Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder with significant cognitive and functional impacts. Genetic factors, particularly the APOE gene and its allelic variants (ε2, ε3, ε4), play a critical role in AD susceptibility. This study analyzed the allelic frequency and distribution of APOE polymorphisms in three provinces of Castilla y León (León, Soria, Salamanca), Spain, to explore their potential relationship with AD risk. Genotypes were determined using polymerase chain reactions, and statistical analyses revealed significant regional variations. The ε3/ε3 genotype was the most prevalent overall, while the ε3/ε4 genotype predominated in specific areas like Ponferrada. The absence of homozygous ε4 individuals in Soria contrasts sharply with higher frequencies in Salamanca. These differences suggest historical and migratory influences on genetic variability. Identifying regional genetic patterns enhances our understanding of AD risk and supports the development of targeted preventive strategies. Early detection of high-risk alleles could improve patient outcomes, reduce healthcare burdens and inform public health policies.
Similar content being viewed by others
Introduction
First described by Alois Alzheimer in 1907, Alzheimer’s disease (AD) is a progressive and irreversible neurodegenerative condition that mainly affects cognitive functions, memory and behaviour, gradually rendering individuals more vulnerable and dependent. This progression places a continuous burden on both family members and caregivers1.
According to the World Health Organization (WHO), an estimated 55.2 million people worldwide were affected by AD and other dementias in 2019. This number is expected to rise to 75 million by 2030 and 132 million by 2050. Although the risk of dementia may decrease as social conditions improve, due to coordinated efforts to reduce risk factors and promote protective measures, it is important to note that the European region currently has the second highest number of dementia cases, with a total of 14.1 million2.
Research conducted in Spain estimates the prevalence of AD to be approximately 0.05% among individuals aged 40–64 years; 1.07% in the 65–69 age range; 3.4% for those aged 70–74 years; 6.9% in the 75–79 age group; 12.1% for individuals aged 80–84 years; 20.1% in the 85–89 age group; and 39.2% among those over 90 years of age3.
When analyzing the brain of a patient with AD from a pathological anatomy perspective, generalized, bilateral and symmetrical atrophy is observed, with prominent involvement of the hippocampus, amygdala and fronto-temporo-parietal areas, which are recognized as association areas4.
At the microscopic level, AD is characterized by two types of abnormal structures. Senile plaques primarily consist of deposits of β-amyloid (Aβ). A 36–43 amino acid peptide precursor of amyloid precursor protein (APP), which abnormally folds into extracellular nest-like lattices. The Aβ42 variant is strongly associated with early amyloid deposition in the extracellular neocortex, which subsequently spreads to subcortical structures such as the hippocampus and entorhinal cortex, impairing cognitive function5,6. Neurofibrillary tangles (NFBs) are composed of Tau protein, which becomes abnormally folded and hyperphosphorylated. These tangles consist of long unbranched neurofilaments located within the cytoplasm of neurons. The distribution of NFBs follows the Braak and Braak Scheme, with the highest concentration found in the limbic system, which affects physiological and emotional responses5,7,8.
Recent research indicates that in 95% of AD cases, classified as late-onset AD (LOAD), can be attributed to two key categories of risk factors: modifiable and non-modifiable9 . Modifiable risk factors include conditions such as type 2 diabetes mellitus, high blood pressure and obesity, while non-modifiable risk factors encompass age, gender, ethnic background and the presence of the apolipoprotein epsilon (APOE)10,11.
Over the past decade, significant research has uncovered various biomarkers, such as Tau protein, detectable in urine, blood, cerebrospinal fluid, tears and saliva, that aid in the early diagnosis of AD. Among these, the APOE gene has been prominently identified in the scientific literature as a crucial possible biomarker and a key indicator of the disease12,13,14.
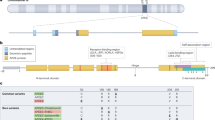
The APOE gene is located within a 45kb gene cluster on chromosome 19, at the q13.2-q13.3 locus15. It encodes APOE, a 34kDa glycoprotein composed of 299 amino acids, with if primarily produced by astrocytes under physiological conditions. Its essential function is to protect neurons from oxidative stress16.
