Abstract
Precocious puberty is characterized by early sexual maturation in children before 8 years in girls and 9 years in boys. While puberty is initiated by the activation of the hypothalamic–pituitary–gonadal axis, precise mechanisms triggering the early activation of this axis in children with central precocious puberty (CPP) remain elusive. Here, we aimed to identify variables that may influence the risk of CPP. This retrospective cohort study utilized data from the Korean National Health Insurance Service and National Health Screening Program for Infants and Children and included 43,952 children with CPP and 854,749 controls. Participants were followed up until 2020 for CPP development to determine their height, weight, and head circumference measurements, as well as evaluate their physiological, emotional, cognitive, and social development. The birth weights for boys and girls with CPP were 0.09 and 0.06 kg lower than those of controls, respectively. Breastfeeding rates for children with CPP were lower than those for controls. Children with low birth weights (boys: odds ratio [OR] = 1.71, P < 0.0001; girls: OR 1.30, P < 0.0001) and those who were overweight (boys: OR 1.33, P = 0.0006; girls: OR 1.30, P < 0.0001) or obese (boys: OR 1.60, P < 0.0001; girls: OR 1.14, P < 0.0001) were more likely to develop CPP. Breastfeeding exerted a significant protective effect against CPP in girls (OR 0.95, P = 0.0003). Low birth weight and high body mass index were associated with CPP development.
Similar content being viewed by others
Introduction
While normal puberty typically begins at the ages of 8–13 years in girls1,2 and 9–14 years in boys3,4, precocious puberty is a condition characterized by early sexual maturation in children before the ages of 8 years in girls and 9 years in boys5. Puberty is initiated by the activation of the hypothalamic–pituitary–gonadal (HPG) axis, leading to the development of secondary sexual characteristics6. This can substantially impact a child’s physical7,8,9 and emotional development10,11. Therefore, it is essential to comprehensively clarify factors contributing to its onset for developing effective treatment strategies.
Despite attempts to identify the etiology of central precocious puberty (CPP), its exact cause remains unclear. During puberty, pulsatile secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus is a critical hormonal event that is regulated by the emergence of activators and suppression of inhibitors of GnRH secretion12. Several studies have documented the potential impact of early life stressors13, obesity14, high-fat diet14, and endocrine disrupting chemicals15 on the onset of CPP. However, despite these efforts, underlying mechanisms that trigger the early activation of the HPG axis in children with CPP remain unclear.
The medical community is yet to fully understand the etiology of CPP. CPP may have a multifactorial etiology, with different factors contributing to its onset. Thus, in this study, we aimed to assess early life factors influencing pubertal development, which may be crucial for effectively stratifying the risk of CPP and devising management strategies for children at high risk.
Methods
Data sources
The National Health Insurance Service (NHIS) provides medical insurance coverage to all Korean citizens to ensure a basic standard of living and improve their overall quality of life. Each individual is registered with a health insurance identification number and is offered insurance coverage for medical expenses. In Korea, the National Health Screening Program for Infants and Children (NHSPIC) is a periodic health examination developed to target the major causes of infant mortality and health issues throughout the lifespan16. This screening tool is completed by caregivers and is indicated for infants and children aged 4–6, 9–12, and 18–24 months and 2.5–3, 3.5–4, 4.5–5, and 5.5–6 years. The main examination items include height, weight, and head circumference measurements; physiological, emotional, cognitive, and social developmental evaluations; visual acuity measurement; hearing screening; nutrition; safety accidents; and critical developmental milestones. These check-ups have been successfully implemented nationwide as one of the primary clinical services for infants and children.
In this retrospective population-based cohort study, we examined enrollment and claims data from the population covered by the NHIS between 2008 and 2020 and those included in the first to seventh NHSPIC.
