Abstract
Electrical-conductivity anomalies in subduction zones are believed to be strongly connected with global water cycling, volcanism and seismicity. However, the causal atomic-scale processes related to conductivity of rock-forming minerals in subducting rocks are virtually unknown. Here, in situ simultaneous high-temperature Raman spectroscopy and resistivity measurements on riebeckite as a model Fe-rich amphibole in subduction zones show that (1) electronic small polarons, with high mobility along the c-axis of the amphibole structure, activate above 500 K; (2) H+ starts diffusing within the crystal above 650 K, although electron transport via polaron hopping is still the dominant mechanism of charge transfer; (3) the anisotropy in the conductivity is enhanced with increasing temperature, emphasizing the dominant role of e− over H+ in causing the high conductivity (above 0.01 S/m) of Fe-rich amphiboles. We show that conductivity data obtained via magnetotelluric measurements are best modelled by considering the effect of stress-driven alignment of amphiboles during plate motion. Our results thus link atomic- and Earth-scale conductivity processes, significantly improving our understanding of subduction processes.
Similar content being viewed by others
Introduction
Anomalously high electrical conductivity (σ > 0.01 S/m) in the lithosphere-asthenosphere system is well known yet poorly understood. Values up to 0.1 and 1 S/m have been measured in asthenosphere and back-arc/fore-arc regions, respectively1,2,3,4. In some localities (e.g., East Pacific Rise), the electrical conductivity of the asthenosphere increases along the direction of plate motion4,5. There are two main models concerning the anisotropic conductivity observed in the Earth’s interior: (1) stress-driven alignment of interconnected ion-rich fluids3,4,5,6,7,8, and (2) intrinsic activation of charge carriers with anisotropic mobility in preferentially oriented and mutually aligned rock-forming minerals. Examples of the latter process are provided by H+ diffusion in olivine9 and electron hopping in talc and serpentinite10.
Amphiboles have gained significant attention in studies of rock conductivity because they are among the primary hydrous phases in the lithosphere, making up as much as 30–40% of continental mid-to-lower crustal rocks and metamorphosed oceanic crust11. Furthermore, amphiboles are stable across a broad range of pressures-temperatures12,13,14, and are thus major constituents in rocks spanning a large spectrum of geological conditions from the lower crust to the upper mantle. The composition of these ribbon silicates is extremely variable; their general formula15 is written as AB2C5T8O22W2, where A = Na, K, ☐ (vacancy); B = Na, Ca, Li, Mg, Fe2+, Mn2+; C = Mg, Fe2+, Fe3+, Mn2+, Al, Ti, Cr, V; T = Si, Al, Ti; and W = OH, F, Cl, O2−. Their structure is strongly anisotropic, consisting of ribbons of edge-sharing MO6 octahedra sandwiched between electrically insulating ribbons of SiO4 tetrahedra to form I-beams (Fig. 1a): natural nanorods aligned parallel to the crystallographic c axis (Fig. 1b). This atomic arrangement results in very anisotropic physical properties which strongly affect the behaviour of amphibole-bearing rocks. For example, elastic P-waves propagate faster along the c axis than normal to the c axis (the direction of amphibole-crystal elongation); thus stress-driven crystal preferred orientation (CPO) of amphiboles is assumed to be the main source of the seismic anisotropy observed worldwide in the deep crust, subducting slab and mantle-wedge regions16,17,18,19,20,21.
The extremely anisotropic structure of riebeckite. (a) View along the c axis of the amphibole structure, which consists of I-beams held together by cations at the M(4) site. The I-beam consists of conductive ribbons of edge-sharing FeO6 octahedra sandwiched between two insulating silicate ribbons, forming a natural nanorod ( ∼1 × 2 nm2 cross section) along c. (b) A sketch of electronic-polaron hopping (black arrows) along ribbons of FeO6 octahedra, resulting in strongly anisotropic conductivity. Atomic structure plotted with Vesta 3.5.8 (https://jp-minerals.org/vesta/en/)65.
Experiments on Fe-bearing amphiboles and amphibole-bearing rocks show high electrical conductivity at high temperature22,23,24,25,26. Moreover, the conductivity along the I-beams (i.e., along the c axis, see Fig. 1b), σ||, is about five to six times larger than the conductivity normal to c (σ⊥)24,27, in agreement with the findings of Zhou et al.28 which show that at 1.0 GPa, amphibole-containing rocks have strong anisotropy of both wave (Vp and Vs) velocity and electrical conductivity, the latter increasing with rising temperature (from 11.1% at 523 K to 25.2% at 973 K). The stress-driven CPO of Fe-amphiboles is thus expected to contribute to both electrical and seismic anisotropy within subducting oceanic crusts, as is the case for other hydrous minerals such as talc and serpentine10.
Based on the low activation energy (< 1 eV, see Table 1), the conductivity of amphiboles has been assumed for decades to be due to Fe-related small electronic polarons, i.e., hopping electrons coupled with longitudinal optical (LO) phonons22,24,25,27,29. This inference is consistent with the low mobility of H+ within the amphibole structure30. Wang et al.23 showed that the enhanced electrical conductivity of amphibolites for temperatures above 800 K (at 0.5 GPa) is due to Fe²⁺ ↔ Fe³⁺ charge transfer, rather than the production of aqueous fluids. Similarly, Della Ventura et al.26 observed a rise of electrical conductivity in a single crystal of riebeckite due to Fe2+ → Fe3+ + e− exchange induced by thermal treatment (above 500 K). The activation of Fe-related electron hopping in amphiboles leads to high conductivity (up to 10−² to 10−¹ S/m) at relatively modest temperatures without the need to invoke partial melting or the involvement of aqueous fluids26,30. A notable exception is tremolite (Table 1). The activation energy for conductivity in this Fe-poor amphibole is 3.79 eV, suggesting a different conduction mechanism such as ion (Mg²⁺, Ca²⁺) diffusion29,31.