The APOE gene locus is polymorphic, presenting three possible isoforms: APOE2, APOE3 and APOE4. These correspond to three alleles of the gene, ε2, ε3 and ε4, which result in six possible genotypes that are inherited in a codominance manner11. Although the isoforms differ only by the position of two amino acid residues, their structural conformations vary significantly17.
APOE4 is associated with a heightened risk of AD (Fig. 1), with heterozygous carriers having a 3- to fourfold increased risk and homozygous carriers facing an 8- to 12-fold increase. In contrast, APOE2 is considered protective against AD, linked to reduced hippocampal atrophy and lower concentrations of Tau protein 17.
Effects of APOE4 in Alzheimer’s Disease. The presence of the APOE4 isoform promotes the formation of β-amyloid, which in turn increases the formation of senile plaques and the aggregation of Tau protein. This leads to heightened neuroinflammation, resulting in clinical consequences such as neuronal loss and cognitive decline. Abbreviation: APOE: Apolipoprotein E;
The geographical distribution of APOE gene in Spain reflects a complex phenomenon shaped by a long and intricate history18,19. Castilla y León is one of the autonomous communities with the oldest population in Spain20, making it a relevant setting for studying AD and its genetic risk factors. Despite the significance of genetic influences on AD, studies on the APOE polymorphism in Spain remain scarce. Due to its geographical location at a crossroads, Castilla y León has received significant genetic contributions from Northern Europe, the Mediterranean area, and Northern Africa. These historical influences may have shaped the distribution of APOE alleles in this region, highlighting the importance of analyzing their impact on AD risk. Understanding the APOE gene polymorphism in this population is crucial for assessing its potential role in AD susceptibility. Given the demographic characteristics of Castilla y León and its genetic background, examining the distribution of APOE alleles in the provinces of León, Soria and Salamanca (Fig. 2) may provide valuable insights for developing targeted prevention and care strategies. Characterizing the APOE gene polymorphism in this population will not only help identify individuals at higher genetic risk but also support the development of personalized prevention and care approaches in the region’s daycare centers. Therefore, this study aims to expand the current knowledge regarding the presence, allelic frequency and genetic distribution of the APOE gene in the provinces of León, Soria and Salamanca, while comparing the data obtained with results from similar studies conducted in other regions of Spain and Europe.
Methods
Study design
An analytical observational case–control study carried out in the "Mensajeros de la Paz" Residences and Asociaciones de Enfermos de Alzheimer (AFA) in Castilla y León, following the guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement were followed. This study setting was selected due to their broad patient population, providing a suitable sample for the study. Additionally, Castilla y León’s historical position as a geographical crossroads has contributed to a diverse genetic background, reinforcing the relevance of analyzing the distribution of APOE polymorphisms in these regions.
Ethical considerations
Prior to the initiation of this study, approval was obtained from the Ethics Committee of the University of León (code ETICA-ULE-021–2022), ensuring full compliance with the legal requirements set forth by Law 31/1995 on the Prevention of Occupational Hazards and Law 14/2007 on Biomedical Research concerning the use of human biological samples. The research adhered to the ethical standards outlined in the Declaration of Helsinki, including its subsequent revisions, and was registered in ClinicalTrials.gov under code NCT06275243.
Study cohort
In the case group, a total of 260 people diagnosed with AD were included, sourced from the AFA of Castilla y León, as well as users of the "Mensajeros de la Paz" Residences located in Mansilla de las Mulas (León) and in La Bañeza (León).
In the control group, a total of 251 people without a diagnosis of AD were included. These participants were recruited in the AFA of León and Ponferrada, as well as in the University of Experience in León and Ponferrada and in the Maintenance Gymnastics classes offered by the City Council of León.