Participants
Children with CPP were defined as those who had been diagnosed with CPP (E22.8 or E30.1) and prescribed GnRH agonists. In the context of the health insurance criteria in Korea, children with CPP are eligible for treatment coverage by health insurance if (1) the Tanner stage is 2 or above; (2) the skeletal age is advanced compared with the chronological age; and (3) the luteinizing hormone peak during the GnRH stimulation test is ≥ 5.0 mIU/mL before the ages of 9 years in girls and 10 years in boys.
To account for the approximately 1.5-year lag time between parents recognizing puberty signs and the hospital diagnosis of CPP17, we only included girls and boys diagnosed before the ages of 9 and 10 years, respectively, when estimating CPP incidence. The control group included children who were not diagnosed with CPP. Owing to the use of anonymous identifying codes in compliance with South Korea’s Bioethics and Safety Act, the Institutional Review Board of Severance Hospital waived the requirement for review board approval for this study (approval number: 4-2021-0752). Due to the retrospective nature of the study, the Institutional Review Board of Severance Hospital waived the need for obtaining informed consent. All experiments were performed in accordance with relevant guidelines and regulations.
Intervention
In all participating cohorts, trained personnel determined the weight and length (if age ˂ 2 years) or standing height (if age ≥ 2 years) of the children following the standard protocols, which were used to derive the body mass index (BMI). Considering potential etiological relevance to health outcomes in later years, the growth of children across the following age periods was examined in the first 5 years of life: early infancy (4–6 months), late infancy (9–12 and 18–24 months), and early childhood (2.5–3, 3.5–4, 4.5–5, and 5.5–6 years). To determine the potential association between demographic characteristics and early-life factors in children, the model considered birth weight, household income, prematurity, breastfeeding, height, weight, and BMI.
Variables
This study was performed using data from the NHSPIC. Breastfeeding was determined by the response “YES” in the first check-up survey. Prematurity was defined as birth before 37 weeks of gestational age, and low birth weight was defined as < 2.5 kg at birth. For each age group, BMI was categorized as “underweight (below the 5th percentile),” “normal weight (between the 5th and 85th percentiles),” “overweight (between the 85th and 95th percentiles),” and “obese (above the 95th percentile).” We also reviewed additional variables from the NHSPIC, such as the consumption of solid food, milk, fruit juices, or beverages containing sugar; breakfast eating patterns; and daily habits related to eating behaviors. Additionally, we examined variables such as daily screen time for television or computer usage and playtime activities. However, given that no significant differences were observed in these variables between the CPP and control groups, they were excluded from the analysis. Also, to investigate the increasing prevalence of CPP in recent years, time trend by birth year was incorporated into the analytical model.
The Korean NHIS premium is based on the concept of taxation determined by income; therefore, household income is included in the NHIS dataset. The income-related information within the dataset was divided into 20 percentiles. For the current study, the top 5 percentiles (16th–20th percentiles) were designated as “high income,” whereas the remaining percentiles were classified as “middle and low income.”
Statistical analyses
First, the crude distributions of weight, height, and BMI were recorded by computing the mean of the total number of children in the reported national health screening. BMI is calculated by dividing an individual’s weight in kilograms by the square of their height in meters. To account for known sex differences in pubertal onset, we decided a priori to conduct separate analyses for boys and girls18. Multivariate logistic regression models were used to identify factors associated with CPP. Statistical analyses were performed using R software version 3.1.1 (R Foundation, Vienna, Austria) and SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). A P-value < 0.05 was considered statistically significant.
Results
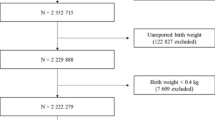
A total of 43,952 children with CPP were identified between 2008 and 2020. The control group comprised 854,749 children (Fig. 1). Among the children with CPP, 41,218 (93.8%) were girls, and 2734 (6.2%) were boys. The mean ages at CPP diagnosis were 8.43 ± 0.69 (range 1.75–9.00) and 9.73 ± 0.77 (range 3.42–10.00) years in girls and boys, respectively (Table 1).
