Abstract
Left ventricular dysfunction is a known risk factor for morbidity and mortality in hemodialysis patients. The prognostic value of left ventricular global longitudinal strain (LV GLS) among those with preserved left ventricular ejection fraction (LVEF) remains uncertain. Subjects with end-stage renal disease initiated hemodialysis at Taipei Veteran General Hospital between 2015 and 2018 were registered. All participants received annually echocardiographic studies thereafter. Left ventricular end-systolic volume (LVESV), end-diastolic volume (LVEDV) and internal diameter in systole (LVIDs), LVEF, and LV GLS were measured. A LV GLS of > – 15.9% was defined as reduced LV GLS. Clinical outcomes of mortality and hospitalization for heart failure (HHF) were followed. A total of 319 patients with preserved LVEF (66.3 ± 15.1 years, 48.6% men) were recruited in the study. Subjects with reduced LV GLS had more coronary artery disease (CAD), higher LVESV and LVIDs, but were similar in age, gender, co-morbidities, biochemistries and other echocardiographic parameters as the counterpart. Both CAD [(odds ratio (OR) and 95% confidence intervals (CIs): 1.669, 1.023–2.724], and LVESV (OR per-1 mL and 95% CIs: 1.073, 1.004–1.146) were independent determinants of reduced LV GLS. Kaplan-Meier analysis indicated that patients with reduced LV GLS had a significantly lower event-free survival rate compared to those with preserved GLS. The multivariate Cox regression analysis further demonstrated LV GLS as a significant predictor of adverse clinical events (hazard ratio per-1% and 95% CIs: 1.055, 1.002–1.110) after accounting for age, gender, and diabetes. Among the hemodialysis patients with preserved LVEF, LV GLS but not the conventional left ventricular functional indices were associated with long-term mortality and HHF. CAD could be a modifiable risk factor among the subjects with reduced LV GLS.
Similar content being viewed by others
Introduction
Patients with end-stage renal disease (ESRD) are associated with increased risks of cardiovascular diseases and heart failure, and their mortality rate is 10 to 20 times higher than that of healthy individuals1,2. Numerous data have shown left ventricular systolic or diastolic function is not unusual among subjects with ESRD2,3, and there has been an inverse correlation between left ventricular ejection fraction (LVEF) and cardiovascular death or total mortality4,5. However, the inverse correlation effect is less pronounced in patients with an ejection fraction greater than 45%6. The intrinsic limitations of ejection fraction may restrict its effectiveness in detecting mild left ventricular systolic dysfunction7.
Given that LVEF is a volume and load-dependent measure, which varies significantly in subjects with ESRD underwent hemodialysis. In contrast, the two-dimensional speckle tracking echocardiography provides an angle-independent measurement of myocardial deformation, offering a precise evaluation of both systolic and diastolic function across all myocardial segments8,9. Left ventricular global longitudinal strain (LV GLS) quantifies the longitudinal shortening of the myocardial fibers10, which has outperformed LVEF as a prognostic indicator for adverse clinical events among general population and individuals with heart failure11,12.
There is limited data on the utility and prognostic value of strain in the hemodialysis population. Our study aims to explore the determinants and predictors of LV GLS in hemodialysis patients and examine the association between post-hemodialysis LV GLS and clinical outcomes.
Methods
Patients
From March 2015 to October 2018, patients more than 18 years old receiving chronic hemodialysis for at least 3 months at Taipei Veteran General Hospital were eligible for the present study. The study participants would undergo transthoracic echocardiography annually. Subjects with rhythm other than sinus (n = 32), suboptimal image quality for speckle tracking analysis (n = 66), significant valvular heart disease (n = 27), and LVEF < 50% (n = 52) were excluded from this study. The flowchart of the study participants was demonstrated in Fig. 1. The anthropometric and demographic data, and comorbidities were recorded. Hemogram and biochemistries were obtained from the overnight-fasting blood samples, following echocardiographic studies. All subjects provided written informed consent for the use and publication of their clinical data, including anthropometric and demographic details, blood samples, electrocardiograms, and echocardiographic studies. This consent fulfills the study protocol approved by the research ethics committee. The investigation has followed the Declaration of Helsinki with the approval of the Institutional Review Board of Taipei Veteran General Hospital.
Study subjects enrollment flowchart. From 2015 to 2018, we enrolled 496 patients who began hemodialysis. Several criteria led to the exclusion of some patients. Those who received an echocardiographic evaluation either one month prior or within three months after starting hemodialysis were included. Our study ultimately analyzed a cohort of 319 patients.
