Abstract
To evaluate the feasibility and safety of using colorectal mucosal grafts (CMG) harvested via endoscopic submucosal dissection (ESD) for ureteral reconstruction in patients with ureteral stricture. Eight patients with ureteral stricture underwent robotic ureteral reconstruction using CMG harvested by ESD. Preoperative assessments included clinical history, physical examination, and various imaging studies. The ESD procedure was performed with standard precautions to obtain sufficient graft material. Postoperative follow-up involved endoscopic examinations and retrograde pyelograms to assess colorectal complication and ureteral patency. The average age of patients was 44 years, and the median BMI was 24.6 kg/m². The causes of stricture included ureteral stones, urinary tract infections, and ureteral polyps. The median stricture length was 3.5 cm. The average size of the harvested CMG was 4 × 2.4 cm. The success rate of the grafting procedure was 100%, with no gastrointestinal complications observed. Endoscopic examination one week postoperatively revealed well-healed wounds. No recurrent ureteral strictures were noted during a median follow-up period of 5 months. The average glomerular filtration rate (GFR) of the affected kidneys was 61 ml/min. Harvesting CMG via ESD for ureteral reconstruction is feasible and safe, with minimal complications and promising short-term outcomes. This technique provides a viable alternative for patients contraindicated for oral mucosal grafts, potentially reducing morbidity associated with traditional intestinal mucosa harvesting methods.
Similar content being viewed by others
Introduction
Complex ureteral stricture presents a significant challenge in urological surgery. Mismanagement or unrecognized ureteral stricture can lead to severe complications, including functional obstruction and renal failure1. Traditional treatment methods primarily involve ileal ureter substitution and kidney transplantation2,3. However, with recent advancements in endoscopic and laparoscopic techniques, minimally invasive ureteroplasty has become increasingly refined. Currently, widely accepted ureteral grafts are derived from oral mucosa, including lingual and buccal mucosa4,5. When oral mucosal grafts (OMG) are contraindicated—such as in cases of oral lichen planus, leukoplakia, oral cancer, prior radiation exposure to the OMG sites, or previous OMG harvesting—alternative graft materials are required6.
Colorectal mucosa has emerged as a promising ureteral graft material. In canine models, transplanted colonic mucosa survived for up to 12 weeks post-transplantation and was found to be nearly completely covered by transitional epithelium through metaplasia7. In clinical trials involving human subjects, patients who received colorectal mucosa grafts (CMG) exhibited a mucosal survival rate exceeding 86% over follow-up periods ranging from 1 to 5 years, with no colorectal-related complications reported8. Endoscopic submucosal dissection (ESD) is an established technique for resecting intraluminal lesions9. Harvesting intestinal mucosa via ESD offers potential advantages, such as being painless, avoiding oral sequelae, and providing abundant graft material. Therefore, we have pioneered the use of ESD to obtain colonic mucosa in combination with robotic ureteral reconstruction to treat ureteral stricture.
In this study, we applied this novel technique to 8 patients with ureteral stricture. We evaluated patient data from the preoperative, intraoperative, and postoperative follow-up periods to identify key considerations for harvesting intestinal mucosal grafts using ESD and to assess the feasibility and safety of this minimally invasive treatment approach.
Materials and methods
From 2023 to 2024, we prospectively enrolled 8 patients (mean age 43 years, range 35–52 years) for CMG urethroplasty. Preoperative assessments included clinical history, physical examination, urine culture, urinary system ultrasound, antegrade or retrograde urethrography, and colonoscopy. Inclusion criteria were: (1) diagnosis of ureteral stricture confirmed by retrograde or antegrade urography; (2) in the presence of contraindications for OMG, the patient agreed to undergo CMG ureteral reconstruction; (3) absence of severe cardiovascular, pulmonary, or systemic diseases, with physical fitness to endure surgery; (4) renal scintigraphy indicating the salvageable function of the affected kidney; (5) ability to comply with postoperative follow-up and reviews; (6) absence of coagulation disorders. Exclusion criteria were: (1) OMG ureteral reconstruction is feasible; (2) refusal to undergo surgery; (3) presence of severe cardiovascular, pulmonary, or systemic diseases rendering surgery intolerable; (4) severe renal insufficiency; (5) inability to comply with postoperative follow-up and reviews; (6) presence of coagulation disorders. The ureteral reconstruction surgeries were performed by a reconstructive surgeon with over 10 years of experience, and CMG harvesting was carried out by an endoscopist with more than 10 years of experience in endoscopic submucosal dissection ESD.