The birth weights of boys and girls with CPP were 0.09 and 0.06 kg lower than those of children without CPP, respectively (boys: CPP = 3.13 kg, without CPP = 3.22 kg, P < 0.0001; girls: CPP = 3.11 kg, without CPP = 3.17 kg, P < 0.0001). The prevalence of premature birth was higher in boys with CPP than in those without CPP (CPP = 5.60%, without CPP = 3.79%, P < 0.0001). Breastfeeding rates were lower among boys and girls with CPP than among those without CPP (boys: CPP = 59.18%, without CPP = 62.69%, P = 0.0002; girls: CPP = 62.08%, without CPP = 63.91%, P < 0.0001). Height, weight, and BMI proportions in each group were similar throughout infancy to early childhood.
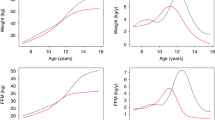
Children with and without CPP demonstrated significant differences in BMI from late infancy to early childhood (Table 2). Across all surveys, the CPP group had a small proportion of underweight and normal-weight children and a high proportion of children who were overweight and obese. Additionally, the CPP group exhibited a significantly faster timing of adiposity rebound (lowest BMI) (boys: without CPP, 6th check-up [54–60 months]; CPP, 5th check-up [42–48 months] [Fig. 2]; girls: without CPP, 6th check-up [54–60 months]; CPP, 4th check-up [30–36 months] [Fig. 3]).
In multivariate logistic regression models (Table 3), children with low birth weight (boys: odds ratio [OR] 1.71, P < 0.0001; girls: OR 1.30, P < 0.0001) and those who were overweight (boys: OR 1.33, P = 0.0006; girls: OR 1.30, P < 0.0001) or obese (boys: OR 1.60, P < 0.0001; girls: OR 1.14, P < 0.0001) were more likely to develop CPP than children with normal weight. Breastfeeding exerted a significant protective effect against CPP in girls (OR 0.95, P = 0.0003). Prematurity did not appear to be a significant risk factor for CPP; rather, it had a protective effect in girls (OR 0.88, P = 0.0011). Furthermore, the high-income group had a significantly lower probability of CPP occurrence than the middle- and low-income groups.
Discussion
In this retrospective population-based cohort study, which used data from the NHIS and NHSPIC from 2008 to 2020, we observed that several early life factors could impact the development of CPP. Factors associated with the increased risk of CPP were low birth weight, high BMI, and early BMI rebound. Conversely, breastfeeding appeared to exert a protective effect against CPP in girls.
The global prevalence of overweight, obesity, and metabolic syndrome in children and adolescents has increased considerably in recent years19,20,21,22. An association between higher childhood BMI and puberty development has been reported in both boys and girls23,24,25,26, and various mechanisms have been proposed as hypotheses. Adipose tissue is rich in aromatase, which can produce estrogens from adrenal androgen precursors. Also, obesity is associated with insulin-induced reductions of sex hormone binding globulin (SHBG), which increases bioavailability of sex steroids27. Moreover, obese children have high leptin levels, which accelerates gonadotropin-releasing hormone (GnRH) pulsatility in hypothalamic neurons and has a direct effect on the anterior pituitary gland28.
In the current study, we analyzed the association between obesity and precocious puberty by analyzing the timing of adiposity rebound. Adiposity rebound refers to the period when a child’s BMI reaches its lowest point before beginning to increase again. Typically, this occurs around 6 years of age in normal development. An early adiposity rebound, occurring before age 5.5 years, has been associated with several negative outcomes such as obesity and faster pubertal development29. Our study results also showed that the timing of adiposity rebound was faster in the CPP group compared to control for both boys and girls. In addition, multivariate logistic regression analysis reconfirmed that overweight and obesity are risk factors for CPP.