Despite the paramount implications for planetary-scale phenomena (e.g., high conductivity, water cycling, earthquake activity, arc magmatism, and ore-forming processes), the formation of thermally activated FeO₆-related polarons with high mobility along the I-beam (Fig. 1b) has only recently been proven directly in several amphibole species26,32,33,34,35,36. This was done using the direction-dependent appearance of resonance Raman scattering (RRS) at elevated temperatures32,33,34,35,36 and in situ simultaneous measurement of electrical resistivity and Fe oxidation state26. Polarons arise from the coupling between conduction electrons and longitudinal optical (LO) polar phonons, carrying local structural distortions due to Fe²⁺ ↔ Fe³⁺ charge transfer. Resonance Raman scattering is widely used in solid-state physics to obtain detailed information on specific electronic states and elementary excitations involved in transport phenomena37. In dielectric materials, RRS occurs via Fröhlich interactions38, i.e., electrostatic interactions between electrons and phonons. Under resonance conditions, a photon from the laser beam interacts with both the phonon and the electron, altering the symmetry selection rules. As a result, the polar optical modes associated with the atomic species involved in the electron transition (specifically the Fe–O modes in our case) are enhanced39. In centrosymmetric crystals such as amphiboles, this leads to distinct spectral changes: normal Raman-active (nonpolar) modes are suppressed while infrared-active modes are enhanced. RRS thus provides direct and robust evidence for the activation of small electronic polarons32,33,34,35,36. The temperatures of beginning and completion of polaron activation (T’ and T’’, respectively), the average magnitude of polaron dipoles, and the degree of polaron-dipole alignment may vary with amphibole composition33. For example, a high Fe2+/Mg ratio in the ribbons of MO6 octahedra or a filled A site promote the activation of polaron hopping32,33,34,35,36. However, the appearance of RRS in actinolite34 has shown that even a minor amount of Fe2+ in magnesian amphiboles is sufficient to trigger polaron hopping, albeit at significantly higher temperatures compared to Fe2+-rich amphiboles33. Riebeckite (A☐BNa2C((Fe2+> Mg)3(Fe3+> Al)2)Si8O22(OH)2) is an Fe-rich analogue of glaucophane (A☐BNa2C((Mg ≥ Fe2+)3(Al ≥ Fe3+)2)Si8O22(OH)2), a common amphibole in blueschist-facies rocks associated with subducted oceanic crust40,41. In riebeckite, T’ and T’’ match the temperatures at which high-conductivity layers (HCLs) are observed in warm and cold subduction environments35. High-temperature polarized Raman spectroscopy also revealed the activation of a second type of charge carrier near T’’: delocalized H+ ions32,34,35,36,42. However, Raman spectroscopy alone cannot quantify the contribution of the different types of charge carriers to the overall conductivity.
Here we report our results from in situ high-temperature polarized Raman spectroscopy coupled with resistivity measurements on riebeckite up to ~ 720 K, a temperature above which irreversible Fe oxidation and dehydrogenation take place in the presence of O242. This approach allowed us to monitor the development of mobile charge carriers and simultaneously to quantify the evolving electrical conductivity along (σ||) or perpendicular (σ⊥) to the I-beams of the structure. The measured σ|| and σ⊥ values were then used to address the effect of stress-driven alignment of amphiboles on the electrical structure of subducting plates, providing a key to improve the interpretation and modelling of Earth-scale conductivity data obtained via magnetotelluric (MT) measurements.
Results
In situ temperature-dependent parallel-polarized \(\:\stackrel{-}{y}\left(zz\right)y\) Raman spectra (Porto’s notation, see experimental methods) collected with an applied dc electric field parallel (E||) or perpendicular (E⊥) to the I-beam (see schematic Fig. 2a and b) are shown in Fig. 2c and d, respectively. For Fe-bearing amphiboles, this Raman-scattering geometry provides the temperature of onset of polaron activation (T’) via the minimum in the temperature dependence of the wavenumber of the SiO4 ring mode at 665 cm− 1 and the temperature of cessation of polaron activation (T’’) via the appearance of RRS conditions at which all SiO4-, Fe2+O6- and OH-related peaks disappear and a strong scattering arising from LO polar Fe3+O6 phonon modes appears at ~ 565 cm− 1, see ref32,33,35,36. For riebeckite, T’ = 500 K and T’’ = 650 K33,35. As shown in Fig. 2c and d, temperature-activated RRS occurs exactly at T’’ = 650 K, independent of the direction of the applied external electric field. This evidence provides direct and robust proof for the complete activation of polarons, with dipoles aligned along the amphibole I-beam32,33,35,36.
Atomistic interpretation of the electrical conductivity of riebeckite. A sketch of the experimental set-up to measure σ|| (a) and σ⊥ (b); E is the applied dc electric field; in situ temperature-dependent \(\:\stackrel{-}{y}\left(zz\right)y\) polarized Raman spectra of riebeckite measured at 300 K and T’’= 650 K with E||c axis (c) and E⊥c axis (d); temperature evolution of conductivity measured with E||c, σ|| (e) and with E⊥c, σ⊥, (f); white triangles are σ(T) of silica glass as a reference insulator to mark the zero level. The activation energy (in eV) derived from data between T’ and T’’ (shaded in yellow) and above T’’, (shaded in cyan) are also indicated in the plots; the blue dotted lines in (e) and (f) mark the temperature above which σ⊥ stops increasing. Atomic structure plotted with Vesta 3.5.8 (https://jp-minerals.org/vesta/en/)65.
The temperature evolution of σ|| and σ⊥ is shown in Fig. 2e and f, respectively. Within uncertainties, σ|| begins to deviate from the zero level (σ(T) of silica glass as a reference insulator, white triangles in Fig. 2e and f) at T’ = 500 K, which is the onset of polaron activation33,35 and increases rapidly with increasing temperature. The conductivity in the direction perpendicular to the I-beams (σ⊥) also increases above T’, but at a considerably lower rate (note that the scales in both panels are the same). The difference between σ|| and σ⊥ becomes even larger above T’’= 650 K, which is the temperature of complete polaron activation accompanied by delocalization of H+ 35,42. For T > 700 K, σ|| continues to increase, whereas σ⊥ flattens out (Fig. 2e and f).
The activation energies for the conductivity were calculated by applying the Arrhenius equation σ = σ0\(\:{e}^{-\frac{{E}_{a}}{{k}_{\text{B}}T}}\) (where σ is the conductivity in S.m− 1, kB is the Boltzmann constant = 8.314 × 10− 3 kJ.mol− 1K− 1, T is the temperature in K, and Ea is the activation energy in kJ.mol− 1) to σ||(T) and σ⊥(T) within two temperature ranges: (1) T’ ≤ T1 ≤ T’’, and (2) T’’ ≤ T2 ≤ 700 K (see Fig. 3). The conductivity can be thus described as: σ = σ1\(\:{e}^{-\frac{{E}_{a1}}{{k}_{\text{B}}T1}}\) + σ2\(\:{e}^{-\frac{{E}_{a2}}{{k}_{\text{B}}T2}}\) where Ea1 and Ea2 are the activation energies for each temperature range. The values derived from σ||(T) and σ⊥(T) in the (T’,T’’) range are 44.5 ± 1.8 kJ.mol− 1 (0.46 ± 0.02 eV) and 49.6 ± 2.1 kJ.mol− 1 (0.51 ± 0.02 eV), respectively (Fig. 3). The activation energies in the range between T’’ and 700 K for both experimental configurations are the same within uncertainties: 51.2 ± 1.1 kJ/mol (0.53 ± 0.01 eV) for σ||(T) and 48.6 ± 1.7 kJ/mol (0.50 ± 0.02 eV) for σ⊥(T) (Fig. 3). The activation energy derived from σ||(T) data in the entire temperature range (300–720 K) is 51.2 ± 0.6 kJ/mol (0.53 ± 0.01 eV).