Transthoracic echocardiography
Transthoracic echocardiography was conducted according to the recommendations of the American Society of Echocardiography, utilizing the GE Vivid Untrasound system (Healthcare, Horten, Norway). Left ventricular internal dimensions at end-diastolic (LVIDd) and end-systolic (LVIDs), left ventricular end-diastolic (LVEDV) and end-systolic volume (LVESV), LVEF, early (E) and late (A) ventricular filling flow velocities, the mitral annulus tissue velocity (e’), left atrial volume, and the peak velocity of tricuspid regurgitation (TRV) were measured accordingly13. The left ventricular mass index (LVMi), the left atrial volume index (LAVi) and right ventricular systolic pressure (RVSP) were calculated. LVH was defined as LVMi > 95 g/m2 for women and > 115 g/m2 for men. Left ventricular diastolic dysfunction was defined when ≥ 3 of the following indicators were met: (1) septal e’ velocity < 7 cm/s, (2) septal E/e’ ratio > 14, (3) TRV > 2.8 m/s, and (4) LAVi > 34 ml/m214.
Speckle-tracking was conducted across three successive cardiac cycles, capturing two-dimensional left ventricular visuals from the standard triad of apical view. Two-dimensional speckle-tracking assessments were executed by utilizing EchoPAC software (version 10.8, GE Vingmed Ultrasound AS, Horten, Norway). Peak systolic longitudinal strain was evaluated from six segments in apical long-axis, 4-chamber, and 2-chamber perspectives. LV GLS was calculated by averaging regional peak longitudinal strain values captured prior to aortic valve closure, as determined within the apical long-axis field. Longitudinal strain computations were achieved by averaging across eighteen segments: six basal, six mid-ventricular, and six apical segments within the left ventricle15. A LV GLS greater than − 15.9% was classified as reduced LV GLS, indicating impaired left ventricular systolic function16,17.
Study endpoints
The adverse clinical events of mortality and hospitalizations for heart failure (HHF) were identified at the outpatient clinics, which was further conformed by reviewing medical recorded and linking the database to the National Death Registry. All the study participants were followed for up to 5 years.
Statistical analysis
The study population were stratified into preserved and reduced LV GLS. The baseline characteristics were compared by using the chi-square test for categorical variables and the Student’s t-test for continuous variables. The Kaplan-Meier survival curve analysis was conducted to examine the difference in survival probability between groups. The univariate and multivariate logistic regression analyses were utilized to evaluate the determinants of LV GLS. The prognostic values of LV GLS were analyzed by Cox regression analysis. A two-tailed P value of < 0.05 was deemed statistically significant. All statistical analyses were performed using SPSS version 17.0 (IBM, Chicago, IL, USA).
Results
Baseline characteristics
A total of 319 patients (age 66.3 ± 15.1 years, 48.6% men) were recruited in this study (Table 1). In general, Age, gender distribution, body mass index, comorbidities, H2FPEF score, laboratory tests, and dialytic efficiency were not different between subjects with preserved or reduced LV GLS. But subjects with reduced LV GLS were more likely to have coronary artery disease (CAD). In addition, subjects with reduced LV GLS had greater LVIDs and LVESV, and lower LVEF than those with preserved LV GLS. Otherwise, LVIDd, LVEDV, E and A wave velocity, e’ velocity, E/e’ ratio, LVMi, LAVi, TRV, and RVSP were similar between the two groups.
In contrast, subjects with preserved LV GLS truly had greater apical longitudinal strains, including AP4L strain, AP2L strain, and AP3L strain, as well as LV GLS than their counterpart.
Predictors of reduced LV GLS
CAD, LVIDs, and LVESV were crudely associated with the presence of reduced LV GLS. In the multivariate logistic regression analysis, CAD [(odds ratio (OR) and 95% confidence intervals (CIs): 1.669, 1.023–2.724], and LVESV (OR per-1 mL and 95% CIs: 1.073, 1.004–1.146) remained related to reduced LV GLS. (Table 2)
Prognostic values of LV GLS
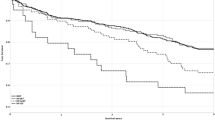
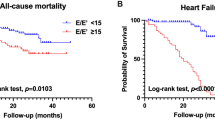
During a mean follow-up period of 4.7 years, there were 44 (13.7%) deaths and 10 (3.1%) HHF across the entire cohort. Among patients with preserved LV GLS, 23 (11.6%) of them encountered HHF or mortality, while 28 (22.9%) of those with reduced LV GLS had adverse clinical outcomes. The Kaplan-Meier survival curve analysis showed better event-free survival among subjects with preserved LV GLS than the others (Fig. 2A), which was mainly driven by the lower risk related to preserved LV GLS (Fig. 2B). However, the rates of HHF were not different between patients with reduced and preserved LV GLS (Fig. 2C).