Inclusion and exclusion criteria
Recruitment inclusion criteria were: (i) Age 60–90 years; (ii) Case group with diagnosis of AD; (iii) Control group without diagnosis of AD; (iv) Voluntarily participation and free of charge. The exclusion criteria were: (i) Participants who, during sampling, presented behavioural and oral alterations or other conditions that impeded the process.
Sample collection and processing
The Canvax® Buccal swab collection & stabilization kit was used to collect buccal cheek cells using a swab. The sample was suspended in an Eppendorf tube with stabilisation fluid and centrifuged at 13,000 RPM for 5 min. Then, 10–20 µl of residual liquid was left for preservation.
Samples were processed with the Canvax® Buccal swab genomic DNA extraction kit to purify the DNA. 3 ml of proteinase K and 200 µl of buffer were added to the sample, homogenised and incubated at 55°C for 1 h. Afterwards, S3 buffer was added, vortexed and centrifuged. The resulting liquid was mixed with isopropanol and buffer S4, centrifuged and the supernatant removed. The supernatant was washed with 70% ethanol and centrifuged again. Finally, the supernatant was removed and allowed to dry.
To amplify APOE2, APOE3 and APOE4 isoforms by polymerase chain reactions (PCR), the sequence-specific primer method described by Pantelidis et al.21 was followed. Three PCR reactions were performed with different combinations of E2, E3 and E4 primers and control primers. The results were analysed by 2% agarose gel electrophoresis with ultraviolet visualisation, with the presence of a 173 bp band being specific and a 785 bp band being control.
Statistical analysis
A descriptive statistical analysis was performed by tabulating the data according to their distribution by absolute and relative frequencies, and they were represented graphically to obtain visual conclusions.
In addition, an inferential study was carried out to test hypotheses about differences between individuals with AD and the control group. To assess significant differences in quantitative variables, the t-test for independent samples was used, assuming normal populations and verifying normality with Kolmogorov–Smirnov and Shapiro–Wilk tests. In the case of several groups, the alternative ANOVA method was used, applying the Sheffé’s test for multiple comparisons. The chi-square test or exact test was used for qualitative variables.
Statistical significance was considered at a p-value of less than 0.05.
All analyses were performed with IBM SPSS Statistics for Windows, version 26.0 (Armonk. NY: IBM Corp.).
Results
The study population comprised 511 individuals, with 260 diagnosed with AD and the remaining 251 identified as healthy controls. The 260 participants with AD were recruited from various AFA located in Soria, Salamanca, León and Ponferrada, as well as from the "Mensajeros de la Paz" Residences in Mansilla de las Mulas (León) and La Bañeza (León).
Socio-demographic data
After obtaining the population sample and conducting personal interviews with the 260 individuals in the case group and 251 in the control group about their socio-demographic characteristics, the following results were obtained and are shown in Table 1.
In both groups, the percentage of women is higher than that of men, being more notable in the control group with 83.67% of women. The AD case group has a higher proportion of widowers (52.30%) compared to the control group (20.24%), while the control group has a higher proportion of married people (63.56%) compared to the case group (41.84%).
In the case group, 74.74% have primary education, while in the control group, the highest level is secondary education with 41.70%. The majority of both groups reside in urban areas, being higher in the control group (95.56%) compared to the case group (72.92%).
An 45.60% of the case group had a family history of AD, in contrast to 26.75% of the control group.
Distribution of APOE genotypes by provinces in Castilla y León
In this section, we analyze how genotypes are distributed across the provinces of Castilla y León. To do so, we consider the sample of AD cases, as the focus is on studying the provincial distribution of genotypes among patients with AD.
First, we describe the sociodemographic characteristics of this sample of patients, which are presented in Table 2, analyzing potential differences by genotype.
As shown, no significant differences are observed by genotype in terms of sociodemographic variables, except for age, where significant differences are found at a level below 0.001. Applying Scheffé’s test, the groups with the greatest differences are the ε3/ε3, ε3/ε4 and ε4/ε4 genotypes.