Activation energies for the electrical conductivity of riebeckite. Conductivity (σ|| and σ⊥) data as a function of temperature plotted in Arrhenius space. The values for the activation energy for “pure” polaronic (T’,T’’) and polaronic plus H+-diffusion conductivity (T’’,700 K) are indicated. The activation energy derived from σ||(T) data in the entire temperature range (300–720 K) is 0.42 ± 0.01 eV. Error bars are smaller than the symbol size.
Discussion
Atomic-scale phenomena in riebeckite as a model Fe-amphibole at high temperatures
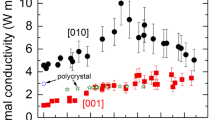
Both σ|| and σ⊥ begins to deviate from the zero level at T’ = 500 K (Fig. 2e and f), signalling the onset of polaron activation33,35. However, σ|| increases much faster than σ⊥ with increasing temperature and at T’’ = 650 K σ|| ~ 3.7σ⊥. The increase in anisotropic conductivity of riebeckite with increasing temperature, derived from dc measurements, accords with the findings from impedance spectroscopy on amphibolite by Zhou et al.28. Given that below T’’, polarons are the main activated charge carriers35, this result clearly shows that polaron conductivity in our model amphibole is predominantly along the c axis, consistent with the strong structural anisotropy of the amphibole (Fig. 1). The ratio σ||/σ⊥ at T’’ is in complete agreement with those available for arfvedsonite, another Fe-rich amphibole24, and with the data of Tolland27 for an amphibole with Fe2+/(Fe2++Mg) = 0.19 (see Fig. 4). Strong direction-dependent conductivity with σ|| > σ⊥ was also observed for a bundle of crocidolite (riebeckite) fibres by Littler and Williams43, though they did not provide specific values for the degree of anisotropy.
Comparison of electrical conductivity of single crystals of amphibole and amphibolites with anomalous high conductivity (HC) in continental crust/forearc, slab–wedge interface, and deeper regions in subduction zones. Conductivity data for single crystals of amphibole from this work (blue and green solid lines) and from Shen et al.31 [1], Schmidbauer et al.25 [2], Tolland27 (σ|| and σ⊥) [3], Della Ventura et al.26 [4], Schmidbauer et al.24 [5], Hu et al.22 [6]. Conductivity data for amphibole-bearing rocks (*) from Wang et al.23 [7] and Zhou et al.28 [8]. The Fe/(Fe + Mg) ratio is indicated for each data set. Note that electrical conductivity increases with the increase of Fe/(Fe + Mg) ratio (red arrow).
Above T’’, where most H+ is delocalized35, σ|| increases steadily up to 720 K (Fig. 2e), whereas σ⊥ flattens out at 700 K (Fig. 2f) and at 720 K σ|| ~ 4.7σ⊥. The constant value of σ⊥ above 700 K indicates no further formation of charge carriers with mobility preferentially normal to the I-beams. Note that up to ~ 720 K, delocalization of both e− and H+ is reversible, and only for T > ~ 720 K and in the presence of external O2, H+ and e− are ejected from the crystal and riebeckite irreversibly oxidizes35,42. Therefore, both electronic polarons and diffusing H+ cations contribute to the conductivity between T’’ = 650 K and 720 K. However, previous infrared spectroscopic analysis suggests that H+ diffusion occurs preferentially perpendicular to the I-beams44. Hence, the fact that σ|| is much larger than σ⊥ supports a model in which polaronic conductivity dominates over conductivity due to H+ diffusion. Moreover, density-functional-theory simulations on grunerite (nominally A☐BFe2+2CFe2+5Si8O22(OH)2) indicate that the experimentally determined T’’ corresponds (in energy units) to the electron energy gap Eg, which is determined by hybridized d-orbitals of CFe2+ and O p-orbitals32. Thus, above T’’, Fe-rich amphiboles should behave as semiconductors and the number of intrinsic charge carriers (electrons in the conduction band and holes in the valence band) should increase with temperature, which also emphasizes the minor contribution of mobile H+ to the amphibole conductivity.
To gain deeper insight into the individual contribution of the two types of charge carriers, polarons and H+, to the conductivity anisotropy, we have calculated the activation energies within two temperature ranges (Fig. 3): (1) between T’ and T’’, where mainly FeO6-related electronic polarons are activated, and (2) between T’’ and 700 K, where the polaron activation is complete and mobile H+ cations are activated, along with intrinsic charge carriers (electrons and holes) in semiconductors. Thus, the former range is indicative of ‘pure’ polaronic activation energy, whereas the latter gives hints on the H+-diffusion activation energy (yellow and blue areas in Fig. 3, respectively).
The “pure” polaronic activation energies derived from σ||(T) and σ⊥(T) in the (T’,T’’) temperature range are 0.46 ± 0.02 eV and 0.51 ± 0.02 eV, respectively (Fig. 3). Our data align with those measured by Tolland27 prior to amphibole dehydrogenation (T < 600 K): 0.54 eV along and 0.57 eV perpendicular to the I-beams (see Fig. 4 and Table 1). The smaller Ea for σ||(T) compared to that for σ⊥(T) provides additional strong support of higher polaron mobility along the I-beams of the structure (Fig. 1b). The activation energies derived from the temperature range between T’’ and 700 K for both experimental configurations are the same within uncertainties: 0.53 ± 0.01 eV for σ||(T) and 0.50 ± 0.02 eV for σ⊥(T) (Fig. 3). This confirms that the mobile charge carriers activated in this temperature range, which include delocalized H+, contribute equally to both σ|| and σ⊥. Above 700 K, σ|| steadily increases, whereas σ⊥ flattens out (Fig. 2e and f), indicating no further formation of charge carriers with mobility normal to the I-beams. Note that the activation energy derived from σ||(T) data between 300 K and 720 K (0.53 ± 0.01 eV) is compatible with that obtained on Fe-containing amphibole single crystals via complex impedance spectroscopy by Hu et al.22 (0.7–0.8 eV) and Schmidbauer et al.25 (between 0.48 and 1.06 eV, depending on the Fe content of their samples), and that obtained via dc measurements by Littler and Williams43 (0.69 eV), Parkhomenko45 (0.9 eV), Della Ventura et al.26 (0.77 eV) and Tolland27 (0.54–0.57 eV) (see Table 1). Our data are also consistent with those obtained via complex impedance spectroscopy on amphibole-containing rocks by Wang et al.23 (0.62 eV) and Zhou et al.28 (0.71–0.75 eV).