The relationship between birth weight and the risk of developing CPP has been reported previously. For example, children with a lower birth weight were found to be at a higher risk of developing CPP than those with a normal birth weight30. In a large population-based study on postnatal growth of Swedish children, girls who were underweight at birth or short for their gestational age reached puberty at a younger age (underweight, 10.7 ± 1.0 years; short, 10.6 ± 1.2 years) than normal-weight girls (11.1 ± 1.0, P = 0.02)31. Lazar et al. reported that children born small for gestational age reached puberty (girls = 10.4 ± 0.9; boys = 12.0 ± 0.9) significantly earlier than those born appropriate for gestational age (girls = 11.4 ± 1.3, P < 0.01; boys = 13.0 ± 1.1, P < 0.01)32. The adiposity rebound hypothesis suggests that children who experience rapid weight gain during infancy and early childhood may have a higher risk of developing CPP33. According to this hypothesis, children with low birth weight may experience catch-up growth during the first few years of life. Catch-up growth can lead to an increased risk of obesity34, which has been highlighted as a risk factor for CPP. In a previous nationwide population-based study conducted in Korea, any rapid weight gain from birth to 3 years of age was shown to contribute to an increased risk of CPP in girls35. Our data found similar findings regarding the difference in the timing of the BMI nadir between the control and CPP groups.
Prematurity (birth before 37 weeks of gestation) is reportedly associated with long-term health concerns, including growth failure, intellectual disabilities, and an increased risk of developing CPP36. Previous reports have supported the potential association between prematurity and CPP. Premature infants may be at an increased risk of developing CPP owing to the immaturity of their HPG axis, which regulates puberty. However, other studies have failed to establish a clear association between prematurity and CPP. Several studies have reported no difference in the timing of menarche between the preterm-born and term-born groups37,38,39, with one study even identifying a delay in menarche in the preterm-born group40. Likewise, the current study also revealed that prematurity was not a risk factor for CPP in either gender.
Recent studies have suggested that breastfeeding may be associated with a lower risk of developing CPP than formula feeding41,42. Breast milk contains various hormones and growth factors, including insulin-like growth factor-1, ghrelin, and leptin, that may help regulate appetite and body mass43. Upon comparing 6-day-old formula-fed and breastfed infants44, higher insulin levels were detected in formula-fed infants, demonstrating the role of formula-feeding in body fat accumulation. Breastfeeding provides healthy body fat levels by maintaining a balance between nutrients and bioactive substances, which may delay the onset of puberty and reduce the risk of CPP. Conversely, findings from a Hong Kong Chinese birth cohort study did not detect any notable association between breastfeeding or cow milk consumption at approximately 6 months, 3 years, or 5 years of age and at the onset of puberty45. In the current study, we observed that breastfeeding could exert a protective effect only in girls (OR 0.95, P = 0.003), with no clear association detected in boys (OR 0.92, P = 0.1073). This may be due to the substantially smaller sample size of boys than that of girls, indicating the need for further research with a larger sample.
According to the life history theory, humans are believed to have evolved a sensitivity to specific elements in their early childhood environments46. Exposure to diverse environments tends to influence children toward distinct reproductive strategies, potentially leading to early pubertal timing46. Household-level socioeconomic position (SEP) is often used as an indicator of childhood disadvantages47. The earlier reported inverse socioeconomic gradient observed in the National Health Examination Survey I (1959–1962) was found to have reversed, with SEP now associated with a younger age at menarche in the 2005–2008 National Health and Nutrition Examination Surveys48. Braithwaite et al. recently explored the variations in age at menarche based on socioeconomic status, which was determined by household income, parental education, and race49. In addition, cumulative exposure to highly unfavorable household SEP from birth was found to be associated with an increased risk of early puberty in boys and girls when compared with individuals from a more favorable background50. Consistent with previous studies, the current study indicates that exposure to highly unfavorable household SEP independently predicts an elevated likelihood of developing early precocious puberty.
