were recruited from Henan Provincial People’s Hospital between January 1, 2023, and May 31, 2024. The inclusion criteria for the AIS group included: (1) complete clinical records; (2) age over 18 years; (3) admission within 72 h of symptom onset, as per the “Chinese Acute Ischemic Stroke Diagnosis Guidelines 2018”; (4) first-time diagnosis of AIS without prior treatment; (5) diagnosis confirmed via cranial CT or MRI. The exclusion criteria encompassed: patients with (1) malignant tumors or those undergoing postoperative chemotherapy; (2) hematologic disorders; (3) other ischemic conditions; (4) severe hepatic or renal impairment; (5) autoimmune disorders; (6) a history of stroke; (7) prior targeted stroke treatments; (8) pregnancy or lactation; and (9) cranial trauma.
The control group (TIA group) included 99 patients diagnosed with TIA and posterior circulation ischemia, non-related to stroke. Patients were selected based on the diagnostic criteria set forth by the AHA/ASA. Exclusion criteria for the TIA group included: (1) patients with infarction lesions or intracranial hemorrhage as confirmed by CT or MRI; (2) presence of malignant tumors, hematologic, or systemic immune diseases; (3) severe hepatic or renal dysfunction; and (4) other ischemic conditions. All AIS and TIA diagnoses adhered to the “Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018”14. All participants in this study provided written informed consent and the study was approved by the Ethics Committee of Henan Provincial People’s Hospital (approval number: 2024LRC-16).
Instruments, reagents, and methods
Venous blood (3–5 mL) was collected on an empty stomach either on the second day post-hospitalization or on the morning of the physical examination. It was centrifuged using a Zhongjia vertical KD- 1044 centrifuge at 1728 × g for 5 min. On the same day, serum levels of TG, TC, HDL-C, LDL-C, GLU, IMA, and Hcy were measured using an Abbott C1600 biochemical analyzer, and non-HDL-C was calculated according to the instructions provided by the kit (Ningbo Meikang Biotechnology Co., Ltd.). All test items had undergone inter-laboratory quality assessment under the clinical inspection center of the Ministry of Health to ensure accuracy and comparability with other laboratories. According to the inpatient medical records, systolic and diastolic pressures measured at admission were documented. Ten baseline data points including hypertension, family history of hypertension, diabetes, family history of diabetes, heart disease, dyslipidemia, hyperhomocysteinemia, smoking history, alcohol consumption history, and family history of cerebrovascular disease were collected. Diagnoses of all conditions were made based on clinical guidelines, and family history was determined if the patient, the patient’s parents, siblings, or children had two or more of these conditions. Reference intervals were as follows: TG: (0 ~ 1.7) mmol/L, TC: (2.33 ~ 5.17) mmol/L, HDL-C: (1.04 ~ 1.74) mmol/L, LDL-C: (1.9 ~ 3.12) mmol/L, GLU: (3.88 ~ 6.11) mmol/L, IMA: (0 ~ 85) U/mL, Hcy: (5 ~ 15) µmol/L, systolic pressure: < 120 mmHg, diastolic pressure: < 90 mmHg. All experiments were conducted in accordance with the relevant guidelines and regulations, and was approved by the Ethics Committee of Henan Provincial People’s Hospital.
Construction and validation of a diagnostic model
A nomogram diagnostic scoring model was developed using clinical data, with optimal parameters identified through least absolute shrinkage and selection operator (LASSO) regression models. LASSO regression (R-package glmnet) screened model parameters, employing a 10-fold orthogonal method to minimize coefficient Lambda based on the principle of minimum standard deviation. Clinical indicators without coefficients were excluded, and those remaining were included in a multivariate logistic regression to construct the predictive nomogram model. The model’s performance was assessed using a receiver operating characteristic (ROC) curve, a calibration curve, and the Hosmer-Lemeshow goodness of fit test. Clinical decision curve analysis (DCA) was performed to evaluate the model’s clinical utility.