Implications of the anisotropic conductivity of amphiboles at subduction zones conditions
Bernardini et al.35,36 show that due to the local charge-buffering provided by delocalization of H+ (Fig. 1a), external oxygen (exO2) is not required to trigger the hopping of polarons in riebeckite. This conclusion has paramount implications in geology because it implies that polaronic conductivity may occur in blue-schist facies rocks associated with subducting oceanic crust at any depth where T > 500 K, regardless the oxygen fugacity. Results from experimental petrology indicate that amphiboles are stable in the mantle lithosphere up to approximately 4 GPa and 1250 K and that their stability is influenced by both water activity and bulk chemical composition12,13. Compositions richer in alkalis tend to stabilize the structure at the highest pressures and temperatures13. However, as water activity decreases, the stability field shifts to lower pressures while expanding to higher temperatures14. In cold subduction zones with lower thermal gradients, amphiboles remain stable to greater depths than in hotter subduction environments (> 150 versus ~ 70 km depths)46. As shown by Goddat et al.47, small-polaron conduction increases with increasing pressure because of its negative activation volume, stressing the role of electron hopping in subducted amphibole-bearing rocks. For riebeckite, in particular, the conductivity increases by approximately one order of magnitude for a pressure increase of 2 GPa in the temperature interval 473 to 873 K45. Hu et al.22 showed with impedance spectroscopy measurements on a single crystal of edenite that pressure in the range 0.5–2.0 GPa (equivalent to depths of up to ~ 65 km) has a very weak effect on conductivity compared to temperature. Similarly, Shen et al.31 observed the same weak pressure effect on the conductivity of tremolite in the range 1.0–2.0 GPa. Moreover, inspection of all data available in the literature shows that there is a clear increase in conductivity with increase of the Fe2+/(Fe2++Mg) ratio in the amphibole (Fig. 4), highlighting the dominant role of FeO6-related electron hopping for reaching high conductivities (above 0.01 S/m). In summary, the above discussion shows that the main contribution of amphiboles to the bulk conductivity of rocks at lithosphere conditions (e.g., continental crusts, forearc regions, and slab-wedge interface, see Fig. 4) is related to the strongly anisotropic electron hopping along the structural I-beams (Fig. 1b), which can be triggered even by minor amounts of Fe34.
It is now important to explore the implications of the strongly anisotropic electrical properties of amphiboles for the interpretation of anomalous conductivity measured via MT methods worldwide in subduction zones2. In the following discussion, we will consider one of the most studied areas in the world: SW Japan. The calculated depth-temperature conditions for the subducting Philippine Sea plate are shown in Fig. 5a and compared with the temperature evolution of σ|| and σ⊥ of riebeckite (Fig. 5b). According to our experimental results, the development of polaronic conductivity with σ|| above 0.01 S/m is expected at the top of the subducting oceanic crust at ~ 15 km depth (point 1 in Fig. 5a). This scenario is in excellent agreement with the electrical conductivity structures observed via MT measurements48,49,50.
Comparison of the thermal ranges for the activation of polarons as determined by our experiments and the calculated P-T conditions for the oceanic crust subducting beneath SW Japan. Temperature and depth conditions for the oceanic crust subducted beneath SW Japan (modified after Peacock and Wang46) (a). Temperature evolution of electrical conductivity of riebeckite in different crystallographic directions (σ|| and σ⊥) (b). The yellow shaded areas denote the temperature range of pure polaronic conductivity in our model amphibole, while the cyan areas denote the range of polaronic plus H+-diffusion conductivity. HCL: high-conductivity layer; RRS: temperature condition for the appearance of resonance Raman scattering in the spectra in Fig. 1; black dashed vertical line (T’=500 K): onset of activation of polarons; red dashed vertical line (T’’=650 K): complete development of polarons and complete delocalization of H+ cations.
However, there are two important issues to consider for a proper interpretation of Earth-scale geophysical data: (1) amphiboles subjected to shear stress exhibit strong CPO16,17, and (2) MT methods provide primarily the horizontal component of rock conductivity.
The CPO of amphiboles depend on both temperature and differential stress16,17. The most common CPO in nature, i.e., type-I, with the c-axis subparallel to the shear direction and the (100) pole subnormal to the shear plane, develops in the temperature range of activation of anisotropic polarons and under a broad range of stress conditions16,17. Anisotropic growth of amphibole grains with this fabric has been also observed in metamorphic rocks from both the Ryoke and Sambagawa belts in SW Japan51,52. Hence, for a scenario in which the stress-driven alignment of amphiboles occurs in response to slab descent, the horizontal component can be derived from the in-lab measured σ||(T) and σ⊥(T) as:
where θ is the angle between the c axis and the direction normal to the Earth’s surface (see Fig. 6a).
The angular dependence of \(\:{\sigma\:}_{h}^{MT}\left(T,\theta\:\right)\) at selected temperatures is given in Fig. 6b, which shows that the effect of θ on \(\:{\sigma\:}_{h}^{MT}\)strongly increases with temperature e.g., at 720 K, 0.02 < \(\:{\sigma\:}_{h}^{MT}\) < 0.14 S/m for 0° < θ < 90°. Hence, for a hypothetical amphibole-bearing subducting slab, \(\:{\sigma\:}_{h}^{MT}\) changes because of: (1) increase of temperature along the descending slab, and (2) variation of the θ angle. What is extremely important here is that the CPO of the amphibole (expressed by the θ angle) definitively controls \(\:{\sigma\:}_{h}^{MT}\)at any given temperature (Fig. 6b). In other words, the evolving stress-driven CPO of the amphibole affects strongly the electrical properties of descending slabs measured via MT methods.
