Abstract
Guava cultivation is significantly affected by various diseases, with wilt disease being one of the most prominent. This disease results from a complex interaction between the phytophagous nematode Meloidogyne enterolobii and the fungus Fusarium sp. To study the menace (on per cent wilt incidence and plant growth parameters) caused by their interaction in aggravating the wilt, the pathogens were inoculated individually and as well as in different combinations to the established air layered seedlings of guava under greenhouse conditions. The experimental study revealed the synergistic interaction among the pathogens, as the study showed in sequential inoculation of nematode 14 days prior to fungus inoculation exacerbated the disease severity and significantly reduced the plant growth parameters. This was followed by combined application of pathogens at the same time which was on par with the results from sequential inoculation of nematode seven days prior to fungus; this signifies the role of phytophagous nematodes in paving the way for susceptibility of crop for secondary infection.
Similar content being viewed by others
Introduction
Guava (Psidium guajava L.) is an economically significant tropical fruit crop, often referred to as the “poor man’s apple.” Native to tropical America, guava cultivation is severely impacted by various biotic and abiotic stresses, with guava wilt emerging as a major constraint to production. The disease is caused by a complex interaction of fungal and nematode pathogens, which synergistically enhance wilt incidence. Several studies have documented the role of Fusarium sp. and Meloidogyne sp. in disease progression1. Notably, a newly identified root-knot nematode species, Meloidogyne enterolobii, has been reported in Tamil Nadu as an emerging threat to guava cultivation and is now spreading across India2.
Guava wilt is widespread across India, causing substantial yield losses. In Uttar Pradesh, losses of 5%–15% have been reported in 12 districts3. In West Bengal, affected orchards have experienced up to 80% yield reduction4. In the Lucknow region, losses ranging from 5 to 60% have been documented5. Additionally, root-knot nematodes (Meloidogyne sp.) alone have been estimated to cause yield losses of approximately 28% in guava6.
Given the economic significance of guava and the substantial losses attributed to pathogen interactions, a deeper understanding of the disease dynamics is essential. The complexity of guava wilt poses challenges for effective management, necessitating detailed investigations into the synergistic and antagonistic relationships among causal agents. Elucidating these interactions will provide critical insights into disease progression and inform the development of targeted management strategies. This study aims to characterize the intricate nature of the guava wilt complex and its impact on plant health, contributing to sustainable guava production.
Materials and methods
Isolation and identification of pathogens associated with guava wilt
Rhizosphere soil and root samples were collected from guava plants exhibiting characteristic wilt symptoms. Fusarium sp. were isolated using standard tissue isolation techniques, followed by single-spore isolation for pure culture7,8. Root-knot nematodes (Meloidogyne sp.) were extracted using Cobb’s sieving and decanting method9 and Baermann’s funnel technique10. Fungal identification was based on microscopic morphology, while nematodes were identified through perineal pattern analysis11. For interaction studies, Fusarium was mass-multiplied using the giant culture method12 on a sorghum–sand bait medium, and nematodes were cultured on coleus plants to ensure adequate inoculum.
Preparation and inoculation of Fusarium culture
Fungal inoculum was prepared using the giant culture method with a 90:10 sorghum- sand meal medium. A total of 400 g of the medium was autoclaved in a 1000 mL conical flask at 1.5 kg/cm2 pressure and 121 °C. After cooling, a 5 mm mycelial disc from a seven-day-old Fusarium culture was inoculated into the flask under aseptic conditions. Flasks were incubated at 25 ± 1 °C for 2–3 weeks, with periodic shaking to ensure uniform fungal growth. Colonized fungal biomass was used as inoculum at a ratio of 1 kg per 5 kg of soil, where guava seedlings were grown.
Multiplication and inoculation of Meloidogyne enterolobii
Root-knot nematodes were extracted from infected guava roots and cultured on coleus plants. Infected plants were maintained until they exhibited typical M. enterolobii symptoms. Second-stage juveniles (J2) were extracted from root fragments using Baermann’s funnel technique. For inoculation, four holes were made around the root zone of guava seedlings in pots, and a suspension (two juveniles/g soil) was evenly distributed. The soil was then covered and watered to maintain optimal moisture.