Statistical analysis
Statistical analyses were conducted using SPSS 27.0, and graphical representations were created with GraphPad Prism 9.4.1. The Shapiro-Wilk normality test determined the distribution of measurement data, which, if normal or approximately normal, was expressed as mean ± standard deviation (SD). Frequency matching was employed to align the distributions of gender and age, thereby minimizing information bias. Univariate and multivariate logistic regression analyses were subsequently conducted for adjustment. The F test compared multiple groups, while the t-test was used for comparisons between two groups; non-normally distributed data were expressed as median and interquartile range [M (25%, 75%)], with the Kruskal-Wallis rank sum test and Mann-Whitney test used for multiple and two-group comparisons, respectively; categorical data were expressed as percentages and analyzed using the χ2 test. Univariate and multivariate binary logistic regressions analyzed correlations between indices and AIS and evaluated risk factors for AIS, respectively. The diagnostic efficacy of each index, alone and in combination, was evaluated by the area under the ROC curve (AUC), sensitivity, and specificity to assist in distinguishing between AIS and TIA. P < 0.05 was considered statistically significant.
Results
Demographic characteristics
A total of 207 AIS patients were diagnosed and treated at Henan Provincial People’s Hospital between January 1, 2023, and May 31, 2024. The cohort comprised 140 males (67.7%) and 67 females (32.4%), ranging in age from 27 to 92 years, with an average age of 58.82 ± 11.578 years. These patients were categorized into three groups based on the time of onset: 73 in the ≤ 12-hour group, 87 in the ≤ 24-hour group, and 47 in the > 24-hour group. The control group (TIA group) included 99 cases of TIA, posterior circulation ischemia, and non-stroke conditions, consisting of 66 males (66.7%) and 33 females (33.3%), aged between 27 and 93 years, with an average age of 57.56 ± 13.224 years. No significant differences were observed in gender distribution or age composition between the AIS and TIA groups (Table 1).
Comparison of basal parameters
Initial comparisons of basal parameters between the AIS and TIA groups revealed significant differences in hypertension, diabetes, family history of diabetes, and smoking history (all with P < 0.05). No significant differences were observed in family history of hypertension, heart disease, dyslipidemia, hyperhomocysteinemia, alcohol consumption history, and family history of cerebrovascular disease (all with P > 0.05; Table 1). Statistical analysis also confirmed no significant differences in age and gender between the groups (t = 0.854, P = 0.394; χ2 = 0.028, P = 0.866), ensuring comparability.
Subsequent analyses of 10 indicators, including TG, TC, HDL-C, LDL-C, GLU, IMA, Hcy, non-HDL-C, systolic pressure, and diastolic pressure, showed that values for TG, TC, LDL-C, Hcy, non-HDL-C, systolic pressure, and diastolic pressure were significantly higher in the AIS group compared to the TIA group (all P < 0.05). There were no significant differences in HDL-C, GLU, and IMA between the two groups (P > 0.05). Additionally, no significant differences were found among the ≤ 12-hour, ≤ 24-hour, and > 24-hour onset groups regarding these 10 indicators (all P > 0.05, Tables 2 and 3; Figs. 1 and 2).
Logistic regression analysis of risk factors for AIS
Univariate logistic regression analysis was performed to assess the correlation between various indices and the occurrence of AIS. The TIA group was assigned a value of 0, and the AIS group was assigned a value of 1. A binary logistic regression model was constructed using the single variables from 10 items: TG, TC, HDL-C, LDL-C, GLU, IMA, Hcy, non-HDL-C, systolic pressure, and diastolic pressure. Results indicated that TG, TC, LDL-C, GLU, Hcy, non-HDL-C, systolic pressure, and diastolic pressure were significantly associated with the occurrence of AIS (all with OR > 1, and P < 0.05; Table 4).