The strength of this study lies in the use of national data drawn from a large sample, including 112,026 children diagnosed with CPP in South Korea during the analysis period. This study has the largest sample size among all studies conducted to date on the etiology of precocious puberty. Furthermore, a notable advantage is that the empirical analysis considers a range of explanatory variables that have been identified as primary factors influencing CPP in previous studies. However, it is important to acknowledge the limitations of this retrospective cohort study, which relied on recall surveys. Potential limitations include recall bias, incomplete data, loss to follow-up, and possible inaccuracies in exposure assessment. For example, gestational age and birth weight values were frequently missing; hence, they were treated as separate, independent variables in the analysis. Additionally, the data provided by NHIS lacked information on specific bone age and tanner stage, which made it difficult to conduct a detailed analysis of the study group.
In Korea, the NHIS defines CPP based on the following criteria: onset of secondary sexual characteristics before the ages of 8 years in girls and 9 years in boys, advanced skeletal age compared to chronological age, and sex hormone levels that have risen to pubertal levels before the ages of 9 years in girls and 10 years in boys. However, because it is difficult to clearly prove the exact time of first appearance of secondary sexual characteristics, NHIS defined the diagnostic criteria for CPP as 9 years of age for girls and 10 years of age for boys based on the time of the GnRH stimulation test. This is slightly different from the internationally accepted standard, which reflects the difference between the time when secondary sexual characteristics are observed and the time when actual tests and diagnoses are conducted. In Korea, treatment costs are low, and access to treatment is good, ensuring that most patients diagnosed with CPP receive treatment. However, this assumption has not been statistically confirmed, potentially leading to bias between diagnosis and treatment. Given that the data was limited to diagnoses and medication administration, it was not possible to assess disease progression. This limitation could introduce bias, particularly because, in CPP, treatment decisions are markedly influenced by disease progression.
This study provides insights indicating that early life factors, such as low birth weight, and high BMI, are associated with the development of CPP, while breastfeeding appears to have a protective effect. The findings underscore the importance of early life experiences in shaping future health outcomes of children, emphasizing the relevance of precise history-taking and regular monitoring from birth. However, it is crucial to recognize the inherent limitations of this study, which adopts a retrospective cohort design relying on recall surveys. Given the retrospective nature of the study design, further research is warranted to meticulously assess the causal relationship between these identified early life factors and the onset of CPP.
Data availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
Biro, F. M. et al. Onset of breast development in a longitudinal cohort. Pediatrics 132(6), 1019–1027 (2013).
Marshall, W. A. & Tanner, J. M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 44(235), 291–303 (1969).
Herman-Giddens, M. E. et al. Secondary sexual characteristics in boys: data from the Pediatric Research in Office Settings Network. Pediatrics 130(5), e1058–e1068 (2012).
Marshall, W. A. & Tanner, J. M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 45(239), 13–23 (1970).
Parent, A. S. et al. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr. Rev. 24(5), 668–693 (2003).
Latronico, A. C., Brito, V. N. & Carel, J. C. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. 4(3), 265–274 (2016).
Carel, J. C., Lahlou, N., Roger, M. & Chaussain, J. L. Precocious puberty and statural growth. Hum. Reprod. Update. 10(2), 135–147 (2004).
Prentice, P. & Viner, R. M. Pubertal timing and adult obesity and cardiometabolic risk in women and men: A systematic review and meta-analysis. Int. J. Obes. (Lond). 37(8), 1036–1043 (2013).
Lakshman, R. et al. Early age at menarche associated with cardiovascular disease and mortality. J. Clin. Endocrinol. Metab. 94(12), 4953–4960 (2009).
Mendle, J., Turkheimer, E. & Emery, R. E. Detrimental psychological outcomes associated with early pubertal timing in adolescent girls. Dev. Rev. 27(2), 151–171 (2007).
Mensah, F. K. et al. Early puberty and childhood social and behavioral adjustment. J. Adolesc. Health. 53(1), 118–124 (2013).
Avendano, M. S., Vazquez, M. J. & Tena-Sempere, M. Disentangling puberty: novel neuroendocrine pathways and mechanisms for the control of mammalian puberty. Hum. Reprod. Update. 23(6), 737–763 (2017).