Fusarium sp. and Meloidogyne enterolobii interaction studies in guava wilt disease complex
Three-month-old air-layered guava seedlings, sourced from the University of Horticultural Sciences, Bagalkot, were used for pot experiments. The study aimed to assess the interaction effects of Fusarium sp. and M. enterolobii on plant growth, wilt incidence, and nematode reproduction.
Experimental design
A Completely Randomized Design (CRD) was followed with eight treatments with three replications each:
-
T1: Fusarium sp. alone
-
T2: M. enterolobii alone
-
T3: Fusarium sp. inoculated one week before M. enterolobii
-
T4: M. enterolobii inoculated one week before Fusarium sp.
-
T5: Fusarium sp. inoculated two weeks before M. enterolobii
-
T6: M. enterolobii inoculated two weeks before Fusarium sp.
-
T7: Simultaneous inoculation of Fusarium sp. and M. enterolobii
-
T8: Untreated control
Sterilized soil and farmyard manure (3:1) were used to fill medium-sized pots. Optimum moisture and nutrient levels were maintained throughout the experiment. After seedling acclimatization, inoculation was performed as per the treatment schedule, with three replications per treatment.
Data collection and analysis
Plant growth parameters
-
Shoot and root length (cm)
-
Fresh and dry weight of shoot (g)
-
Fresh and dry weight of root (g)
Meloidogyne enterolobii multiplication parameters
1. Gall count: Number of root galls per plant was recorded, and averages were calculated.
2. Root rot scoring: Severity was assessed using the CIAT (1987) scale:
Score | Description |
|---|---|
1 | No visible symptoms |
3 | Light discoloration, minor necrotic lesions (~ 10% affected) |
5 | Moderate lesions (~ 25% affected), minor root deterioration |
7 | Severe lesions (~ 50% affected), softening, root reduction |
9 | Extensive necrosis (~ 75% affected), advanced rotting |
3. Nematode population: Counts were recorded from root (per 10 g) and soil (per 200 cc) samples.
4. Female and egg mass count: Number of females and egg masses per gram of root sample.
5. Root-knot index: Assessed using the Taylor and Sasser (1978)13 scale.
Grade | Number of galls per plant |
|---|---|
0 | No galls |
1 | 1–2 galls |
2 | 3–10 galls |
3 | 11–30 galls |
4 | 31–100 galls |
5 | > 100 galls |
Statistical analysis
The information and data obtained in the present experiment for various parameters are subjected to statistical analysis applying ANNOVA for a completely randomized design. The statistical analysis was done by using Web based Agricultural statistics Package (WASP) 2.0 version and HAU-OPSTAT.
Results
Interaction between Fusarium sp. and Meloidogyne enterolobii in guava wilt disease complex
Effect of Fusarium sp. and Meloidogyne enterolobii on wilt incidence in guava seedlings
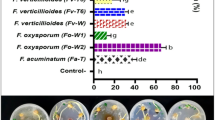
The effect of Fusarium sp. and Meloidogyne enterolobii on wilt incidence in guava seedlings was assessed 150 days post-inoculation (DPI) (Table 1 and Figs. 1, 2). ANOVA revealed significant variation among treatments (p < 0.01), as confirmed by the critical difference at 1% (CD@1% = 1.60) and a low coefficient of variation (CV = 1.63%).
At 90 DPI, the highest wilt incidence (100%) was recorded in T6, where M. enterolobii was inoculated two weeks before Fusarium sp. (Fig. 1). By 150 DPI, a similar trend was observed in T4, where M. enterolobii was inoculated one week before Fusarium sp., also resulting in 100% wilt incidence. Simultaneous inoculation (T7) led to an incidence of 83.33% (p < 0.01). T5 (Fusarium sp. introduced two weeks before M. enterolobii) exhibited 66.66% incidence, while T3 (Fusarium sp. inoculated one week prior) and T1 (Fusarium sp. alone*) showed significantly lower wilt incidences (50%, p < 0.01). These results underscore the influence of inoculation timing on disease progression.