The effect of the CPO on the horizontal component of conductivity in our model sodium amphibole. (a) Preferred orientation of σ|| of the amphibole in the simple-shear zone; red paired arrows denote the direction of shearing force. (b) Isolines of temperature (black lines) showing the relation between \(\:{\sigma\:}_{h}^{MT}\) and the θ angle between 300 and 720 K. T’: onset of polaron activation. T’’: complete development of polarons.
A plot of the data extrapolated from the 2D MT imaging of Kasaya et al.48 shows a steep increase in conductivity along the shear direction of the Philippine Sea Plate subducting beneath SW Japan, between ~ 13 and 19 km depth (yellow shaded area in Fig. 7a). At this depth, the subducted crustal rocks pass through the greenschist metamorphic facies46 dominated by amphibole, chlorite, epidote, plagioclase and quartz53. It is worth noting that at the temperature conditions of this HCL (see Fig. 5a), the conductivity of our model amphibole is several orders of magnitude greater than that of chlorite (~ 10− 5 S/m)54, epidote (~ 10− 3 S/m)55, plagioclase (< 10− 8 S/m)56, and quartz (~ 10− 5 S/m)57. This suggests that amphiboles have a more dominant role than other rock-forming minerals in affecting the electrical properties of the descending slab. We note also that the amphiboles constituting the rocks from the Sambagawa belt in central Shikoku (SW Japan) are hornblendes with high Fe content (Fe2+ and Fe3+ ~ 1.5 and 0.6 apfu, respectively)58.
The effect of stress-driven CPO of amphiboles on the electrical structure of a subduction zone. (a) Variation of conductivity with depth in SW Japan derived from the transverse MT profile of Kasaya et al.48 (see Fig. 7c). The yellow-shaded areas denote the expected depth of development of electronic polarons in our model sodium amphibole (see Fig. 5a). (b) Modelling of the conductivity measured in SW Japan (orange diamonds) at the depth of development of polarons. The evolution of \(\:{\sigma\:}_{h}^{MT}\) with depth is obtained by integrating the temperature-depth data of Peacock and Wang46 (see Fig. 5a) with our in-lab results at different θ values. (c) Atomistic interpretation of the regional conductivity model across the Nankai Trough and Kii Peninsula (data from Kasaya et al.48). The increase of conductivity between 13 and 19 km (blue to orange area below the plate boundary) closely matches the development of polaronic conductivity in preferentially oriented amphibole crystals during subduction of the Philippine Sea Plate beneath the overlying Eurasian plate.
We can use the evolution of \(\:{\sigma\:}_{h}^{MT}\left(T,\theta\:\right)\) of our model Fe-amphibole to simulate the sharp increase in conductivity observed via MT (yellow shaded area in Fig. 7a). However, it must be emphasized that \(\:{\sigma\:}_{h}^{MT}\left(T,\theta\:\right)\) is different for amphiboles of different composition, particularly with different Fe/Mg ratio and A-site population32,33,34,35,36. Moreover, the slab is a polymineralic system in which several rock-forming minerals affect its electrical properties. Therefore, the following discussion must be considered as a simplified model directed toward increasing our understanding of more complex systems.
The evolution of \(\:{\sigma\:}_{h}^{MT}\left(T,\theta\:\right)\) for our amphibole at depths of 13–19 km (calculated using the variation of temperature with depth in Fig. 5a) is given in Fig. 7b and compared with the MT data from Kasaya et al.48 (orange diamonds in Fig. 7b). The \(\:{\sigma\:}_{h}^{MT}\left(T,\theta\:\right)\) has been calculated at θ values between two extreme situations, i.e., θ = 0° (assuming the amphibole crystals are oriented vertically during subduction) and θ = 90° (assuming the amphiboles are oriented horizontally). Both situations are clearly unrealistic and there is no fit between the experimental data and the simulated data (Fig. 7b). On the other hand, Fig. 7b shows that \(\:{\sigma\:}_{h}^{MT}\left(T,\theta\:\:=32.5^\circ\:\right)\) matches very well the MT profile from Kasaya et al.48. We need to consider here that in a rock subjected to a simple-shear-stress field (see schematic grey plane in Fig. 6a), the amphibole crystals align progressively with their a and c axes parallel to the minimum and maximum stress vector, respectively16,17,51,52. This being the case, only the xx and zz components of the conductivity 2nd -rank tensor σ will contribute to \(\:{\sigma\:}_{h}^{MT}\left(T,\theta\:\right)\). The in-lab measured σ|| corresponds exactly to σzz, whereas σ⊥ is dominated by σxx but contains a small contribution from σyy due to the inclination of σ⊥ to the reciprocal-space axis a*. To estimate the influence of this issue on our results, we have averaged σ⊥ over an angle ϕ ∈ [0, π/2], which represents the extreme situation of randomly oriented b and a* axes around the [001] direction. In this case, the best match between \(\:{\sigma\:}_{h}^{MT}\left(T,\theta\:\right)\) and the MT profile is obtained for \(\:\theta\:\:=37.5^\circ\:\), a value that is very similar (within uncertainties) to that obtained with the formula given above, supporting the validity of our assumption.
Basic structural-geology arguments show that the shallow part of the slab is dominated by simple-shear deformation59. Under such conditions, minerals with strong morphological anisotropy (such as amphiboles) grow aligned with their prismatic elongation (|| the c crystallographic axis) oriented at ~ 45° to the direction of shearing force60. According to our simulation, the dipping angle of the slab should thus be 45°-32.5° = 7.5° (or 45°-37.5° = 12.5° if the angle average of σ⊥ is used), which is in close agreement with the value of 7–11° given by Nakanishi et al.61. Our simulation is also consistent with the pervasive stress-driven alignment of amphiboles (type-I CPO) observed in the metamorphic rocks from SW Japan51,52. Therefore, the stress-driven CPO of the amphibole elegantly explains the increase in conductivity observed between 13 and 19 km beneath SW Japan (blue-to-orange area below the plate boundary in Fig. 7c). This consideration thus implies that preferential orientation of amphibole crystals provides a strongly anisotropic contribution to the bulk electrical conductivity of subducted crustal rocks, with higher conductivity expected along the direction of plate motion.
Conclusions
This study shows that (1) the onset of activation of FeO6-related electronic polarons in riebeckite (at ~ 500 K) perfectly matches the increase in anisotropic conductivity with σ|| > σ⊥; (2) electron transport via polaron hopping is the dominant mechanism of charge transfer between 500 and 720 K; (3) anisotropy in the conductivity is strongly enhanced by increasing temperature (at 720 K σ|| ~ 4.7σ⊥); (4) conductivity data from MT measurements are best modelled by considering the effect of stress-driven alignment of amphibole crystals during plate motion.
