Abstract
The immune function is suspected to play an important role in the health effects of air pollution but it remains poorly investigated in pregnant women. One-week personal measurements of exposure to nitrogen dioxide (NO2), particulate matter with an aerodynamic diameter of ≤ 2.5 µm mass concentration (PM2.5) and PM2.5 oxidative potential (OP) were assessed in 270 pregnant women from the French cohort SEPAGES. PM filters were analyzed for PM2.5 OP using the dithiothreitol (DTT) and the ascorbic acid (AA) assays. From a blood sample withdrawn at the end of the exposure measurement week, levels of 29 cytokines and chemokines were measured at baseline and after T cell and dendritic cell activation with phytohemagglutinin (PHA) and resiquimod (R848), respectively. Associations between each air pollutant and each cytokine were assessed using adjusted linear regression models. An increase in NO2 exposure was associated with higher interleukin 10 (IL-10) and lower PHA-activated tumor necrosis factor (TNF). No association with PM2.5 concentration was observed, but increased exposure to PM \({\text{OP}}^{{{\text{AA}}}}\) was associated with lower baseline and R848-activated IL-8 and increased exposure to PM \({\text{OP}}^{\text{DTT}}\) was associated with higher PHA-activated IL-17A. Our study provides insights into the relationships between air pollution exposure and immune function among pregnant women.
Similar content being viewed by others
Introduction
Exposure to atmospheric pollutants, such as particulate matter (PM) and nitrogen dioxide (NO2), affects a number of human systems and organs, and contributes to a significant part of premature death worldwide1. Oxidative stress and the modulation of the immune system are two inter-related mechanisms through which air pollutants exert their deleterious effects on health2,3,4. Oxidative stress, by an excess of reactive oxygen species, can damage lipoproteins or lipids in membranes, leading to the formation of oxidation-specific epitopes recognized by pattern recognition receptors of the innate immune system5. This can enhance inflammatory mechanisms, thereby leading to a cyclic generation of oxidative stress and inflammation6.
Most of the research carried out on immune system has focused on the assessment of ambient particles, notably PM10 and PM2.5, and gases (NO2). Epidemiological studies showed that exposure to NO2 was associated with increased pro-inflammatory cytokines, such as IL-6 and TNF-α7,8. Previous studies highlighted that ambient exposure to fine particles was associated with an intensification of inflammatory responses and an increase in B lymphocytes9,10. The oxidative potential (OP) of PM, a metric developed to mimic oxidative stress generation in the interstitial lung fluid after PM inhalation, was assessed in very few epidemiological studies addressing its relationship with human immune system. OPAA, OPDTT and OPDCFH from ambient PM were found associated with elevated expression of pro-inflammatory biomarkers in lung epithelial cells11,12. To the best of our knowledge, four studies estimated associations between OP of PM and immune system biomarkers in humans and showed that OP of PM was positively associated with plasma IL-613, blood IL-6 expression14 and IL-6 levels in nasal fluids15, while Steenhof et al.16 did not find any association with blood IL-6, nor with nasal IL-6 and IL-8. Overall, most studies tend to converge towards a pro-inflammatory effect of OP exposure, but they mostly relied on the expression of IL-6 and a relatively small sample size.
Pregnant women constitute a sensitive population to air pollution, primarily due to the modifications that occur in their immune systems during pregnancy, including a modification on cytokine production17. However, there is a notable lack of available data regarding effects of exposure to air pollution on the immune system in this particular population. Furthermore, it is especially relevant to delve into the mechanisms involved in the health effects of air pollution in pregnant women, as prenatal exposure to air pollution influences the development of child health and immune function18,19,20.
The aim of this study was to assess the associations between personal exposure to air pollutants (NO2, PM2.5 concentration and PM OP) during pregnancy and immune function measured both at the baseline state and after T cell and dendritic cell activation.
Materials and methods
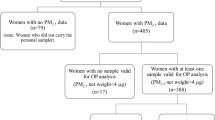
Study population
This study is based on data from the SEPAGES French parent–child cohort. The population of the study lives within 80 km around the center of Grenoble in the Alpes, France. Briefly, 484 pregnant women, with pregnancy duration less than 19 weeks and with a singleton pregnancy, older than 18 years old and affiliated to the French national security system, were recruited between 2015 and 201721. Their partner and children were also recruited. Sociodemographic and health data were collected using a combination of questionnaires, interviews, and clinical examinations during and after pregnancy. Exposure information were collected using personal samplers and immunological information were collected using blood samples. The present analysis is based on 270 mothers with exposure assessed to at least one of the measured air pollutants during pregnancy and immunological measurements at the end of the exposure assessment week (Fig. 1). To be included in the analysis, mothers had to have the blood samples collected within 2 days after the pollutant sampling period to assess the immune system’s status in relation to the exposure measurements. Participants signed an informed consent and the study protocol received approval from the French data privacy institution (Commission Nationale de l’Informatique et des Libertés, CNIL—n°914138) and the Comité de Protection des Personnes Sud-Est V (CPP—2013-A01491-44). All methods were performed in accordance with the relevant guidelines and regulations.