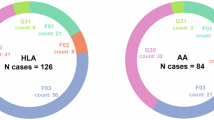
Information on the distribution of genotypes by geographical area is shown in Fig. 3, as well as in Table 3.
As can be seen, the most frequent genotype in the three provinces is the homozygous ε3, especially in Soria, with a percentage of over 60%. This is followed by the ε3/ε4 genotype, which in the case of Salamanca presents a percentage of cases quite close to the previous genotype (38.10% compared to 33.33%). As for León, the situation is similar to the other two provinces, with 55.60% of individuals carrying the ε3 homozygote and 34.80% carrying the ε3/ε4 genotype.
However, when considering the area of León and Ponferrada separately, some differences are observed. The area of León shows the same trend as described above, with percentages similar to those of Soria (59.70% and 31.34% for each genotype, respectively). In Ponferrada, on the contrary, the most numerous genotypes are ε3/ε4, followed by ε3/ε3, although the percentages are similar (45.45% and 43.18%, respectively) as shown in Fig. 4. To confirm there are no differences between these two areas, a z-test for independent proportions was applied. In the case of genotype ε3/ ε4, a p-value of 0.7083 was obtained, whereas for the genotype ε4/ ε4, the p-value was 0.8366. Therefore, the percentage of individuals with these two genotypes can be considered as similar.
Finally, the homozygous ε4 genotype is not present in the sample of individuals from Soria. In the province of León, it is found in 3.90% of cases, although the percentage is higher in the Ponferrada area than in León (6.82% compared to 2.99%). In Salamanca it is 19.05%.
These results are consistent with those obtained by González et al.22, who considered a sample of patients from the Castilla y León region. Applying a z-test for the difference in proportions between the percentages of AD patients in our study and those in González et al.22 for each genotype, no significant differences were observed, as the p-value was greater than 0.05 in all cases.
Another study that analyzed the distribution of genotypes by province was conducted by Reales et al.23, focusing on the Andalusia region, specifically the provinces of Granada and Huelva. Applying the same test, significant differences were observed between the two studies. Specifically, in both Granada and Huelva, there is a significantly lower percentage of individuals with the ε3/ε4 genotype (p-value < 0.001***), while the percentage of cases with the ε3/ε3 genotype is significantly higher (p-value < 0.01** and p-value < 0.001***, respectively). Additionally, in the case of Granada, there is a significantly lower percentage of individuals with the ε4/ε4 genotype compared to our study (p-value < 0.05*).
Discussion
The aim of this study was to analyze the allelic and genotypic frequencies and genetic distribution of the APOE gene in the provinces of León, Soria, and Salamanca, with a focus on understanding its implications for the prevention and management of AD. To provide a broader context, the findings were compared with similar studies conducted in other regions of Spain and Europe. Given the growing relevance of APOE in biomedical research, particularly in neurodegenerative diseases like AD, a genetic analysis was performed on individuals from the AFA associations in Soria, Salamanca, León, and Ponferrada, as well as from ‘Mensajeros de la Paz’ Residences in Mansilla de las Mulas and La Bañeza (León).
A total of 511 individuals were evaluated, of whom 260 had been diagnosed with AD and 251 were healthy. In both groups, the proportion of women was higher, reflecting the general trend of longer life expectancy in women and their greater susceptibility to AD24.
The higher proportion of widowed and the lower proportion of married people in the case group, as well as the reverse situation in the control group, indicate that marital status has a significant impact on the incidence of AD. Widowhood is associated with an increased risk of social isolation and emotional stress, factors that may contribute to cognitive decline. Conversely, being married may offer a protective effect, due to increased social support and continuous cognitive stimulation25,26.
The higher prevalence of lower educational levels in the case group suggests a correlation between lower educational level and higher risk of AD. The results obtained support the cognitive reserve hypothesis, which posits that higher education provides a greater ability to compensate for pathological changes in the brain27,28. Related to this is the higher proportion of AD cases residing in rural settings.