Colonic mucosa graft harvest and prepare
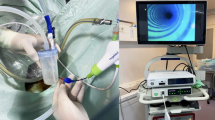
The patients underwent standardized bowel preparation the night before surgery and received perioperative prophylactic antibiotics, including cefazolin, ciprofloxacin, and metronidazole. On the day of surgery, after induction of general anesthesia, patients were positioned in the right or left lateral decubitus position to facilitate the subsequent ureteral reconstruction (Fig. 1). A colonoscopy was performed to exclude any inflammatory bowel disease or incidental findings, such as polyps, that would contraindicate the use of colonic mucosa as a graft. To preserve normal anal function, the mucosal harvesting site was located at least 7 cm above the dentate line. Due to the rich vascular network in the rectal mucosa, its use as a graft was avoided. As a result, colonic mucosa was preferentially selected. Colonic mucosa harvesting was typically performed at the beginning of the surgical procedure.
After measuring the length of the ureteral stricture laparoscopically with robotic assistance, ESD was initiated to harvest the colonic mucosa. This procedure utilized an electronic fiber-optic colonoscope and imaging system (Olympus Co, Ltd, Japan) (Fig. 1). A custom-made ruler, tailored to the required graft size, was placed on the target colorectal mucosal surface (Fig. 2A). An endoscopic electrocautery knife was used to mark the colonic mucosa, with the marking points positioned approximately 5 mm outside the edges of the ruler. Methylene blue-infused saline was injected into the submucosal layer at the marked sites. Mucosal dissection was initiated at the distal submucosa using a bipolar needle knife. The submucosal layer was thoroughly exposed using the traction method (Figs. 2B-C). Throughout the procedure, we avoided prolonged heat application and physical damage to the graft mucosa. The dissected graft mucosa was then extracted transanally. CMG was prepared for ureteral reconstruction. After ESD, hemostasis was achieved using an electrocautery knife and hemostatic clips to address bleeding points and exposed small vessels. The wound was irrigated with saline and left open for secondary healing.
Harvesting and preparing CMG. A Cut sterile medical pads to create custom-made rulers. B Place the custom-made ruler on the appropriate colonic mucosa, then use an endoscopic electrocautery knife to mark around it. C The submucosal layer was thoroughly exposed using the traction method. D The graft was removed transanally and was trimmed.
CMG was placed on clean gauze and rinsed with a hypertonic citrate-purine solution. The mucosa was then trimmed using sterile scissors to remove any damaged tissue resulting from the endoscopic dissection (Fig. 2D). Finally, the graft was rinsed with a polymyxin solution.
Ureteral reconstruction
Laparoscopic ureteral reconstruction was performed using the Da Vinci robotic system. During the procedure, the dilated ureter was identified and carefully dissected from the surrounding tissue. Ureteral perfusion was assessed using intravenous indocyanine green (ICG) dye. The location of the ureteral stricture was determined based on the fluorescence imaging of the dilated ureter. Based on the anatomical blood supply of the ureter, which varies along its length, a longitudinal incision was made along the ventral aspect of the ureter at the stricture site. This approach was chosen to minimize disruption to the posterior vascular network, which is primarily responsible for the ureter’s blood supply, thus ensuring sufficient perfusion and promoting optimal healing of the anastomosis. The incision extended 5 mm into the healthy ureter on both sides of the stricture. The ureteral patency was checked using a 10-Fr urinary catheter. The length of the proximal ureteral stricture was then measured by a guidewire with length markings to guide the size of the colonic mucosal graft to be dissected using ESD (Fig. 3A).
Robot-assisted CMG ureter reconstruction. A The edges of the spatulated ureter and CMG were anastomosed in a running fashion using 5 − 0 Monocry1 sutures. B To provide sufficient blood supply, the reconstructed ureteral segment was wrapped with the omental flap. C Preoperative retrograde ureterography. D Postoperative retrograde ureterography.