Interactive effects of Fusarium sp. and Meloidogyne enterolobii on plant growth parameters of guava seedlings
The impact of Fusarium sp. and M. enterolobii on guava seedling growth is summarized in Table 2 and Figs. 3 and 4. ANOVA indicated a significant reduction in shoot and root length, as well as fresh and dry biomass across treatments (p < 0.01), confirmed by CD@1% values for shoot length (0.83 cm), root length (0.57 cm), fresh shoot weight (1.21 g), fresh root weight (0.96 g), dry shoot weight (1.04 g), and dry root weight (0.56 g). The CV values ranged from 2.56% to 4.54%.
T6 (M. enterolobii inoculated two weeks before Fusarium sp.) exhibited the most significant decline, with shoot and root lengths of 7.50 cm and 3.77 cm (p < 0.01). T7 (simultaneous inoculation) resulted in 8.83 cm and 6.67 cm, closely matching T4 (8.00 cm and 6.43 cm). T5 (Fusarium sp. inoculated two weeks before M. enterolobii) exhibited moderate suppression (12.67 cm and 8.53 cm), while T3 (Fusarium sp. inoculated one week prior*) showed a lesser reduction (15.47 cm and 8.88 cm). The highest values were recorded in T1 (Fusarium sp. alone*, 19.43 cm and 9.60 cm) and T2 (M. enterolobii alone*, 19.10 cm and 8.77 cm). Control plants recorded the maximum shoot and root lengths (23.07 cm and 13.60 cm, p < 0.01).
Fresh biomass followed a similar pattern, with T6 exhibiting the lowest fresh shoot and root weights (7.03 g and 4.83 g, p < 0.01), followed by T4 (7.67 g and 6.50 g) and T7 (9.17 g and 8.50 g). The highest fresh biomass was observed in control plants (49.50 g and 20.83 g, p < 0.01). Dry biomass data mirrored this trend, with T6 showing the lowest shoot and root dry weights (4.23 g and 3.17 g, p < 0.01), followed by T4 (4.60 g and 4.00 g) and T7 (5.23 g and 4.67 g). Control plants exhibited the highest values (29.67 g and 9.50 g). These findings highlight the suppressive effects of M. enterolobii on plant growth, particularly when nematodes establish themselves before fungal colonization.
Interactive effects of Fusarium sp. and Meloidogyne enterolobii on nematode multiplication and root rot in guava seedlings
The influence of Fusarium sp. and M. enterolobii on nematode reproduction and root rot severity is presented in Table 3 and Fig. 5 and 6. Significant differences among treatments were confirmed via ANOVA (p < 0.01), with CD@1% values for gall number (2.47), female count (1.58), egg masses per gram of root (1.12), nematode population per 10 g root (2.12), and per 200 cc soil (1.50). CV values ranged from 0.64% to 11.27%, confirming data reliability.
T2 (M. enterolobii alone) showed the highest gall formation (270.67 galls per plant) and gall index (5.00, p < 0.01). This was followed by T5 (Fusarium sp. inoculated two weeks prior to M. enterolobii), which resulted in 199.67 galls and a gall index of 5.00. T7 (simultaneous inoculation) exhibited a gall index of 5.00 with 106.67 galls. In contrast, T6 (M. enterolobii inoculated two weeks before Fusarium sp.) significantly curtailed gall formation (21.67 galls, gall index = 2.67, p < 0.01).
Regarding nematode reproduction, T2 recorded the highest values (58.00 females and 43.00 egg masses per gram of root, p < 0.01), followed by T5 (46.00 females, 34.67 egg masses). Fusarium sp. significantly reduced nematode reproduction in T6, with the lowest female count (7.33) and egg mass number (5.33, p < 0.01). Nematode populations in root (per 10 g) and soil (per 200 cc) followed a similar trend, with T2 recording the highest values (499.00 and 299.00) and T6 significantly reducing nematode multiplication (20.00 and 100.00, p < 0.01).
With respect to root rot severity, T6 displayed the highest root rot index (9.00), underscoring the aggravating impact of Fusarium sp. in the presence of nematodes. This was followed by T4 and T7 (both 7.00), while moderate root rot was recorded in T3 and T5 (both 5.00). The lowest root rot severity was observed in T1 (Fusarium sp. alone*, 3.00) and T2 (M. enterolobii alone*, 3.67, p < 0.01). These results confirm the significant (p < 0.01) role of M. enterolobii in enhancing nematode multiplication and Fusarium sp. in exacerbating root rot severity, particularly under sequential inoculation conditions.