Multivariate logistic regression analysis was also conducted, with the TIA group assigned a value of 0 and the AIS group a value of 1. Indicators related to non-HDL-C such as TG, TC, HDL-C, and LDL-C were excluded. The model was adjusted for biases in hypertension, diabetes, family history of diabetes, and smoking history, considering differences in gender, age, and baseline data. GLU, Hcy, non-HDL-C, systolic pressure, and diastolic pressure, which had shown a significant impact on AIS in the univariate analysis, were included to construct the multivariate logistic regression. It was determined that non-HDL-C and systolic pressure were independent risk factors for AIS (OR = 1.663, 95% CI 1.239–2.234, P < 0.01; OR = 1.035, 95% CI 1.012–1.057, P < 0.01; Table 5).
Diagnostic performance of separate and combined detection of 10 indicators to aid in distinguishing AIS from TIA
The ROC curve analysis revealed that the single-item test for systolic pressure offered the best auxiliary differentiation, with a diagnostic AUC of 0.686, a sensitivity of 78.7%, and a specificity of 51.5%; when combined, systolic pressure and non-HDL-C achieved a diagnostic AUC of 0.736, with sensitivity and specificity of 75.4% and 64.6%, respectively. When all 10 items were combined, the sensitivity was 82.6%, and the specificity 59.6%, corresponding to an AUC of 0.770 (Table 6; Fig. 3).
Construction of a nomogram diagnostic scoring model and its diagnostic efficacy in distinguishing AIS from TIA
The LASSO regression method was employed to filter model parameters for constructing a nomogram diagnostic scoring model. Univariate and multivariate logistic regression analyses were conducted on predictors with non-zero coefficients identified by LASSO regression to develop the diagnostic model. The analysis indicated that smoking habit (OR = 1.096, P = 0.176), systolic pressure (OR = 1.006, P < 0.001), LDL (OR = 1.132, P < 0.001), GLU (OR = 1.035, P = 0.033), and Hcy (OR = 1.005, P = 0.071) were independent risk factors for AIS (Fig. 4A and B). The selection process of variables is shown in Fig. 4C and D. In the nomogram of the diagnostic model training set, systolic pressure had the longest line segment, indicating the greatest contribution to the diagnosis, followed by LDL, GLU, Hcy, and smoking habit (Fig. 4E). The ROC curve for the training set of the nomogram diagnostic model showed an AUC of 0.769, while the AUC reached 0.704 in the validation set, confirming the model’s high efficacy in distinguishing AIS from TIA (Fig. 4F). The Hosmer-Lemeshow goodness-of-fit test and a calibration curvue (Bootstrap method, n = 1 000) were utilized to assess the model’s calibration, with results in both the internal and external validation sets yielding P > 0.05, indicating consistent model performance (Fig. 4G). The calibration curve for the training set demonstrated good agreement between the predicted and actual probabilities (Fig. 4G). Additionally, the DAC curve of the model based on the five parameters revealed that the curve was significantly distant from the two extreme lines, suggesting practical application value (Fig. 4H).
Predictive model distinguishing AIS from TIA based on the LASSO algorithm. (A, B) Variable Selection. (C, D) Outcome of LASSO regression for vital parameter. Using a tenfold cross-validation approach, the coefficient lambda is minimized based on the criterion of the smallest standard deviation, ultimately selecting clinical indicators with non-zero coefficients. In the cross-validation process, the function of the binomial deviance values is represented by log(lambda), with the Y-axis depicting binomial deviance values. The lower X-axis represents log (lambda), while the upper X-axis shows the average number of parameters. (E) Columnar graph of the predictive model. (F) Diagnostic AUCs for the training and validation sets. (G) Calibration Curves for the training and validation Sets. (H) Clinical Decision Curves for the training and validation sets.