Our work definitively demonstrates that the CPO of amphiboles, which is assumed to be among the main sources of seismic anisotropy observed worldwide in subduction zones, also strongly affects the electrical properties of subducting crustal rocks. This result emphasizes the further need for in situ HT Raman spectroscopy coupled with electrical-conductivity measurements on metamorphic rock-forming minerals to constrain the atomic-scale mechanisms of rock conductivity.
Experimental methods
The sample used for the experiments described in this work is a near-end-member riebeckite from Mt. Malosa, Zomba District, Malawi. It has been comprehensively characterized in previous papers by a combination of analytical methods, including electron probe microanalysis, X-ray diffraction, Mössbauer spectroscopy, Fourier transform infrared (FTIR) spectroscopy, Raman spectroscopy, and X-ray absorption spectroscopy35,36,42,62,63. It has monoclinic C2/m symmetry at ambient conditions and its exact crystal-chemical formula is: A(☐0.9K0.06Na0.04)B(Na1.82Ca0.13\(\:{\text{F}\text{e}}_{0.05}^{2+}\))C[M(1)(\(\:{\text{F}\text{e}}_{1.84}^{2+}{\text{M}\text{g}}_{0.16}^{}\))M(2)(\(\:{\text{F}\text{e}}_{0.21}^{2+}{\text{M}\text{g}}_{0.04}^{}{\text{F}\text{e}}_{1.64}^{3+}{\text{A}\text{l}}_{0.10}^{}{\text{T}\text{i}}_{0.01}^{}\))M(3)(\(\:{\text{F}\text{e}}_{0.89}^{2+}{\text{M}\text{g}}_{0.06}^{}{\text{M}\text{n}}_{0.05}^{2+}\))]T(Si7.97Al0.03)O22W[(OH)1.90\(\:{\text{F}}_{0.10}^{}\)], that simplifies in the A☐BNa2C(Fe2+3Fe3+2Si8O22(OH)2 stoichiometry.
Pristine fragments of a euhedral crystal (size ~ 1.5 × 1.5 × 2.5 mm3) were used for in situ high-temperature experiments under an external direct-current (dc) electric field applied with a Keithley 2410 high-voltage source meter (voltage basic accuracy of 0.012% and a low-current accuracy of 300 pA). Electrical contacts were glued to natural faces parallel either to the crystallographic (001) or (110) plane of an amphibole crystal using a PELCO high-performance silver paste and then annealed at 93 °C for 2 h. Hence, the applied dc electric field was either parallel (E||) or perpendicular (E⊥) to the crystallographic c axis, i.e., to the ribbons of FeO6 octahedra (see Fig. 2a and b). The area of the electrical contacts was measured under an optical microscope by moving the sample along the XYZ coordinates using a computer-controlled motorized stage. The structural stability of the sample was monitored throughout the experiment by examining the evolution of features in the Raman spectra. The conductivity of a specimen of commercial amorphous silica (Suprasil) with a size similar to that of the amphibole samples was used as a reference insulator to establish the “zero”-conductivity level for the experimental set-up. In situ temperature-dependent experiments were done in air using a LINKAM THMS-E600 stage with a temperature accuracy of 0.1 K and a heating rate of 10 K/min. Previous experiments64 on wide-band insulators using the same set-up verified the absence of ionic surface leakage up to 800 K. The stabilization time prior to collecting Raman spectra at the desired temperature was 10 min.
Raman spectra were collected with a Horiba Jobin-Yvon T64000 triple-monochromator spectrometer equipped with an Olympus BX41 microscope and a liquid-N2-cooled charge-couple-device detector, using the 514.532-nm line of a Coherent Innova 90 C FreD Ar+ laser. The spectrometer was calibrated to the Raman peak of Si at 520.5 cm− 1. The spectral resolution was ~ 2 cm− 1, whereas the instrumental precision in determining the peak positions was ~ 0.35 cm− 1.
Parallel polarized spectra (polarization of the incident light parallel to the polarization of scattered light) were collected in backscattering geometry with the c-axis parallel to the polarization of the incident light (\(\:\stackrel{-}{y}\left(zz\right)y\) scattering geometry in Porto’s notation, with z || c and y ⊥ c and lying in the plane of b- and reciprocal-space a*-axes). The as-measured spectra were baseline-corrected, temperature-reduced for the Bose-Einstein population factor, and fitted with pseudo-Voigt functions to derive the phonon wavenumbers ω, full widths at a half maximum, integrated intensities I, and weight coefficients q of the Lorentzian contribution to the peak shape.
Data availability
The datasets generated and analysed during the current study are available from the corresponding authors on reasonable request.
References
Ichiki, M., Baba, K. & Toh, H. Fuji-ta, K. An overview of electrical conductivity structures of the crust and upper mantle beneath the Northwestern Pacific, the Japanese Islands, and continental East Asia. Gondwana Res. 16, 545–562 (2009).
Pommier, A. Geophysical assessment of migration and storage conditions of fluids in subduction zones. Earth Planet Space. 66, 1–11 (2014).
Naif, S., Key, K., Constable, S. & Evans, R. L. Melt-rich channel observed at the lithosphere–asthenosphere boundary. Nature 495, 356–359 (2013).
Evans, R. et al. Geophysical evidence from the MELT area for compositional controls on oceanic plates. Nature 437, 249–252 (2005).
Manthilake, G. et al. Tracking slab surface temperatures with electrical conductivity of glaucophane. Sci. Rep. 11, 18014 (2021).
Manthilake, G., Koga, K. T., Peng, Y. & Mookherjee, M. Halogen bearing amphiboles, aqueous fluids, and melts in subduction zones: insights on halogen cycle from electrical conductivity. J. Geophys. Res. : Solid Earth. 126, 1–14 (2021).
Chen, S. et al. Dehydration of phengite inferred by electrical conductivity measurements: implication for the high conductivity anomalies relevant to the subduction zones. Geology 46, 11–14 (2018).
Pommier, A. et al. Experimental constraints on the electrical anisotropy of the lithosphere–asthenosphere system. Nature 522, 202–206 (2015).
Dai, L. & Karato, S. I. High and highly anisotropic electrical conductivity of the asthenosphere due to hydrogen diffusion in olivine. Earth Planet. Sci. Lett. 408, 79–86 (2014).