Analysis of APOE gene genotype frequencies in the provinces of León (including León and Ponferrada), Salamanca and Soria provides valuable information on the public health of Castilla y León in terms of the distribution of ε2, ε3 and ε4 allelic variables.
The most prevalent genotype in all populations was ε3/ε3, corresponding to 55.60% in León, 38.10% in Salamanca and 61.54% in Soria. The results show the expected correlation with what has been studied so far about the expression of the APOE locus, since the ε3 allele is considered to be the most prevalent29. As expected, the ε2/ ε2 genotype was found at the lowest percentage in all populations (0%), coinciding with the results obtained for Castilla y León by González et al.22 in 2020, and being similar to those included in the study by Reales23 for the populations of Huelva and Granada (0.66% and 0.59%, respectively). These results contradict the pre-established notion of a positive clinal distribution of the ε2 allelic variant towards southern Europe30.
Regarding the distribution of the ε4/ε4 genotype, the values observed in Leon (3.90%) and especially in Soria (0%), reflect the latitudinal trend in the European continent, where there is a positive gradient from south to north 30. It is worth noting that the 19.05% of the sample studied in Salamanca for this genetic variant represents one of the highest percentages reported to date in Spain.
Our findings regarding the distribution of the APOE alleles align with previous studies conducted in Spain and other European populations. For instance, our results confirm that the ε3 allele is the most prevalent in all studied provinces, consistent with findings from González et al.22 in Castilla y León. Additionally, the frequency of the ε4 allele follows the expected latitudinal trend observed in Europe, where its prevalence increases from south to north, as reported by Corbo & Scacchi30. However, our study also highlights some unexpected variations, such as the notably high ε4/ε4 genotype frequency in Salamanca (19.05%), which differs from previous reports in other Spanish regions, such as Barcelona, where Haddy et al.31 reported an ε4 allele frequency of 11%, or Galicia, where Garcés et al.32 defined this frequency as 8.30%. This indicates that the distribution of APOE gene polymorphism in the Iberian Peninsula is less predictable from a geographical perspective, as no clear meridional or latitudinal gradient is discernible.
Spain’s strategic location at the crossroads between Europe and Africa has facilitated significant interactions among diverse ethnic and cultural groups. As a result, each population that settled in Spain throughout various historical periods has left both a historical and genetic legacy. Prior research has suggested that the risk associated with the ApoE4 allele may be attenuated in individuals of African ancestry compared to those of European descent. Specifically, Rajabli et al.33 identified that African genetic ancestry at certain loci (17p13.2) may reduce the risk of AD in African American populations, though the overall proportion of African ancestry alone does not fully explain differences in AD prevalence. However, in our study, we did not assess the genetic ancestry of participants, and therefore, we cannot determine whether the African genetic legacy in different Spanish regions influences the distribution of APOE4 or its association with AD risk. Future studies incorporating ancestry-informative markers could provide valuable insights into how genetic background modulates the effects of APOE4 on AD risk across different regions.
The ε2/ε4 genotype is particularly rare due to the low frequency of the ε2 and ε4 alleles individually and the low probability of both being present in the same individual. This explains the percentages of 0% in Soria, 0.75% in León, 2.27% in Ponferrada and 4.76% in Salamanca.
The alleles of the APOE gene (ε2, ε3 and ε4) have different evolutionary origins. The ε4 allele is the most ancestral, present in the earliest human populations. With dispersal and adaptation to new environments, the ε3 and ε2 alleles emerged. Currently, the ε3 allele is the most common and provides an adaptive advantage in lipid metabolism. The ε2 allele, being more modern and less common, is associated with life extension and increased responsiveness to dietary fat34.
The frequency of the ε4 allele is determined by the percentages of the ε4/ ε4 and ε3/ ε4 genotypes. The difference between the León and Ponferrada areas for these two genotypes is particularly relevant: 2.99% and 6.82% respectively for ε4/ε4, as well as 31.34% and 45.45% respectively for ε3/ ε4. This may suggest a persistence of ancestral characteristics in this population due to factors such as geographical isolation and limited migratory flow from the Ponferrada area.