Discussion
Ischemic stroke and TIA represent the predominant forms of cerebrovascular disease11,22,23,25. Globally, the incidence of stroke ranges between 150 and 200 cases per 100,000 individuals annually, with AIS constituting more than 80% of these instances1. TIA is characterized by temporary or mild clinical symptoms stemming from localized brain dysfunction due to diminished regional blood flow, or ischemia. Early recognition of ischemic events is crucial, given that 20% of ischemic stroke cases are preceded by a TIA within the preceding hours to days3,4,6,7. Furthermore, up to 80% of subsequent strokes post-TIA are preventable, highlighting the importance of prompt diagnosis and intervention for both AIS and TIA4,6. However, limited clinical methods exist to distinguish between AIS and TIA. In this study, the level of non-HDL-C was calculated based on serological indicators such as TG, TC, LDL-C, GLU, IMA, Hcy, non-HDL-C, and combined with systolic and diastolic BP. The diagnostic efficiency of these indicators for distinguishing AIS from TIA and their correlation with AIS were analyzed. A diagnostic model for distinguishing AIS from TIA was constructed using LASSO regression analysis.
Research indicates that dyslipidemia often complicates AIS33, with patients typically exhibiting elevated levels of TG, TC, LDL-C, and reduced HDL, often accompanied by hyperhomocysteinemia15,34,35. Some studies have identified HDL as a protective factor against stroke, and the TC/HDL ratio has been used to assess the relationship between dyslipidemia and ischemic stroke36,37,38. Additionally, hypertension is prevalent among AIS patients19,20. A meta-analysis indicated that ischemic stroke was the most prevalent hypertension-mediated organ damage among all patients presenting in emergency department with hypertensive crisis, with a proportion of 28.1%39. This study focused on evaluating the performance of non-HDL-C and BP in confirming AIS and TIA. Calculations revealed that non-HDL-C, TG, TC, LDL-C, Hcy, systolic BP, and diastolic BP levels in the AIS group were significantly higher than those in the TIA group, with TC, TG, LDL, and TC/HDL associated with the occurrence of AIS. Furthermore, non-HDL-C was significantly linked to adverse outcomes in AIS patients. Jiao et al. also noted that high serum non-HDL-C levels might significantly increase the risk of cognitive dysfunction post-AIS40. Similarly, a study by Lu et al. identified high serum non-HDL-C levels, age, education, Hcy levels, and HAMD scores as independent risk factors for cognitive impairment in AIS patients41. Consistent with these findings, our study identified non-HDL-C and systolic BP as independent risk factors for AIS.
We evaluated the diagnostic performance of the included parameters for distinguishing AIS from TIA and found that the single systolic BP test had the highest AUC of 0.686 among all single tests. For parallel detection, the combination of systolic BP, LDH-C, and non-HDL-C reached an AUC of 0.737, while the AUC for 10 combined tests reached 0.770. These results suggest that the diagnostic performance of these parameters is still limited. Moreover, the specificity of blood pressure alone is reported to be 51.5%, implying that nearly half of individuals without AIS may be misidentified as having the condition (false positives). Although the specificity of measuring systolic pressure in isolation is confined, raising awareness among primary care physicians and promoting the combined use of this indicator—especially when CT or MRI is not an option—can diminish the risk of overlooking diagnoses of AIS42. This holds greater significance than preventing false-positives, given that the hazards and repercussions of an undiagnosed AIS are substantially elevated43. Furthermore, considering that the majority of patients first approach community healthcare settings when suspecting AIS or TIA, the integration of straightforward and readily accessible measures like systolic pressure monitoring and non-HDL-C testing becomes specially crucial.