Guo, X., Yoshino, T. & Katayama, I. Electrical conductivity anisotropy of deformed Talc rocks and serpentinites at 3 GPa. Phys. Earth Planet. Inter. 188, 69–81 (2011).
Christensen, N. I. & Mooney, W. D. Seismic velocity structure and composition of the continental crust: a global view. J. Geophys. Res. : Solid Earth. 100, 9761–9788 (1995).
Grove, T. L., Chatterjee, N., Parman, S. W. & Médard, E. The influence of H2O on mantle wedge melting. Earth Planet. Sci. Lett. 249, 74–89 (2006).
Mandler, B. E. & Grove, T. L. Controls on the stability and composition of amphibole in the Earth’s mantle. Contrib. Mineral. Petrol. 171, (2016).
Juriček, M. P. & Keppler, H. Amphibole stability, water storage in the mantle, and the nature of the lithosphere-asthenosphere boundary. Earth Planet. Sci. Lett. 608, 118082 (2023).
Hawthorne, F. C. & Oberti, R. Amphiboles: crystal chemistry. In: (eds Hawthorne, F. C., Oberti, R., Della Ventura, G. & Mottana, A.) Amphiboles: Crystal Chemistry, Occurrence, and Health Issues, Reviews in Mineralogy and Geochemistry, 67. Min. Soc. Am, Chantilly, Virginia, 1–54 (2007).
Jung, H. Crystal preferred orientations of Olivine, orthopyroxene, serpentine, chlorite, and amphibole, and implications for seismic anisotropy in subduction zones: a review. Geosci. J. 21, 985–1011 (2017).
Ko, B. & Jung, H. Crystal preferred orientation of an amphibole experimentally deformed by simple shear. Nat. Commun. 6, 6586 (2015).
Bezacier, L. et al. Elasticity of glaucophane, seismic velocities and anisotropy of the subducted oceanic crust. Tectonophysics 494, 201–210 (2010).
Faccenda, M. et al. Fault-induced seismic anisotropy by hydration in subducting oceanic plates. Nature 455, 1097–1100 (2008).
Long, M. D. & Wirth, E. A. Mantle flow in subduction systems: the mantle wedge flow field and implications for wedge processes. J. Geophys. Res. : Solid Earth. 118, 583–606 (2013).
Tatham, D. J. et al. Amphibole and lower crustal seismic properties. Earth Planet. Sci. Lett. 267, 118–128 (2008).
Hu, H. et al. Effect of dehydrogenation on the electrical conductivity of Fe-bearing amphibole: implications for high conductivity anomalies in subduction zones and continental crust. Earth Planet. Sci. Lett. 498, 27–37 (2018).
Wang, D., Guo, Y., Yu, Y. & Karato, S. Electrical conductivity of amphibole-bearing rocks: influence of dehydration. Contrib. Mineral. Petrol. 164, 17–25 (2012).
Schmidbauer, E., Kunzmann, T., Fehr, T. & Hochleitner, R. Electrical conductivity, thermopower and 57Fe Mössbauer spectroscopy on an Fe-rich amphibole, arfvedsonite. Phys. Chem. Min. 23, 99–106 (1996).
Schmidbauer, E., Kunzmann, T., Fehr, T. & Hochleitner, R. Electrical resistivity and 57Fe Mössbauer spectra of Fe-bearing Calcic amphiboles. Phys. Chem. Min. 27, 347–356 (2000).
Della Ventura, G. et al. In situ high-temperature reversible Fe oxidation and resistivity of riebeckite: implications for geophysical anomalies in subduction zones. Geochemistry 84, 1 (2024).
Tolland, H. G. Mantle conductivity and electrical properties of Garnet, mica and amphibole. Nat. (London) Phys. Sci. 241, 35–36 (1973).
Zhou, W., Fan, D., Liu, Y. & Xie, H. Measurements of wave velocity and electrical conductivity of an amphibolite from Southwestern margin of the Tarim basin at pressures to 1.0 GPa and temperatures to 700°C: comparison with field observations. Geophys. J. Int. 187, 1393–1404 (2011).
Katsura, T., Yoshino, T., Manthilake, G. & Matsuzaki, T. Electrical conductivity of the major upper mantle minerals: a review. Russ Geol. Geophys. 50, 1139–1145 (2009).
Karato, S. I. & Wang, D. Electrical Conductivity of Minerals and Rocks, in Physics and Chemistry of the Deep Earth (ed Karato S)145–182 (Wiley, 2013).
Shen, K., Wang, D. & Liu, T. Electrical conductivity of tremolite under high temperature and pressure: implications for the high-conductivity anomalies in the Earth and Venus. Contrib. Mineral. Petrol. 175, 52 (2020).
Mihailova, B. et al. Atomistic insight into lithospheric conductivity revealed by phonon–electron excitations in hydrous iron-bearing silicates. Commun. Mater. 2, 57 (2021).
Mihailova, B. et al. Polarons in rock-forming minerals: physical implications. Condens. Matter. 7, 68 (2022).
Rösche, C. et al. Oxidation processes and thermal stability of actinolite. Phys. Chem. Min. 49, 47 (2022).
Bernardini, S., Della Ventura, G., Schlüter, J. & Mihailova, B. Thermally-activated electron hopping in Fe-rich amphiboles: implications for the high-conductivity anomalies in subduction zones. Geochemistry 83, 1 (2023).
Bernardini, S., Della Ventura, G., Schlüter, Hawthorne, F. C. & Mihailova, B. The effect of A-site cations on charge-carrier mobility in Fe-rich amphiboles. Am. Mineral. 109, 1545–1553 (2024).
Weber, W. H. & Merlin, R. Raman Scattering in Materials Science (Springer, 2000).
Yu, P. Y. & Cardona, M. Fundamentals of Semiconductors (Springer, 2010).
de la Flor, G. et al. Resonance Raman scattering of perovskite-type relaxor ferroelectrics under nonambient conditions. Phys. Rev. B. 90, 064107 (2014).
Wintsch, R. E., Byrne, T. & Toriumi, M. Exhumation of the Sanbagawa blueschist belt, SW Japan, by lateral flow and extrusion: evidence from structural kinematics and retrograde P-T-t paths. In: Ring, U., Brandon, M.T., Lister, G.S., Willett, S.D. (Eds.), Exhumation Processes: Normal Faulting, Ductile Flow and Erosion, 154. Geological Society, London, Special Publications, pp. 129–155 (1999).
Deer, W. A., Howie, R. A. & Zussman, J. Double-chain Silicates, 2nd edition. The Geological Society, London (1997).