In a multivariate Cox regression model, presence of diabetes and reduced LV GLS were all independently predictive of worse outcomes in subjects with ESRD and preserved LVEF (Fig. 3). None of the other measures of LV structure or function, including LVIDs, LVEDV, LVESV, LVEF, and diastolic dysfunction correlated with the clinical outcomes. With adjustments for age, gender, and diabetes, LV GLS (HR per-1% and 95% CIs: 1.055, 1.002–1.110) remained significant predictors of adverse clinical events (Table 3).
Discussion
The study investigated the prognostic roles of left ventricular function in subjects with ESRD receiving chronic hemodialysis. The results demonstrated LV GLS but not LVEF or diastolic dysfunction was associated with long-term risks of mortality and HHF. In addition, CAD and LVESV were independent determinants of reduced LV GLS. Consequently, the results may underscore the importance of using LV GLS for clinical risk evaluation among hemodialytic patients who deem had preserved LVEF.
LV GLS has been a sensitive discriminator over clinical risk factors in predicting all-cause mortality in patients with renal impairment and preserved LVEF18. In a prospective cohort study of 88 stable hemodialysis patients, reduced LV GLS was independently associated with mortality during a mean follow-up duration of 26 months19. The present study further extended the long-term prognostic impact of LV GLS on mortality and HHF. Therefore, abnormal LV GLS could serve as an early indicator of uremic cardiomyopathy in dialysis patients when they have normal LVEF. The underlying causes of abnormal LV GLS in ESRD may involve the interstitial fibrosis with myocyte hypertrophy20. In animal models of uremic cardiomyopathy, Rafael et al. illustrated that strain parameters can detect uremic cardiomyopathy and predict cardiovascular mortality among dialysis patients21. Although there were only minor differences in left ventricular structures and functions in this study, LV GLS outperformed other cardiac parameters in the prediction of adverse clinical events. The study results may support LV GLS a favorable index of uremic cardiomyopathy.
LVH is commonly observed among subjects with ESRD, and left ventricular chamber size usually would be small, especially in patients with normal LVEF. Even though, Wu et al. have highlighted LVIDs, but not LVEF an independent risk factor of major adverse cardiovascular events and mortality in chronic hemodialysis patients22. The dependability of LVEF as a functional index is affected by its sensitivity to external elements like preload, afterload, and heart rate23. Based on current understandings, LVEF may be more reflective of LV dilation than myocardial contractility24,25. In contrast, LVESV is less susceptible to cardiac loading conditions and more responsive to variations in contractility, indicating LVESV a better index of left ventricular systolic function26,27. In this study, 87.7% of the population had LVH and left ventricular chamber size were all within normal values. LVESV was a sensitive index related to LV GLS and LVEF, underscoring left ventricular chamber size for risk stratifications28,29,30. However, none of LVIDs, LVESV or LVEF was associated with the outcomes.
Increased pressure, volume overload, anemia, hypocalcemia, and hyperphosphatemia may have explain diastolic dysfunction but not systolic dysfunction was more prevalent in patients with ESRD31,32,33. Among patients with HFpEF, LV GLS has been associated with left ventricular end-diastolic pressure, indicating that a decrease in LV GLS may precede early-stage LV diastolic dysfunction14. In this study, we did not demonstrated significant association between diastolic dysfunction and LV GLS, neither the diastolic dysfunction correlated with the outcomes34. The results may suggest LV GLS a better indicator of left ventricular abnormalities beyond systolic and diastolic dysfunction.
The study also demonstrated CAD as a key determinant of LV GLS in hemodialysis patients. This could be attributed to the susceptibility of the endocardium to the adverse effects of reduced blood flow or ischemia. Given that the endocardial myofibers predominantly have a longitudinal orientation, LV GLS tends to deteriorate quickly under ischemic conditions35. The results may alert the clinical physicians to survey for CAD among subjects with reduced LV GLS but normal LVEF. However, the clinical benefits of subsequent coronary interventions needs further investigations.
Since conventional echocardiographic parameters are related to volume fluctuations, there’s a concern about the impact of fluid load on LV GLS in patients undergoing hemodialysis. Liu et al. have shown the measures of LV GLS during hemodialysis or on an interdialytic day were consistent9. Moreover, recent researches have indicated that LV GLS is considered to be less influenced by both pre-load and after-load36,37.