The trimmed CMG was introduced into the abdominal cavity through an auxiliary port and positioned on the ventral aspect of the stricture for robot-assisted ureteroplasty. Before suturing, two 4.7-Fr double-J stents were inserted into the ureter using a guidewire. The edges of the incised ureter and the CMG were then continuously sutured with 5 − 0 Monocryl sutures. It is important to note that the CMG was sutured with the mucosal side facing the ureteral lumen and the wound surface facing outward (Fig. 3A). To ensure adequate blood supply, the reconstructed ureteral segment was wrapped with an omental flap (Fig. 3B). Finally, a drainage tube was placed near the anastomosis site, and a Foley catheter was inserted into the urethral meatus. All incisions were closed in layers.
Postoperative management and Follow-Up
After surgery, patients were instructed to remain fasting for 2 days and were typically supported with stool softeners or fiber supplements to facilitate bowel movements. Prophylactic antibiotics were administered for 3 days. An endoscopic examination was scheduled 1 week postoperatively to assess the healing of the ESD site. The Foley catheter was removed on postoperative day 5, and the drainage tube was removed on postoperative day 7. The ureteral stents were extracted at 8 weeks postoperatively. Additionally, a retrograde pyelogram was performed between 9 and 10 weeks. Patients were monitored for subjective urinary symptoms, glomerular filtration rate (GFR), CT Urography, urine flow rate, and postvoid residual urine at 3 and 6 months, and then annually thereafter.
Results
In this study, the average age of patients at the time of surgery was 46 years, and the average body mass index (BMI) was 23.4 kg/m². Among the 8 cases, 6 were caused by ureteral stones, 1 was caused by a urinary tract infection, and 1 was due to a ureteral polyp. The average length of the stricture was 3.7 cm (Table 1). For a patient with bilateral ureteral stenosis, the ureteral reconstruction was performed in two stages. The reconstruction was first completed on the side with more severe stenosis. The patient was then monitored for two months to ensure there were no unforeseen postoperative complications that could worsen renal damage. After confirming the absence of complications, the reconstruction of the contralateral ureter was performed. The average preoperative GFR of the affected kidneys was 23.2 ml/min. The average size of the CMG was 4.3 × 2.2 cm. One patient had previously undergone OMG urethral reconstruction, which later resulted in a secondary stricture. The success rate of the grafting procedure was 100% (Table 2).
During follow-up after the ESD procedure, one patient experienced hematochezia on the third postoperative day. Upon examination, hemorrhoids were identified as the cause, and this was not considered an operation-related complication. Endoscopic examination 1 week postoperatively revealed that all wounds had healed without signs of bleeding, necrosis, or intestinal obstruction. Bowel function returned to normal in all patients, who were able to resume a regular diet. The average postoperative hospital stay was 7.7 days. During follow-up of the ureteral reconstruction, no patients experienced recurrent ureteral stricture. The average postoperative GFR of the affected kidneys was 40.6 ml/min. Retrograde pyelograms showed no evidence of stricture in the reconstructed ureters (Fig. 3C-D). The average follow-up period was 5 months, with no patients lost to follow-up (Table 2).
Discussion
To our knowledge, this study is the first worldwide to utilize the ESD technique for harvesting CMG for ureteral transplantation and reconstruction in patients with ureteral stricture. The results suggest that obtaining colorectal mucosa using this technique is relatively straightforward and feasible. As a minimally invasive procedure, it also exhibits a low incidence of intraoperative and postoperative complications, ensuring good safety.
Although OMG are considered the preferred graft material, with success rates ranging from 90 to 97.6%4,5,6, alternative graft materials are limited when OMG is contraindicated. Previous studies have highlighted several advantages of using intestinal mucosal grafts for ureteral reconstruction. In 2003, Xu et al. were the first to explore the use of circumferential colonic mucosa for repairing recurrent urethral strictures, and histological examination revealed that the transplanted intestinal mucosa could ultimately be replaced by transitional epithelium7. However, subsequent research has shown that intestinal transplantation after bowel resection is associated with a higher incidence of gastrointestinal complications8. As a result, Palmer et al. attempted to use rectal mucosal grafts and transanal endoscopic microsurgery (TEM) for urethral substitution, demonstrating promising early outcomes10. This marked the first attempt to apply minimally invasive intestinal techniques for obtaining mucosal grafts. However, rigid endoscopes, such as the proctoscope used in TEM, have limited operational scope and provide fewer options for intestinal mucosal grafts. In this study, we report the first case of using CMG obtained through the ESD technique for ureteral reconstruction. Since its introduction in 198811, ESD has been widely used for superficial tumor lesions. Experienced surgeons can obtain longer and wider grafts, effectively meeting the needs of ureteral reconstruction. Therefore, we believe that OMG remains the gold standard for long-segment ureteral reconstruction, while ESD provides a promising alternative for obtaining CMG.