We further explored methods to enhance the accuracy of differential diagnosis between AIS and TIA. The LASSO logistic model, a machine learning tool for classification, selects optimal variables to establish a regression model using the LASSO algorithm, determining variable selection based on the contraction of variable coefficients to achieve the optimal combination44. This approach helps simplify models and enhance prediction accuracy45. In this study, smoking habit, systolic BP, LDL, GLU, and Hcy were identified as risk factors for AIS using LASSO regression, and a nomogram model based on these five items was established. This model demonstrated good predictive value and clinical utility, with AUCs of 0.769 and 0.704 in the training and internal validation sets, respectively. Compared to single tests, this approach markedly improved diagnostic efficiency. Alireza Baratloo et al. conducted a cross-sectional diagnostic accuracy study and developed a nomogram-based clinical tool for diagnosing AIS at the prehospital stage, achieving an AUC of 0.893 with an 8-item-based nomogram model46. In our study, the LASSO regression algorithm was employed to automatically select important variables through regularization, thereby reducing model complexity and avoiding overfitting. The nomogram model developed in our study, based on five routinely used clinical indicators for AIS and TIA, demonstrated superior diagnostic performance compared to any individual parameter alone, and decision curve analysis also showed the nomogram’s promising diagnostic utility in aiding the differentiation of AIS. Thus, the generated nomogram may provide an intuitive scoring system, facilitating rapid assessment of disease risk and enhancing clinical decision-making efficiency. The combination of LASSO regression and nomogram in this study offers advantages such as variable selection, strong interpretability, and personalized prediction in the differential diagnosis of AIS and TIA. However, the LASSO regression algorithm is limited by its linearity assumption in constructing predictive models, and it exhibits drawbacks such as data dependency, uncertainty in variable selection, potential neglect of variable interactions, and insufficient external validation. In practical applications, integrating other methods and clinical expertise remains crucial for enhancing diagnostic accuracy. For the LASSO regression model, the development of software tools—including mobile or desktop applications—that may promptly generate predictive outcomes upon inputting pertinent clinical diagnostic information from patients is vital. These tools would aid clinicians in incorporating patient history, thereby facilitating diagnosis and clinical assessment.
Our study has several limitations. First, it is a retrospective analysis with a relatively small patient cohort, leading to inevitable biases in the results. Second, both the training and validation sets of the diagnostic model included limited samples, resulting in relatively low AUCs. Third, the predictive effectiveness of the nomogram depends significantly on the selection of variables, and its predictive capability varies widely across different datasets and groups. Lastly, the evaluation of hypertension is integral to understanding a patient’s overall cerebrovascular risk profile, and its primary utility lies in guiding secondary prevention strategies, rather than acute diagnostic differentiation. Therefore, more stringent exclusion criteria and larger external validation cohorts are required to substantiate the findings of this study in the future.
In summary, we investigated the diagnostic value of non-HDL-C and BP in AIS and TIA. Additionally, we introduced a new nomogram-based diagnostic tool for differentiating between AIS and TIA, which demonstrated acceptable specificity, sensitivity, and AUC. Given that the indicators examined in this study demonstrate potential auxiliary diagnostic value, although the current sample size is limited, they have clarified the trends in these metrics. Building on this foundation, the next step will involve expanding the sample size and incorporating additional clinical data. By leveraging modern AI technology for big data analysis and modeling, it is promising to develop an artificial intelligence (AI)-based model capable of low-cost, high-efficiency differentiation of AIS and TIA in primary care settings, and this approach seeks to address the limitations posed by the lack of imaging resources, optimize clinical decision-making processes, and ultimately enhance diagnostic and treatment standards.
Data availability
The data that support the results of this study are available from the corresponding author upon reasonable request.
References
Saini, V., Guada, L. & Yavagal, D. R. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology 97, S6–S16. https://doi.org/10.1212/wnl.0000000000012781 (2021).
Tang, X. et al. Leukoaraiosis and acute ischemic stroke. Eur. J. Neurosci. 54, 6202–6213. https://doi.org/10.1111/ejn.15406 (2021).
Amin, H. P. et al. Diagnosis, workup, risk reduction of transient ischemic attack in the emergency department setting: A scientific statement from the American Heart Association. Stroke 54, e109–e121. https://doi.org/10.1161/str.0000000000000418 (2023).