Given the nature of an observational study, there were inherent limitations. Firstly, the study demonstrated LV GLS did not correlate with HHF because of the relatively small size of the study cohort and limited events. Therefore, we may not have sufficient statistical power to exclude the prognostic association of traditional left ventricular parameters. Secondly, we did not assess the levels of brain natriuretic peptide (BNP), while BNP has its clinical utility in ESRD, it doesn’t offer the comprehensive cardiac insights provided by echocardiography for these patients.
Conclusion
Among asymptomatic hemodialysis patients with preserved LVEF, we demonstrated LV GLS was independently associated with 5-year mortality and HHF. None of the other indices, including left ventricular chamber sizes, LVEF and diastolic function correlated with the clinical outcomes. In addition, CAD and LVESV were major determinants of LV GLS. The study results may encourage the implement of LV GLS as a routine measure in hemodialysis patients to early detect high-risk subjects even with normal LVEF. And the further survey for CAD among those with reduced LV GLS may offer opportunities to improve prognosis when coronary intervention is indicated.
Data availability
The datasets generated and analysed during the current study are not publicly available due to patient privacy concerns and legal restrictions associated with the confidentiality of medical records. However, they are available from the corresponding author on reasonable request.
Abbreviations
- LV GLS:
-
Left ventricular global longitudinal strain
- LVESV:
-
Left ventricular end-systolic volume
References
Foley, R. N., Parfrey, P. S. & Sarnak, M. J. Epidemiology of cardiovascular disease in chronic renal disease. J. Am. Soc. Nephrol.: JASN. 9(12 Suppl), S16–23 (1998).
Wang, A. Y. M. et al. Sudden cardiac death in end-stage renal disease patients: a 5-year prospective analysis. Hypertension 56(2), 210–216 (2010).
Foley, R. N. et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 47(1), 186–192 (1995).
Yamada, S. et al. Prognostic value of reduced left ventricular ejection fraction at start of Hemodialysis therapy on cardiovascular and all-cause mortality in end-stage renal disease patients. Clin. J. Am. Soc. Nephrology: CJASN. 5(10), 1793 (2010).
Stallworthy, E. J. et al. Do echocardiographic parameters predict mortality in patients with end-stage renal disease? Transplantation 95(10), 1225–1232 (2013).
Curtis, J. P. et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J. Am. Coll. Cardiol. 42(4), 736–742 (2003).
Marwick, T. H. Methods used for the assessment of LV systolic function: common currency or tower of babel?? Heart 99(15), 1078–1086 (2013).
Kim, H. K. et al. Assessment of left ventricular rotation and torsion with two-dimensional speckle tracking echocardiography. J. Am. Soc. Echocardiogr. 20(1), 45–53 (2007).
Liu, Y. W. et al. Left ventricular systolic strain in chronic kidney disease and Hemodialysis patients. Am. J. Nephrol. 33(1), 84–90 (2011).
Amundsen, B. H. et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J. Am. Coll. Cardiol. 47(4), 789–793 (2006).
Cho, G. Y. et al. Global 2-dimensional strain as a new prognosticator in patients with heart failure. J. Am. Coll. Cardiol. 54(7), 618–624 (2009).
Stanton, T., Leano, R. & Marwick, T. H. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ. Cardiovasc. Imaging. 2(5), 356–364 (2009).
Lang, R. M. et al. Recommendations for chamber quantification: a report from the American society of echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European association of echocardiography, a branch of the European society of cardiology. J. Am. Soc. Echocardiogr. 18(12), 1440–1463 (2005).
Nagueh, S. F. et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur. J. Echocardiogr. 17(12), 1321–1360 (2016).
Negishi, K. et al. Practical guidance in echocardiographic assessment of global longitudinal strain. JACC: Cardiovasc. Imaging. 8(4), 489–492 (2015).
DeVore, A. D. et al. Impaired left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: insights from the RELAX trial. Eur. J. Heart Fail. 19(7), 893–900 (2017).
Yingchoncharoen, T. et al. Normal ranges of left ventricular strain: a meta-analysis. J. Am. Soc. Echocardiogr. 26(2), 185–191 (2013).
Krishnasamy, R. et al. The association between left ventricular global longitudinal strain, renal impairment and all-cause mortality. Nephrol. Dialysis Transplant. 29(6), 1218–1225 (2014).
Liu, Y. W. et al. Association of left ventricular longitudinal strain with mortality among stable Hemodialysis patients with preserved left ventricular ejection fraction. Clin. J. Am. Soc. Nephrol.: CJASN 8(9), 1564 (2013).