Soriano-Guillen, L. et al. Central precocious puberty in children living in Spain: Incidence, prevalence, and influence of adoption and immigration. J. Clin. Endocrinol. Metab. 95(9), 4305–4313 (2010).
Calcaterra, V., Magenes, V. C., Hruby, C., Siccardo, F., Mari, A., Cordaro, E., et al. Links between childhood obesity, high-fat diet, and central precocious puberty. Children (Basel). 10(2) (2023).
Gore, A. C. et al. EDC-2: The endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 36(6), E1–E150 (2015).
Moon, J. S. Review of national health screening program for infant and children in Korea. JKMA. 53(5), 377–385 (2010).
Xhrouet-Heinrichs, D. et al. Longitudinal study of behavioral and affective patterns in girls with central precocious puberty during long-acting triptorelin therapy. Acta Paediatr. 86(8), 808–815 (1997).
Fechner, P. Y. Gender differences in puberty. J. Adolesc. Health. 30(4 Suppl), 44–48 (2002).
Kim, H. Y. & Kim, J. H. Temporal trends in the prevalence of metabolically healthy overweight and obesity in Korean youth: Data from the Korea National Health and Nutrition Examination Survey 2011–2019. Ann. Pediatr. Endocrinol. Metab. 27(2), 134–141 (2022).
Di Cesare, M. et al. The epidemiological burden of obesity in childhood: A worldwide epidemic requiring urgent action. BMC Med. 17(1), 212 (2019).
Apperley, L. J. et al. Childhood obesity: A review of current and future management options. Clin. Endocrinol. (Oxf). 96(3), 288–301 (2022).
Song, K. et al. Changes in the prevalences of obesity, abdominal obesity, and non-alcoholic fatty liver disease among Korean children during the COVID-19 outbreak. Yonsei Med. J. 64(4), 269–277 (2023).
Huang, A., Reinehr, T. & Roth, C. L. Connections between obesity and puberty: Invited by manuel Tena-Sempere, Cordoba. Curr. Opin. Endocr. Metab. Res. 14, 160–168 (2020).
Aksglaede, L., Sorensen, K., Petersen, J. H., Skakkebaek, N. E. & Juul, A. Recent decline in age at breast development: The Copenhagen Puberty Study. Pediatrics 123(5), e932–e939 (2009).
De Leonibus, C. et al. Timing of puberty and physical growth in obese children: A longitudinal study in boys and girls. Pediatr. Obes. 9(4), 292–299 (2014).
Lee, J. M. et al. Weight status in young girls and the onset of puberty. Pediatrics 119(3), e624–e630 (2007).
Burt Solorzano, C. M. & McCartney, C. R. Obesity and the pubertal transition in girls and boys. Reproduction 140(3), 399–410 (2010).
Shalitin, S. & Phillip, M. Role of obesity and leptin in the pubertal process and pubertal growth—A review. Int. J. Obes. Relat. Metab. Disord. 27(8), 869–874 (2003).
Kang, M. J. The adiposity rebound in the 21st century children: Meaning for what?. Korean J. Pediatr. 61(12), 375–380 (2018).
Deng, X. et al. Association between small fetuses and puberty timing: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health. 14(11), 1377 (2017).
Persson, I. et al. Influence of perinatal factors on the onset of puberty in boys and girls: implications for interpretation of link with risk of long term diseases. Am. J. Epidemiol. 150(7), 747–755 (1999).
Lazar, L., Pollak, U., Kalter-Leibovici, O., Pertzelan, A. & Phillip, M. Pubertal course of persistently short children born small for gestational age (SGA) compared with idiopathic short children born appropriate for gestational age (AGA). Eur. J. Endocrinol. 149(5), 425–432 (2003).
Williams, S. & Dickson, N. Early growth, menarche, and adiposity rebound. Lancet 359(9306), 580–581 (2002).