Discussion
The present study elucidates the interactive effects of Fusarium sp. and Meloidogyne enterolobii on guava seedlings, demonstrating that co-inoculation of both pathogens significantly exacerbates disease severity and negatively impacts plant growth parameters.
Impact on wilt and growth parameters
After 150 days of inoculation, a significant increase in wilt incidence was observed, with the highest percentage recorded in T4 and T6 (100%), followed by T7 (83.33%). This aligns with previous studies indicating that nematodes act as predisposing agents, facilitating fungal infection and accelerating wilt progression14. The correlation between wilt incidence and plant growth parameters in the current study further supports this relationship, where T6 showed the most pronounced reduction in shoot and root length, fresh biomass, and dry biomass.
The mechanism underlying this interaction can be attributed to the physiological and biochemical changes induced by nematode infestation. M. enterolobii disrupts root integrity, creating entry points for fungal invasion and altering root exudates, which enhance fungal colonization1,15. These findings are in agreement with Portar and Powel (1967)16 and Powell (1971)17, who reported that wilt severity increased when nematodes were introduced before fungal pathogens, as the root damage caused by nematodes facilitated fungal establishment and proliferation.
Root rot severity and disease synergism
The present findings also reveal that root rot severity was significantly higher in T6 (9.00, p < 0.01) and T4 (7.00), confirming the synergistic interaction between M. enterolobii and Fusarium sp. In contrast, root rot was moderate in reciprocal treatments (T3 and T5), which recorded a severity index of 5.00, comparable to T1 (4.33). This suggests that nematode-induced stress enhances host susceptibility to fungal colonization, leading to greater disease expression than individual infections14.
Studies have shown that nematode infestation triggers physiological alterations in host plants, such as disrupted nutrient uptake and hormonal imbalances, which enhance fungal pathogenicity15. The wounds created by nematodes not only provide direct entry points but also release plant exudates that promote fungal germination and infection 1. These results are also supported by Bergeson (1972)18, who documented that the combined action of nematodes and fungi significantly impacts plant health across various crops.
Nematode reproduction and gall formation
The interaction between M. enterolobii and Fusarium sp. significantly influenced nematode reproduction. Plants inoculated with the fungus first (T5 and T3) exhibited significantly higher gall numbers (199.67 and 98.00, respectively) compared to other treatments. This could be due to enhanced juvenile penetration into the root system, as reported by Tu and Cheng (1971)19. However, in treatments where nematodes were introduced first (T6 and T4), gall formation was significantly reduced (21.67 and 72.00), likely due to severe root rot impairing root suitability for nematode penetration.
Furthermore, fungal involvement significantly suppressed nematode reproduction, as observed in T6, which recorded the lowest number of females (7.33, p < 0.01) and egg masses (5.33) per gram of root. Similarly, root and soil nematode populations were markedly reduced (20.00 and 100.00, respectively). These results align with findings by Meena et al. (2015)20 in gerbera and Ahmed and Shahab (2018)21 in lentil, where fungal metabolites suppressed nematode reproduction, potentially through toxic secondary metabolites and structural modifications in the root system. El-Shawadfy et al. (1988)22 also reported that fungal colonization adversely affects nematode development by altering root tissue composition, reducing the formation of feeding sites required for nematode sustenance.
Metabolites secreted by Fusarium sp. may directly inhibit nematode juvenile development, while severe root decay caused by fungal infection can deprive nematodes of essential nutrients, thereby reducing their reproductive potential23,24. Additionally, Meloidogyne parasitized cells are more susceptible to fungal invasion than normal cells, further contributing to nematode suppression25.
Comparative analysis with other crops
The nematode-fungus interaction observed in guava has been extensively studied in other crops, further validating our findings. Studies have demonstrated similar synergistic effects in cotton26, muskmelon27, tomato28, Vigna unguiculata29, betelvine30, banana31 and guava32. These studies consistently show that nematodes predispose plants to fungal infections by weakening root defense mechanisms and facilitating pathogen ingress.