Patrick, L. & Halabi, C. Inpatient management of acute stroke and transient ischemic attack. Neurol. Clin. 40, 33–43. https://doi.org/10.1016/j.ncl.2021.08.003 (2022).
Rhoney, D. H. Contemporary management of transient ischemic attack: Role of the pharmacist. Pharmacotherapy 31, 193–213. https://doi.org/10.1592/phco.31.2.193 (2011).
Mendelson, S. J. & Prabhakaran, S. Diagnosis and management of transient ischemic attack and acute ischemic stroke: A review. Jama 325, 1088–1098. https://doi.org/10.1001/jama.2020.26867 (2021).
Yao, Q. et al. Imaging diagnosis of transient ischemic attack in clinic and traditional Chinese medicine. Biomed. Res. Int. 2019, 5094842. https://doi.org/10.1155/2019/5094842 (2019).
Hong, L., Hsu, T. M., Zhang, Y. & Cheng, X. Neuroimaging prediction of hemorrhagic transformation for acute ischemic stroke. Cerebrovasc. Dis. 51, 542–552. https://doi.org/10.1159/000521150 (2022).
Regenhardt, R. W., Potter, C. A., Huang, S. S. & Lev, M. H. Advanced imaging for acute stroke treatment selection: CT, CTA, CT perfusion, and MR imaging. Radiol. Clin. N. Am. 61, 445–456. https://doi.org/10.1016/j.rcl.2023.01.003 (2023).
Nguyen, T. N. et al. Noncontrast computed tomography vs computed tomography perfusion or magnetic resonance imaging selection in late presentation of stroke with Large-Vessel occlusion. JAMA Neurol. 79, 22–31. https://doi.org/10.1001/jamaneurol.2021.4082 (2022).
Hurford, R., Sekhar, A., Hughes, T. A. T. & Muir, K. W. Diagnosis and management of acute ischaemic stroke. Pract. Neurol. 20, 304–316. https://doi.org/10.1136/practneurol-2020-002557 (2020).
Wang, A. et al. Non-HDL-C/HDL-C ratio is associated with carotid plaque stability in general population: A cross-sectional study. Front. Neurol. 13, 875134. https://doi.org/10.3389/fneur.2022.875134 (2022).
Di Angelantonio, E. et al. Major lipids, apolipoproteins, and risk of vascular disease. Jama 302, 1993–2000. https://doi.org/10.1001/jama.2009.1619 (2009).
Li, X. W. et al. Chinese guidelines for the diagnosis and treatment of hand, foot and mouth disease (2018 edition). World J. Pediatr. WJP. 14, 437–447. https://doi.org/10.1007/s12519-018-0189-8 (2018).
Aggarwal, D. J., Kathariya, M. G. & Verma, D. P. K., LDL-C, NON-HDL-C and APO-B for cardiovascular risk assessment: Looking for the ideal marker. Indian Heart J. 73, 544–548. https://doi.org/10.1016/j.ihj.2021.07.013 (2021).
Caprioli, J. & Coleman, A. L. Blood pressure, perfusion pressure, and glaucoma. Am. J. Ophthalmol. 149, 704–712. https://doi.org/10.1016/j.ajo.2010.01.018 (2010).
DeWitt, D. S. & Prough, D. S. Cerebral blood flow and blood pressure: Dependent or codependent? Crit. Care Med. 47, 1007–1009. https://doi.org/10.1097/ccm.0000000000003784 (2019).
Zhao, W., Hausenloy, D. J., Hess, D. C., Yellon, D. M. & Ji, X. Remote ischemic conditioning: Challenges and opportunities. Stroke 54, 2204–2207. https://doi.org/10.1161/strokeaha.123.043279 (2023).
Cho, H. et al. Untreated hypertension and prognosis paradox in acute ischemic stroke. Neurol. Sci. 44, 2087–2095. https://doi.org/10.1007/s10072-023-06653-8 (2023).