The low frequency of the ε2 allele supports the premise of being the most modern. Therefore, it is possible that the adaptive advantages it offers are not yet available to all populations.
The higher frequency of the ε4 allele in a province could be associated with an increased risk of developing AD in its population, as this allele is the main known genetic risk factor for the disease. Several studies have shown that carriers of the ε4 allele have a higher risk of developing Alzheimer’s at earlier ages compared to non-carriers35,36. While genetics play a crucial role, other modifiable and environmental factors also influence the development of AD in populations with a higher prevalence of the ε4 allele. Cardiovascular risk factors, such as high blood pressure, diabetes mellitus and hypercholesterolemia, as well as unhealthy dietary patterns, have been associated with both APOE4 and an increased risk of AD37,38. However, research also suggests that lifestyle interventions can mitigate this genetic risk. Adherence to a healthy lifestyle, including regular physical activity, a balanced diet, such as the Mediterranean diet, maintaining social engagement, participating in cognitively stimulating activities, and avoiding smoking and excessive alcohol consumption, has been associated with a slower rate of memory decline, even among APOE-ε4 carriers39.
Given the increased risk of AD associated with the APOE-ε4 allele, particularly in populations with a higher prevalence, public health strategies should emphasize early screening, lifestyle modifications, and preventive interventions. Evidence suggests that adherence to a structured lifestyle program—including a whole-food, minimally processed plant-based diet, regular aerobic and strength-training exercise, stress management techniques (e.g., meditation, yoga, and relaxation exercises), cognitive stimulation, and social support—can contribute to cognitive health and mitigate genetic risk factors. Additionally, targeted nutritional supplementation (e.g., omega-3 fatty acids, curcumin, vitamin B12, and magnesium) has been explored for its potential neuroprotective effects40. Implementing these interventions through public health initiatives could be particularly beneficial for at-risk populations, promoting brain health and reducing the burden of AD.
Conclusions
The results show significant differences in genotypic frequencies, suggesting genetic variability influenced by historical and migratory factors specific to each region. These findings reflect the persistence of ancestral characteristics in Ponferrada and Salamanca. Furthermore, the low frequency of the ε2 allele suggests reduced genetic protection against AD in the studied population, supporting its classification as a more modern variant.
Additionally, differences in AD prevalence between rural and urban areas underscore the need to enhance access to specialized health services and education about the disease in rural regions. The distribution patterns of APOE gene alleles in Castilla y León are consistent with trends observed in other European populations, facilitating comparisons with genetic studies across Europe.
Early identification of risk alleles enables the implementation of preventive and therapeutic strategies for AD. This, in turn, supports the development of effective prevention programs, improves patient’s quality of life and alleviates the burden on their families and the healthcare system.
Data availability
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. (American Psychiatric Association, 2013). https://doi.org/10.1176/appi.books.9780890425596.
World Health Organization. Global Status Report on the Public Health Response to Dementia: Executive Summary. (Geneva, 2021).
Galende, A. V. et al. Report by the Spanish Foundation of the Brain on the social impact of Alzheimer disease and other types of dementia. Neurología (English Edition). 36, 39–49 (2021).
Tahami, M. A. A., Byrnes, M. J., White, L. A. & Zhang, Q. Alzheimer’s disease: epidemiology and clinical progression. Neurol. Ther. 11, 553–569 (2022).
Jellinger, K. A. Neuropathological assessment of the Alzheimer spectrum. J. Neural. Transm. 127, 1229–1256 (2020).
Lane, C. A., Hardy, J. & Schott, J. M. Alzheimer’s disease. Eur. J. Neurol. 25, 59–70 (2018).
Sheppard, O. & Coleman, M. Alzheimer’s disease: etiology, neuropathology and pathogenesis. in Alzheimer’s disease: drug Discovery 1–23 (Exon Publications, Brisbane, 2020). https://doi.org/10.36255/exonpublications.alzheimersdisease.2020.