Conclusion
The present study demonstrates the synergistic interaction between Meloidogyne enterolobii and Fusarium sp. in guava seedlings, leading to increased wilt incidence, severe root rot, and reduced plant growth. Disease severity was highest when nematodes were inoculated before fungi, highlighting the role of M. enterolobii in predisposing plants to fungal invasion. The findings align with previous studies, confirming that nematode-induced physiological and structural changes facilitate fungal colonization, thereby intensifying disease progression. Conversely, Fusarium sp. significantly suppressed nematode reproduction, possibly due to fungal metabolites affecting nematode development. These results emphasize the need for integrated management strategies to mitigate the combined impact of these pathogens and protect guava crops from wilt disease complexes.
Future directions
-
1.
Influence of agro-meteorological conditions on severity of wilt complex of guava has to be investigated.
-
2.
Need to decipher the molecular and bio chemical basis of host–pathogen interactions during guava wilt disease complex.
-
3.
Need to evaluate different guava root stocks for the resistance against wilt disease complex.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Gomes, V. M., Souza, R. M. D., Almeida, A. M. & Dolinski, C. Relationships between Meloidogyne enterolobii and Fusarium solani: Spatial and temporal dynamics in the occurrence of guava decline. Nematoda 2, 9–14 (2014).
Poornima, K., Suresh, P., Kalaiarasan, P., Subramanian, S. & Ramaraju, K. Root knot nematode, Meloidogyne enterolobii in Guava (Psidium guajava L.) a new record from India. Madras Agric. J. 103(10–12), 359–365 (2016).
Sing, B. & Lal, S. B. Wilt of guava. Agric. Anim. Husb. 3, 78–79 (1953).
Chattopadhyay, S. B. & Sengupta, S. K. Studies on wilt of Psidium guajava L. in West Bengal. Indian J. Hortic. 12, 76–79 (1955).
Misra, A. K. & Shukla, S. K. Assessment of loss due to guava wilt around Lucknow. Nat. Seminar on Production and Post-harvest Technology of Guava 9–10 (Dept. Hort. CSAUA&T, 2002).
Kumar, V., Khan, M. R. & Walia, R. K. Crop loss estimations due to plant parasitic nematodes in major crops in India. Natl. Acad. Sci. Lett. 43(5), 409–412 (2020).
Booth, C. The Genus Fusarium, 237 (Kew Surrey: Commonwealth Mycological Institute, 1971).
Booth, C. Fusarium Laboratory Guide for Identification of Major Species, 58 (Kew Survey: Commonwealth Mycological Institute, 1977).
Cobb, N. Estimating the Nematode Population of the Soil (Agric. Tech. Circ. U.S. Dept. Agriculture 48, 1918).
Baermann, G. Eine einfache Methode zur Auffindung von Ankylostomum- (Nematoden) Larven in Erdproben. Welteureden 41–47 (Geneesk. Lab. Feestbundel, 1917).
Chitwood, B. G. Root knot nematodes I. A revision of genus Meloidogyne goeldi 1887. Proc. Helminthol. Soc. Wash 16, 90–104 (1949).
Misra, A. K. & Pandey, B. K. Advances in horticulture biotechnology diagnostics for horticulture crops: Guava (Psidium guajava L.) (eds Singh, H. P., Anandraj M. & Bhat A. I.), 108–119 (2013).
Taylor, A. L. and Sasser, J. N. Biology, Identification and Control of Root Knot Nematodes (Melodogyne sp.) (North Carolina State University Graphics, 1978).
Al Hazmi, A. S. & Nadary, S. N. Interaction between Meloidogyne incognita and Rhizoctonia solani on green beans (Phaseolus vulgaris). Saudi J. Biol. Sci. 25, 570–574 (2015).
Khan, M. R. & Sharma, R. K. Fusarium-nematode wilt disease complexes, etiology and mechanism of development. Indian Phytopathol. 73, 615–628 (2020).
Porter, D. M. & Powell, N. T. Influence of certain Meloidogyne species on Fusarium wilt development in flue-cured tobacco. Phytopathology 57, 282–285 (1967).