Gąsecki, D., Kwarciany, M., Kowalczyk, K., Narkiewicz, K. & Karaszewski, B. Blood pressure management in acute ischemic stroke (Hypertens. Rep, 2020). https://doi.org/10.1007/s11906-020-01120-7.
Ferrari, F. & Villa, R. F. Brain bioenergetics in chronic hypertension: Risk factor for acute ischemic stroke. Biochem. Pharmacol. 205, 115260. https://doi.org/10.1016/j.bcp.2022.115260 (2022).
Huber, M. & Heiss, W. D. Acute ischemic stroke. Semin. Thromb. Hemost. 22, 53–60. https://doi.org/10.1055/s-2007-998991 (1996).
Herpich, F. & Rincon, F. Management of acute ischemic stroke. Crit. Care Med. 48, 1654–1663. https://doi.org/10.1097/ccm.0000000000004597 (2020).
Tassi, R., Guideri, F., Acampa, M., Domenichelli, C. & Martini, G. Acute ischemic stroke and concomitant massive pulmonary embolism: A challenge. Neurol. Sci. 42, 4777–4780. https://doi.org/10.1007/s10072-021-05494-7 (2021).
Nentwich, L. M. Diagnosis of acute ischemic stoke. Emerg. Med. Clin. N. Am. 34, 837–859. https://doi.org/10.1016/j.emc.2016.06.008 (2016).
Cutting, S., Regan, E., Lee, V. H. & Prabhakaran, S. High ABCD2 scores and in-hospital interventions following transient ischemic attack. Cerebrovasc. Dis. Extra 6, 76–83. https://doi.org/10.1159/000450692 (2016).
Cao, S. et al. ABCD2 score has equivalent stroke risk prediction for anterior circulation TIA and posterior circulation TIA. Sci. Rep. 13, 13993. https://doi.org/10.1038/s41598-023-41260-9 (2023).
Giles, M. F. et al. Early stroke risk and ABCD2 score performance in tissue- vs time-defined TIA: A multicenter study. Neurology 77, 1222–1228. https://doi.org/10.1212/WNL.0b013e3182309f91 (2011).
Zhao, H. et al. ABCD² score may discriminate minor stroke from TIA on patient admission. Transl. Stroke Res. 5, 128–135. https://doi.org/10.1007/s12975-013-0286-x (2014).
Wardlaw, J. M. et al. ABCD2 score and secondary stroke prevention: Meta-analysis and effect per 1000 patients triaged. Neurology 85, 373–380. https://doi.org/10.1212/wnl.0000000000001780 (2015).
Yang, C. et al. Validation of the Canadian TIA score to predict subsequent stroke risk in Chinese TIA patients. Cerebrovasc. Dis. 51, 735–743. https://doi.org/10.1159/000524264 (2022).
Gross, H., Guilliams, K. P. & Sung, G. Emergency neurological life support: Acute ischemic stroke. Neurocrit. Care 23(Suppl 2), S94–S102. https://doi.org/10.1007/s12028-015-0159-0 (2015).
Xu, T. et al. Dyslipidemia and outcome in patients with acute ischemic stroke. Biomed. Environ. Sci. BES 27, 106–110. https://doi.org/10.3967/bes2014.023 (2014).
Holmen, M., Hvas, A. M. & Arendt, J. F. H. Hyperhomocysteinemia and ischemic stroke: A potential dose-response association—A systematic review and Meta-analysis. TH. Open Companion J. Thromb. Haemost. 5, e420–e437. https://doi.org/10.1055/s-0041-1735978 (2021).
Ortiz-Munoz, G. et al. Dysfunctional HDL in acute stroke. Atherosclerosis 253, 75–80. https://doi.org/10.1016/j.atherosclerosis.2016.08.035 (2016).