CMG harvested using the ESD technique offers several potential advantages, including being painless, avoiding oral sequelae, and providing an ample supply of graft material. During and after the procedure, no gastrointestinal complications related to intestinal transplantation were observed. In the short-term follow-up, no significant cases of recurrent ureteral stricture were noted. Additionally, GFR of the affected kidneys significantly increased compared to preoperative levels. Therefore, this technique is both feasible and safe.
ESD is a relatively complex endoscopic technique, and there were no prior reports regarding its applicability to ureteral reconstruction or the precautions needed during the procedure. In this study, we identified several considerations that may serve as a basis for future technological developments: (1) Since ureteral reconstruction determines the required surgical position, the patient was first anesthetized accordingly before ESD. Specifically, for left ureteral stenosis, the right lateral position was chosen to facilitate both the reconstruction and the preceding ESD procedure. Conversely, for right ureteral stenosis, the left lateral position was used. In cases of bilateral ureteral stenosis, we habitually prioritized treating the left-sided stenosis first, and thus, the right lateral position was initially adopted. (2) Due to the extensive network of capillaries in the rectum, it is not recommended as a site for obtaining intestinal mucosa grafts. (3) Similar to any graft, the success of colonic mucosal transplantation depends on the formation of new blood vessels; therefore, it is crucial to avoid intestinal mucosa with scar tissue. (4) To prevent potential intestinal lesions, it is necessary to avoid mucosa with polyps, ulcers, or areas previously treated endoscopically. (5) ESD can cause irreversible thermal damage to the surrounding mucosa grafts; thus, trimming the mucosa before transplantation is essential to prevent the formation of scar adhesions in the abdominal cavity. Additionally, endoscopists need to excise a larger area of mucosa than what is required for the graft. We recommend performing mucosal dissection 0.5 cm outside the measured perimeter of the mucosa graft.
Although the results of this study largely align with our expectations, there are several limitations to consider. First, as this is a preliminary study, the sample size is relatively small, and future research should involve a larger cohort of participants. Second, this study was conducted at a single center, and validation through multicenter trials is needed. Third, medical centers equipped to perform both ESD and robotic ureteral reconstruction with the Da Vinci system are not widely available. To simplify the procedure, we plan to explore the use of traditional laparoscopic techniques in conjunction with ESD for ureteral reconstruction in future studies.
In conclusion, this preliminary study demonstrates that using ESD to obtain CMG for urethral reconstruction is feasible and safe. It effectively addresses ureteral stenosis caused by the underlying disease and rapidly improves renal filtration rate. ESD technology minimizes the morbidity associated with traditional methods of intestinal mucosa harvesting and enables the acquisition of sufficient intestinal mucosa grafts for all patients with ureteral strictures. This approach addresses the limitations previously encountered with OMG and rectal mucosal graft procurement.
Data availability
All data generated or analysed during this study are included in this published article.
Change history
07 January 2026
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41598-025-34580-5
References
Darwish, A. E. et al. Ureteral stricture after ureteroscopy for stones: A prospective study for the incidence and risk factors. Urol. Ann. 11 (3), 276–281 (2019 Jul-Sep). UA.UA_110_18. PMID: 31413506; PMCID: PMC6676818.
You, Y. et al. Oral mucosal graft ureteroplasty versus ileal ureteric replacement: a meta-analysis. BJU Int. 132 (2), 122–131. https://doi.org/10.1111/bju.15994 (2023). Epub 2023 Mar 14. PMID: 36815226.
Knight, R. B., Hudak, S. J. & Morey, A. F. Strategies for open reconstruction of upper ureteral strictures. Urol. Clin. North. Am. 40 (3), 351–361. https://doi.org/10.1016/j.ucl.2013.04.005 (2013). Epub 2013 Jun 18. PMID: 23905933.