Aoki, J. et al. Clinical and pathologic characteristics of dilated cardiomyopathy in Hemodialysis patients. Kidney Int. 67(1), 333–340 (2005).
Kramann, R. et al. Speckle tracking echocardiography detects uremic cardiomyopathy early and predicts cardiovascular mortality in ESRD. J. Am. Soc. Nephrol. JASN. 25(10), 2351 (2014).
Wu, C. K. et al. High inferior Vena Cava diameter with high left ventricular end systolic diameter as a risk factor for major adverse cardiovascular events, cardiovascular and overall mortality among chronic Hemodialysis patients. J. Clin. Med. 11(18), 5485 (2022).
Burkhoff, D., Mirsky, I. & Suga, H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am. J. Physiol. Heart Circ. Physiol. 289(2), H501–H512 (2005).
Konstam, M. A. Systolic and diastolic dysfunction in heart failure? Time for a new paradigm. J. Card. Fail. 9(1), 1–3 (2003).
Maurer, M. S., Rumbarger, L. E. K. & King, D. L. Ventricular volume and length in hypertensive diastolic heart failure. J. Am. Soc. Echocardiogr. 18(10), 1051–1057 (2005).
Sagawa, K. et al. End-systolic pressure/volume ratio: a new index of ventricular contractility. Am. J. Cardiol. 40(5), 748–753 (1977).
Suga, H., Kitabatake, A. & Sagawa, K. End-systolic pressure determines stroke volume from fixed end-diastolic volume in the isolated canine left ventricle under a constant contractile state. Circul. Res. 44(2), 238–249 (1979).
Eckardt, K. U. et al. Left ventricular geometry predicts cardiovascular outcomes associated with anemia correction in CKD. J. Am. Soc. Nephrology: JASN. 20(12), 2651 (2009).
Wu, I. W. et al. Ventricular function and all-cause mortality in chronic kidney disease patients with angiographic coronary artery disease. JN J. Nephrol. 23(2), 181 (2010).
Chen, R. et al. Left ventricular myocardial function in Hemodialysis and nondialysis uremia patients: a three-dimensional speckle-tracking echocardiography study. PLoS One 9(6), e100265 (2014).
Leibowitz, D. Left ventricular hypertrophy and chronic renal insufficiency in the elderly. Cardiorenal. Med. 4(3–4), 168–175 (2014).
Abraham, T. P. et al. Diastolic dysfunction in Familial hypertrophic cardiomyopathy Transgenic model mice. Cardiovascular. Res. 82(1), 84–92 (2009).
Lombardi, R. et al. Myocardial collagen turnover in hypertrophic cardiomyopathy. Circulation 108(12), 1455–1460 (2003).
Wang, Y. C. et al. Preclinical systolic and diastolic dysfunctions in metabolically healthy and unhealthy obese individuals. Circulation: Heart Fail. 8(5), 897–904 (2015).
Reant, P. et al. Experimental validation of circumferential, longitudinal, and radial 2-dimensional strain during Dobutamine stress echocardiography in ischemic conditions. J. Am. Coll. Cardiol. 51(2), 149–157 (2008).
Weidemann, F. et al. Myocardial function defined by strain rate and strain during alterations in inotropic States and heart rate. Am. J. Physiol. Heart Circ. Physiol. 283(2), H792–H799 (2002).
Marwick, T. H. Measurement of strain and strain rate by echocardiography: ready for prime time? J. Am. Coll. Cardiol. 47(7), 1313–1327 (2006).
Acknowledgements
We express our sincere gratitude to the diligent staffs in the cardiovascular center at Taipei Veterans General Hospital for their invaluable efforts and contributions to this project.
Author information
Authors and Affiliations
Contributions
All the listed authors contributed to the manuscript. Shih-Hsien Sung conceived of the study design. Yi-Tsang Fu and Chih-Hsueh Tseng acquired and analyzed the data. Wei-Ming Huang and Hao-Min Cheng interpreted the results. Wen-Chung Yu and Chih-Ching Lin helped to visualize the results. Yi-Tsang Fu drafted the manuscript. Shih-Hsien Sung critically revised the manuscript. Chern-En Chiang and Chen-Huan Chen supervised the planning and execution of this research. Shih-Hsien Sung is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All the authors have read and approved the submitted manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fu, YT., Tseng, CH., Huang, WM. et al. Prognostic impacts of left ventricular strain in hemodialytic patients with preserved left ventricular systolic function. Sci Rep 15, 24723 (2025). https://doi.org/10.1038/s41598-025-97569-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-97569-0