Martin-Calvo, N., Goni, L., Tur, J. A. & Martinez, J. A. Low birth weight and small for gestational age are associated with complications of childhood and adolescence obesity: Systematic review and meta-analysis. Obes. Rev. 23(Suppl 1), e13380 (2022).
Choe, Y. et al. Rapid weight gain in early life is associated with central precocious puberty in girls, not in boys—a nationwide population-based study in Korea. Front. Endocrinol. (Lausanne). 14, 1210995 (2023).
Han, J. H. et al. Application of machine learning approaches to predict postnatal growth failure in very low birth weight infants. Yonsei Med. J. 63(7), 640–647 (2022).
Dossus, L. et al. Determinants of age at menarche and time to menstrual cycle regularity in the French E3N cohort. Ann. Epidemiol. 22(10), 723–730 (2012).
D’Aloisio, A. A., DeRoo, L. A., Baird, D. D., Weinberg, C. R. & Sandler, D. P. Prenatal and infant exposures and age at menarche. Epidemiology 24(2), 277–284 (2013).
Atay, Z., Turan, S., Guran, T., Furman, A. & Bereket, A. Puberty and influencing factors in schoolgirls living in Istanbul: End of the secular trend?. Pediatrics 128(1), e40–e45 (2011).
Hui, L. L., Leung, G. M., Lam, T. H. & Schooling, C. M. Premature birth and age at onset of puberty. Epidemiology 23(3), 415–422 (2012).
Kale, A. et al. Breastfeeding versus formula-feeding and girls’ pubertal development. Matern. Child Health J. 19(3), 519–527 (2015).
Aghaee, S. et al. Breastfeeding and timing of pubertal onset in girls: A multiethnic population-based prospective cohort study. BMC Pediatr. 19(1), 277 (2019).
Lawrence, R. A. Does breastfeeding protect against overweight and obesity in children? A review. Childhood Obes. 6(4), 193–197 (2010).
Lucas, A., Boyes, S., Bloom, S. R. & Aynsley-Green, A. Metabolic and endocrine responses to a milk feed in six-day-old term infants: Differences between breast and cow’s milk formula feeding. Acta Paediatr. Scand. 70(2), 195–200 (1981).
Kwok, M. K., Leung, G. M., Lam, T. H. & Schooling, C. M. Breastfeeding, childhood milk consumption, and onset of puberty. Pediatrics 130(3), e631–e639 (2012).
Belsky, J., Steinberg, L. & Draper, P. Childhood experience, interpersonal development, and reproductive strategy: and evolutionary theory of socialization. Child Dev. 62(4), 647–670 (1991).
Cleland, V. J., Ball, K., Magnussen, C., Dwyer, T. & Venn, A. Socioeconomic position and the tracking of physical activity and cardiorespiratory fitness from childhood to adulthood. Am. J. Epidemiol. 170(9), 1069–1077 (2009).
Krieger, N. et al. Age at menarche: 50-year socioeconomic trends among US-born black and white women. Am. J. Public Health. 105(2), 388–397 (2015).
Braithwaite, D. et al. Socioeconomic status in relation to early menarche among black and white girls. Cancer Causes Control. 20(5), 713–720 (2009).
Sun, Y., Mensah, F. K., Azzopardi, P., Patton, G. C., Wake, M. Childhood social disadvantage and pubertal timing: A national birth cohort from Australia. Pediatrics. 139(6) (2017).
Funding
This study was supported by a grant from Yonsei University College of Medicine (6-2022-0160) and Hankuk University of Foreign Studies Research Fund (2024).
Author information
Authors and Affiliations
Contributions
M.L., J.K.: Conceptualization, methodology, writing-original draft preparation; H.K.: Data curation, software, validation; J.S., J.S.: Supervision, writing-reviewing and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Institutional Review Board of Severance Hospital (approval number: 4-2021-0752).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, M., Kim, J., Kim, H. et al. Early life factors of precocious puberty based on Korean nationwide data. Sci Rep 15, 16165 (2025). https://doi.org/10.1038/s41598-025-98529-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98529-4