Della Ventura, G. et al. The dynamics of Fe oxidation in riebeckite: a model for amphiboles. Am. Mineral. 103, 1103–1111 (2018).
Littler, J. G. F. & Williams, R. J. P. Electrical and optical properties of crocidolite and some other iron compounds. J. Chem. Soc. 1182, 6368–6371 (1965).
Della Ventura, G. et al. Kinetics of hydrogen diffusion in riebeckite, Na2Fe3 + 2Fe2 + 3Si8O22(OH)2: an HT-FTIR study. Am. Mineral. 107, 754–764 (2022).
Parkomenko, E. I. Electrical resistivity of minerals and rocks at high temperature and pressure. Rev. Geophys. Space Phys. 20, 193–218 (1982).
Peacock, S. M. & Wang, K. Seismic consequences of warm versus cool subduction metamorphism: examples from Southwest and Northeast Japan. Science 286, 937–939 (1999).
Goddat, A., Peyronneau, J. & Poirier Dependence on pressure of conduction by hopping of small polarons in minerals of the Earth’s lower mantle. Phys. Chem. Minerals. 27, 81–87 (1999).
Kasaya, T. et al. Resistivity image of the Philippine sea plate around the 1944 Tonankai earthquake zone deduced by marine and land 579 MT surveys. Earth Planet Space. 57, 209–213 (2005).
Yamaguchi, S. et al. Preliminary report on regional resistivity variation inferred from the network MT investigation in the Shikoku district, Southwestern Japan. Earth Planet Space. 51, 193–203 (1999).
Fuji-ta, K., Ogawa, Y., Yamaguchi, S. & Yaskawa, K. Magnetotelluric imaging of the SW Japan forearc - a lost paleoland revealed? Phys. Earth Planet. Inter. 102, 231–238 (1997).
Hara, I. et al. Deformation and recrystallization of amphiboles in Sambagawa schist with special reference to history of Sambagawa metamorphism. J. Sci. Hiroshima Univ. Ser. C Geol. Min. 8, 135–148 (1983).
Imon, R., Okudaira, T. & Kanagawa, K. Development of shape- and lattice-preferred orientations of amphibole grains during initial cataclastic deformation and subsequent deformation by dissolution–precipitation creep in amphibolites from the Ryoke metamorphic belt, SW Japan. J. Struct. Geol. 26, 793–805 (2004).
Apted, M. J. & Liou, J. G. Phase relations among greenschist, epidote-amphibolite, and amphibolite in a basalt system. Am. J. Sci. 283-A, 328–354 (1983).
Manthilake, G. et al. Dehydration of chlorite explains anomalously high electrical conductivity in the mantle wedges. Sci. Adv. 2, e1501631 (2016).
Hu, H. et al. Influence of dehydration on the electrical conductivity of epidote and implications for high-conductivity anomalies in subduction zones. J. Geophys. Res. Solid Earth. 122, 1751–2762 (2017).
Yang, X., Keppler, H., McCammon, C. & Ni, H. Electrical conductivity of orthopyroxene and plagioclase in the lower crust. Contrib. Mineral. Petrol. 163, 33–48 (2012).
Wang, D-J. et al. Electrical conductivity of synthetic quartz crystals at high temperature and pressure from complex impedance measurements. Chin. Phys. Lett. 19, 1211–1213 (2002).
Aoki, K. et al. Metamorphic P–T–time history of the Sanbagawa belt in central Shikoku, Japan and implications for retrograde metamorphism during exhumation. Lithos 113, 393–407 (2009).
Fossen, H. Structural Geology (Cambridge Univ. Press, 2016).
Rossetti, F., Storti, F. & Salvini, F. Cenozoic noncoaxial transtension along the Western shoulder of the Ross Sea, Antarctica, and the emplacement of McMurdo Dyke arrays. Terra Nova. 12, 60–66 (2000).
Nakanishi, A. et al. Crustal structure across the coseismic rupture zone of the 1944 Tonankai earthquake, the central Nankai trough seismogenic zone. J. Geophys. Res. 107 (2002).
Susta, U., Della Ventura, G., Hawthorne, F. C., Mihailova, B. & Oberti, R. The crystal chemistry of riebeckite, ideally Na2Fe2 + 3Fe3 + 2Si8O22(OH)2: a multi-technique study. Mineral. Mag. 82, 837–852 (2018).
Oberti, R. et al. The HT behaviour of riebeckite: expansivity, deprotonation, selective Fe oxidation and a novel cation disorder scheme for amphiboles. Eur. J. Mineral. 30, 437–449 (2018).
Steilmann, T., Maier, B. J., Gospodinov, M., Bysmayer, U. & Mihailova, M. Electric-field-induced local structural phenomena in relaxor ferroelectric PbSc0.5Nb0.5O3 near the intermediate temperature T* studied by Raman spectroscopy. J. Phys. : Condens. Matter. 26, 175401 (2014).
Momma, K. & Izumi, F. VESTA: a three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 41, 653–658 (2008).
Acknowledgements
We gratefully acknowledge financial support by the Federal Ministry of Education and Research (BMBF) and the Free and Hanseatic City of Hamburg under the Excellence Strategy of the Federal Government and the Länder, by the Grant to Department of Science, Roma Tre University (MIUR-Italy Dipartimenti di Eccellenza, ARTICOLO 1, COMMI 314-337 LEGGE 232/2016), and by a Discovery Grant to FCH from the Natural Sciences and Engineering Research Council of Canada. We extend our thanks to M. Mookherjee for editorial handling, and G. Manthilake and an anonymous reviewer for their positive criticism and suggestions which greatly improved the clarity of the paper.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Federal Ministry of Education and Research (BMBF) and the Free and Hanseatic City of Hamburg under the Excellence Strategy of the Federal Government and the Länder; Deutsche Forschungsgemeinschaft (Project MI 1127/13-1); MIUR-Italy Dipartimenti di Eccellenza (ARTICOLO 1, COMMI 314–337 LEGGE 232/2016); Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Contributions
B.M., G.D.V. and S.B. planned the original project. S.B. conducted the experiments, evaluated and analysed the data, and wrote the first draft of the paper. B.M., G.D.V., F.C.H., F.S. and A.M. contributed to the analysis and revised the text.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bernardini, S., Della Ventura, G., Hawthorne, F.C. et al. The effect of anisotropic electrical conductivity of amphiboles on geophysical anomalies observed in subduction zones. Sci Rep 15, 14244 (2025). https://doi.org/10.1038/s41598-025-98025-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-98025-9