Powell, N. T. Interactions between nematodes and fungi in disease complexes. Annu. Rev. Phytopathol. 9, 253–274 (1971).
Bergeson, G. B. Concepts of nematode fungus associations in plant disease complexes: A review. Exp. Parasitol. 32, 301–314 (1972).
Tu, C. C. & Cheng, Y. H. Interaction of Meloidogyne incognita and Macrophomina phaseolina in kenafroot rot. J. Nematol. 3, 39–42 (1971).
Meen, K. S., Ramyabharathi, S. A., Raguchander, T. & Jonathan, E. I. Meloidogyne incognita and Fusarium oxysporum interaction in Gerbera. Afr. J. Microbiol. Res. 9(18), 1281–1285 (2015).
Ahmed, D. & Shahab, S. Studies on interaction of Meloidogyne incognita (Kofoid and White) Chitwood and Fusarium solani (Mart.) Sacc forming a disease complex in lentil (Lens culinaris Medik.). Arch. Phytopathol. 51(7–8), 338–348 (2018).
El-Shawadfy, M. M., Nigel, G. & Hague, M. Influence of Fusarium oxysporum f. sp. glycines on the invasion and development of Meloidogyne incognita on soybean. Revue de Nematol. 4, 437–439 (1988).
Fattah, F. A. & Webster, J. M. Ultrastructural modifications of Meloidogyne javanica induced giant cells caused by fungal culture filterates. Rev. Nematol. 12, 197–210 (1989).
Mockbel, A. A. H., Ibrahim, I. K. A., Shehata, M. R. & El-Saedy, M. Interaction between certain root-rot fungal the root-knot nematode, Meloidogyne incognita on sunflower plants. Egypt. J. Phytopathol. 35, 1–11 (2007).
Melendez, P. L. & Powell, N. T. Histological aspects of the Fusarium wilt-root knot complex in flue-cured tobacco. Phytopathology 57, 286–292 (1967).
Atkinson, G. F. Some diseases of cotton. Alabama Agricultural Experiment Station Bulletin No. 41, 1–65 (1892).
Bergeson, G. B. The effect of Meloidogyne incognita on the resistance of four muskmelon varieties to Fusarium wilt. Plant Dis. Rep. 59, 410–413 (1975).
Sidhu, G. & Webster, J. M. Predisposition of tomato to the wilt fungus (Fusarium oxysporum lycopersici) by the root-knot nematode (Meloidogyne incognita). Nematologica 23, 436–442 (1977).
Harris, A. R. & Ferris, H. Interactions between Fusarium oxysporum f. sp. tracheiphilum and Meloidogyne sp. in Vigna unguiculata. Pathogenesis by F. oysporumtracheiphilium as affected by M. javanica and host cultivar. Plant Pathol. 40, 465–475 (1991).
Jonathan, E. I., Sivakumar, M. & Padmanabhan, D. Interaction of Meloidogyne incognita and Phytophthora palmivora on betelvine. Nematol. Medit. 24, 341–343 (1996).
Jonathan, E. I. & Gajendran, G. Interaction of Meloidogyne incognita and Fusarium oxysporum f. sp. cubense on banana. Nematol. Medit. 26, 9–11 (1998).
Sing, N. Emerging problem of guava decline caused by Meloidogyne enterolobii and Fusarium oxysporum f. sp. psidii. Indian Phytopatol. 27 (2020).
Author information
Authors and Affiliations
Contributions
S. (Shravani) conducted the research, performed the experiments, collected and analyzed the data, and prepared the manuscript. K.C.K.K. (K C. Kiran Kumar), R.K.M. (Raghavendra K. Mesta), M.P.B. (M P. Basavarajappa), S.M. (Shankar Meti), and M.A.W. (M A. Waseem) provided guidance, supervised the study, and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shravani, Kiran Kumar, K.C., Mesta, R.K. et al. Unleashing the synergistic interaction between Meloidogyne enterolobii and Fusarium sp. in guava wilt complex disease. Sci Rep 15, 34131 (2025). https://doi.org/10.1038/s41598-025-97767-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97767-w