Liang, C. et al. Lingual mucosal graft ureteroplasty for long proximal ureteral stricture: 6 years of experience with 41 cases. Eur. Urol. 82 (2), 193–200 (2022). Epub 2022 May 23. PMID: 35618522.
Lee, Z. et al. Robotic ureteroplasty with buccal mucosa graft for the management of complex ureteral strictures. J. Urol. 198 (6), 1430–1435. https://doi.org/10.1016/j.juro.2017.06.097 (2017). Epub 2017 Jul 20. PMID: 28736319.
Li, B. et al. Laparoscopic onlay lingual mucosal graft ureteroplasty for proximal ureteral stricture: initial experience and 9-month follow-up. Int. Urol. Nephrol. 48 (8), 1275–1279 (2016). Epub 2016 Apr 26. PMID: 27115158.
Xu, Y. M. et al. One-stage urethral reconstruction using colonic mucosa graft: an experimental and clinical study. World J. Gastroenterol. 9 (2), 381–384. https://doi.org/10.3748/wjg.v9.i2.381 (2003). PMID: 12532472; PMCID: PMC4611352.
Xu, Y. M. et al. Urethral reconstruction using colonic mucosa graft for complex strictures. J. Urol. 182 (3), 1040–1043. https://doi.org/10.1016/j.juro.2009.05.030 (2009). Epub 2009 Jul 18. PMID: 19616803.
Ma, M. X. & Bourke, M. J. Endoscopic submucosal dissection in the West: current status and future directions. Dig. Endosc. 30 (3), 310–320. https://doi.org/10.1111/den.12960 (2018). Epub 2017 Oct 16. PMID: 28884493.
Palmer, D. A., Marcello, P. W., Zinman, L. N. & Vanni, A. J. Urethral reconstruction with rectal mucosa graft onlay: A novel, minimally invasive technique. J. Urol. 196 (3), 782–786. https://doi.org/10.1016/j.juro.2016.03.002 (2016). Epub 2016 Mar 9. PMID: 26968645.
Hirao, M. et al. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. (1988). May-Jun;34(3):264-9 https://doi.org/10.1016/s0016-5107(88)71327-9. PMID: 3391382.
Acknowledgements
Declared none.
Funding
Supported by the Guidance Project of Scientific Research Plan of the Health Commission of Hubei Province (Grant No. WJ2023 F012), the Science and Technology Innovation Cultivation Fund of Zhongnan Hospital of Wuhan University (CXPY2024002), Major Projects of Technological Innovation in Hubei Province (2017 ACA169), Key Project of Discipline and Platform Construction of Zhongnan Hospital of Wuhan University (Grant No. PTXM2023012), National Key Clinical Specialty Construction Project (Grant No. GJLCZD2023002), Zhongnan Hospital of Wuhan University Science and Technology Achievement Transformation Fund (202225 KJCGZH), the National Natural Science Foundation of China (Grant No. 82403279; 82303181), and Hubei Province health and family planning scientific research project (Grant No. WJ2023M065).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Haihang Nie collected all patients’ informations and wrote this original draft. Fan Wang and Yali Yu reviewed and edited this original draft. Jingkai Zhou and Xiubing Chen were responsible for preoperative and postoperative preparations. YunTian Hong and Xianglin Li were responsible for the postoperative follow-up of the patients. Haizhou Wang was responsible for surgical quality control. Chaoqi Liang and Bing Li were responsible for the ureteral reconstruction procedure. The ESD procedures were performed by Jiayan Nie and Hongling Wang. Qiu Zhao was responsible for funding acquisition, supervision of the study. All authors read and approved the final manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Statement of ethics
The study was approved by the Research Ethics Committee of Zhongnan Hospital of Wuhan University, and written informed consent was obtained from all patients (grant no.2024049). All the methods were carried out in accordance with the relevant guidelines under the ethical approval and consent to participate section. All informed consent from all subjects for publication of identifying information/images.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41598-025-34580-5"
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nie, H., Yu, Y., Chen, X. et al. RETRACTED ARTICLE: Minimally invasive robotic ureteral reconstruction using endoscopic submucosal dissection harvested colorectal mucosa graft for ureteral stricture. Sci Rep 15, 12389 (2025). https://doi.org/10.1038/s41598-025-97826-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-97826-2