Papagiannis, A. et al. HDL cholesterol efflux capacity and phospholipid content are associated with the severity of acute ischemic stroke and predict its outcome. Clin. Chim. Acta 540, 117229. https://doi.org/10.1016/j.cca.2023.117229 (2023).
Liu, Y. et al. Non-traditional lipid profiles and the risk of stroke: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. NMCD 33, 698–714. https://doi.org/10.1016/j.numecd.2023.01.003 (2023).
Wei, L. et al. Interaction analysis of abnormal lipid indices and hypertension for ischemic stroke: A 10-year prospective cohort study. Front. Cardiovasc. Med. 9, 819274. https://doi.org/10.3389/fcvm.2022.819274 (2022).
Siddiqi, T. J. et al. Clinical outcomes in hypertensive emergency: A systematic review and meta-analysis. J. Am. Heart Assoc. 12, e029355. https://doi.org/10.1161/jaha.122.029355 (2023).
Jiao, Y., Tian, T., Wei, S., Wang, C. & Wu, L. Association between serum non-high-density lipoprotein cholesterol and cognitive dysfunction after acute ischemic stroke: A cross-sectional study. Brazilian J. Med. Biol. Res. = Revista Brasileira De Pesquisas Medicas E Biologicas 53, e9487. https://doi.org/10.1590/1414-431x20209487 (2020).
Lu, D. et al. Association between serum non-high-density lipoprotein cholesterol and cognitive impairment in patients with acute ischemic stroke. BMC Neurol. 16, 154. https://doi.org/10.1186/s12883-016-0668-2 (2016).
Albers, G. W. Management of acute ischemic stroke. An update for primary care physicians. Western J. Med. 166, 253–262 (1997).
Ozdemir, H., Sagris, D., Abdul-Rahim, A. H., Lip, G. Y. H. & Shantsila, E. Management of ischaemic stroke survivors in primary care setting: The road to holistic care. Intern. Emerg. Med. 19, 609–618. https://doi.org/10.1007/s11739-023-03445-y (2024).
Motamedi, F., Pérez-Sánchez, H., Mehridehnavi, A., Fassihi, A. & Ghasemi, F. Accelerating big data analysis through LASSO-Random forest algorithm in QSAR studies. Bioinformatics 38, 469–475. https://doi.org/10.1093/bioinformatics/btab659 (2022).
Lin, K., Wang, Y., Li, Y. & Wang, Y. Identification of biomarkers associated with pediatric asthma using machine learning algorithms: A review. Medicine 102, e36070. https://doi.org/10.1097/md.0000000000036070 (2023).
Baratloo, A., Ramezani, M., Rafiemanesh, H., Sharifi, M. & Karimi, S. A nomogram-based clinical tool for acute ischemic stroke screening in prehospital setting. Curr. J. Neurol. 22, 58–62. https://doi.org/10.18502/cjn.v22i1.12618 (2023).
Funding
This study was supported by the Foundation of Science and Technology in Henan Province (No. 242102310292), the Backbone Teachers Program of Sanquan College of Xinxiang Medical University (SQ2025GGJS06), the Henan Provincial Department of Education, Henan Province Key Discipline Teaching and Research ([2023] No. 414–1058), and Science and Technology Project of Henan Province (No.252102311009).
Author information
Authors and Affiliations
Contributions
Zhi’an He conceived the experiment. Ya Zhu, Sha Yan, and Yuheng Chen performed the experiments and collected the data. Yuping Fu analyzed and interpreted the data. Yuping Fu wrote the manuscript draft. Zhi’an He revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fu, Y., Zhu, Y., Yan, S. et al. Appraising non-HDL-C, systolic pressure, and a nomogram-based diagnostic model as auxiliary biomarkers in confirming acute ischemic stroke and transient ischemic attack. Sci Rep 15, 13530 (2025). https://doi.org/10.1038/s41598-025-97474-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97474-6