Khan, S., Barve, K. H. & Kumar, M. S. Recent advancements in pathogenesis, diagnostics and treatment of Alzheimer’s disease. Curr. Neuropharmacol. 18, 1106–1125 (2020).
Loeffler, D. A. Modifiable, non-modifiable, and clinical factors associated with progression of Alzheimer’s disease. J. Alzheimers. Dis. 80, 1–27 (2021).
Tcw, J. & Goate, A. M. Genetics of β-amyloid precursor protein in Alzheimer’s disease. Cold Spring Harb. Perspect Med. 7, a024539 (2017).
Alonso Vilatela, M. E., López-López, M. & Yescas-Gómez, P. Genetics of Alzheimer’s disease. Arch. Med. Res. 43, 622–631 (2012).
Villar, G., Canales, M. A. & Espinoza, J. C. Salivary biomarkers for early prognosis of Alzheimer’s disease. Int. J. Morphol 39, 1234 (2021).
Liang, D. & Lu, H. Salivary biological biomarkers for Alzheimer’s disease. Arch. Oral Biol. 105, 5–12 (2019).
Altuna-Azkargorta, M. & Mendioroz-Iriarte, M. Blood biomarkers in Alzheimer’s disease. Neurologia 36, 704–710 (2021).
Bloom, G. S. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 71, 505–508 (2014).
Das, H. K., McPherson, J., Bruns, G. A. P., Karathanasis, S. K. & Breslow, J. L. Isolation, characterization, and mapping to chromosome 19 of the human apolipoprotein E gene. J. Biol. Chem. 260, 6240–6247 (1985).
Koutsodendris, N., Nelson, M. R., Rao, A. & Huang, Y. Apolipoprotein E and Alzheimer’s Disease: findings, hypotheses, and potential mechanisms. Annu. Rev. Pathol. Mech. Dis. 17, 73–99 (2022).
Adroer, R. et al. Apolipoprotein E4 allele frequency in Spanish Alzheimer and control cases. Neurosci. Lett. 189, 182–186 (1995).
Bycroft, C., Fernandez-Rozadilla, C., Ruiz-Ponte, C. et al. Patterns of genetic differentiation and the footprints of historical migrations in the Iberian Peninsula. Nat. Commun. https://doi.org/10.1038/s41467-018-08272-w (2019).
Instituto Nacional de Estadística. Estadísticas de población en Castilla y León. Madrid. Instituto Nacional de Estadística https://ine.es/ (2024).
Pantelidis, P., Lambert-Hammill, M. & Wierzbicki, A. S. Simple Sequence-specific-Primer-PCR Method To Identify the Three Main Apolipoprotein E Haplotypes. Clin. Chem. 49, 1945–1948 (2003).
González, R. D. et al. ApoE variants in an Iberian Alzheimer cohort detected through an optimized sanger sequencing protocol. Genes (Basel) 12, 4 (2021).
Reales, G. et al. New insights into the distribution of APOE polymorphism in the Iberian Peninsula The case of Andalusia (Spain). Ann. Hum. Biol. 41, 443–452 (2014).
Barnes, L. L. et al. Sex Differences in the clinical manifestations of Alzheimer disease pathology. Arch. Gen. Psychiatry 62, 685–691 (2005).
Gerritsen, L. et al. Influence of negative life events and widowhood on risk for dementia. Am. J. Geriatr. Psychiatry 25, 766–778 (2017).
Lyu, J., Min, J. & Kim, G. Trajectories of cognitive decline by widowhood status among Korean older adults. Int. J. Geriatr. Psychiatry 34, 1582–1589 (2019).
Anderson, E. L. et al. Education, intelligence and Alzheimer’s disease: Evidence from a multivariable two-sample Mendelian randomization study. Int. J. Epidemiol. 49, 1163–1172 (2020).
Gonneaud, J. et al. Association of education with Aβ burden in preclinical familial and sporadic Alzheimer disease. Neurology 95, 1554–1564 (2020).
Gharbi-Meliani, A. et al. The association of APOE ε4 with cognitive function over the adult life course and incidence of dementia: 20 years follow-up of the Whitehall II study. Alzheimers Res. Ther. https://doi.org/10.1186/s13195-020-00740-0 (2021).
Corbo, R. M. & Scacchi, R. Apolipoprotein E (ApoE) allele distribution in the world. Is ApoE*4 a ‘thrifty’ allele?. Ann. Hum. Genet. 63, 301–310 (1999).
Haddy, N. et al. The importance of plasma apolipoprotein E concentration in addition to its common polymorphism on inter-individual variation in lipid levels: Results from Apo Europe. Eur. J. Hum. Genet. 10, 841–850 (2002).
Garcés, M. Estudio Sobre Las Enfermedades Neurodegenerativas En España y Su Impacto Económico y Social. (2016).
Rajabli, F. et al. Admixture mapping identifies novel Alzheimer’s disease risk regions in African Americans. Alzheimer’s Dementia 19, 2538–2548 (2023).
Eisenberg, D. T. A., Kuzawa, C. W. & Hayes, M. G. Worldwide Allele Frequencies of the Human apolipoprotein E Gene: Climate, Local Adaptations, and Evolutionary History. Am. J. Phys. Anthropol. 143, 100–111 (2010).
Yamazaki, Y., Zhao, N., Caulfield, T. R., Liu, C. C. & Bu, G. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat. Rev. Neurol. 15, 501–518 (2019).
Roses, A. D. & Saunders, A. M. APOE is a major susceptibility gene for Alzheimer’s disease. Curr. Opin. Biotechnol. 5, 663–667 (1994).
Norwitz, N. G., Saif, N., Ariza, I. E. & Isaacson, R. S. Precision nutrition for Alzheimer’s prevention in ApoE4 carriers. Nutrients 13, 1362 (2021).
Wanleenuwat, P., Iwanowski, P. & Kozubski, W. Alzheimer’s dementia: pathogenesis and impact of cardiovascular risk factors on cognitive decline. Postgrad Med. 131, 415–422 (2019).
Jia, J. et al. Association between healthy lifestyle and memory decline in older adults: 10 year, population based, prospective cohort study. BMJ 380, (2023).
Ornish, D. et al. Effects of intensive lifestyle changes on the progression of mild cognitive impairment or early dementia due to Alzheimer’s disease: a randomized, controlled clinical trial. Alzheimer’s Res. Ther. 16(1), 1–17 (2024).
Acknowledgements
We would like to thank the AFAs of Castilla y León and in the Residences of "Mensajeros de la Paz" for their support, in recruitment process of the individuals.
Funding
This work was supported by the “Consejo General de Enfermería” (grant ID: PNI_CGE11).
Author information
Authors and Affiliations
Contributions
I.P.P. carried out the conceptualization of the study, contributed to the writing of the original draft and participated in the data collection. L.S.V. conceptualized the study, performed the statistical analysis, carried out the formal investigation, supervised the work and secured funding. A.G.G. designed and implemented the methodology, carried out the formal analysis and contributed to the investigation. B.E. critically reviewed and edited the manuscript. I.C.V. supported the formal investigation and contributed to data collection. J.A.F.F. reviewed and edited the manuscript. C.M.M. participated in the review and editing of the manuscript. L.B.C. contributed to the conceptualization of the study, assisted in data interpretation, performed critical review and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study adhered to the principles outlined in the Declaration of Helsinki and received approval from the Institutional Review Board (Ethics Committee) of the University of León (protocol code “ETICA-ULE-021–2022,” approved on May 5, 2022). Additionally, the study was registered at ClinicalTrials.gov under the identifier NCT06275243.
Informed consent
Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pardo-Pérez, I., Sánchez-Valdeón, L., García-Gallego, A. et al. Regional analysis of APOE polymorphism in Alzheimer’s disease in Spain. Sci Rep 15, 14877 (2025). https://doi.org/10.1038/s41598-025-98323-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98323-2