Personal exposure assessment to air pollutants
Women in the SEPAGES cohort wore or kept nearby personal air samplers placed in a wearable backpack to measure their personal exposures to PM2.5 (MicroPEM™ active air sampler; RTI International, USA) and NO2 (Passam AG passive air sampler; Switzerland) for 7 (13%) to 8 (87%) consecutive days22,23,24. The measurement week took place during the second (81%) or the third trimester (19%) of pregnancy (median [min–max] gestational age = 19 weeks14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36).
NO2 concentration was measured using spectrophotometry following established methods25. PM2.5 filters from the MicroPEM were weighed before and after sampling at RTI International (USA), using a microbalance (Mettler Toledo UMX2) placed in an environmental chamber maintained at a temperature of 21 °C and 35% relative humidity. PM2.5 mass concentration was calculated by dividing PM mass collected during the week and measured by gravimetric analysis, by the air volume sampled during the measurement week (µg/m3). PM filters were cold-stored until OP analysis. The protocol for OP measurement was previously published22,24, based on the protocol established by Calas et al.26,27. Briefly, PM2.5 were extracted from filters into a simulated interstitial lung fluid consisting in a mixture of 1,2-dipalmitoylphosphatidylcholine (DPPC), to reach a final concentration of 10 µg/mL. Extracts were incubated at 37 °C for 75 min under vortex agitation prior to analysis using the dithiothreitol (DTT) and the ascorbic acid (AA) assays. A 96-well plate (CELLSTRAR, Greiner-Bio) was used to mix the extracts with DTT or AA solutions. For the AA assay, the absorbance at 265 nm (TECAN spectrophotometer Infinite M200 Pro) is measured over time to evaluate AA consumption by PM2.5 extract, for a total reaction time of 30 min. For the DTT assay, the absorbance at 412 nm measured the formation of the 2-nitro-5-thiobenzoic acid (TNB), which is the reaction product of the remaining DTT and dithionitrobenzoic acid (DTNB), for a total reaction time of 30 min. Samples were analyzed in triplicates, and the mean was calculated for each sample. For both assays, consumption rates were then normalized by the mass of PM of the extract (OPm, in nmol/min/µg), or by the corresponding air volume sampled (OPv, nmol/min/m3).
Maternal immune function
Blood samples were collected by trained field workers, within a maximum of 48 h after the end of the exposure measurement week, following the procedure published by Manches et al.20. Briefly, blood was collected in BD Medical 368886 vacutainer tubes (lithium heparin) for immunological analyses (cell culture and plasma separation), and in BD Medical 368861 vacutainer tubes (EDTA) for cell counting. They were transported to the Etablissement Français du Sang (EFS) in coolers, placed on a rotating device for at least 5 min to ensure homogeneous cell content, and were then processed within 24 h after collection.
Innate and adaptative immunity of the women were measured at baseline and after a 24-h ex vivo activation of whole blood at 37 °C using dendritic cell activator Resiquimod (R848, InvivoGen, 5 μg/mL) and T cell activator phytohaemagglutinin (PHA, Oxoid, 10 μg/mL), as previously described by Manches et al.20.
Briefly, cytokines were measured in the culture supernatant (for activated cells) or in plasma (for baseline cytokines) by cytometric bead arrays (BD™ CBA Human cytokines Flex Set that is a bead-based immunoassay capable of simultaneously measuring several cytokines in biological fluids, BD Biosciences).
Among the 29 cytokines that were measured (12 at baseline, 9 after PHA-activation and 8 after R848 activation) only those with at least 70% of detected values were considered20. Hence, for the samples activated with PHA, the overall activity of T lymphocytes (T helpers Th1, Th2, Th9, Th17, and regulatory Treg) was assessed by quantifying the levels of IL-2, TNF-α, interferon (IFN) γ, IL-13, IL-17a, IL-9 and IL-10. For the samples activated with R848, the overall activity of dendritic cells was evaluated by quantifying TNF-α, IL-10, IL-6, IL-8, IFN-α, IFN-γ, IL-1β, and IL-12p70. For the non-activated sample, the basal state of the immune system was quantified by IL-8, monocyte chemoattractant protein-1 (MCP1) and regulated on activation, normal T cell expressed and secreted (RANTES). The concentrations below the limit of detection were imputed by a fill-in approach, that randomly selects values between 0 and the LOD based on the underlying distribution28,29. Due to their skewed distribution, cytokine concentrations were log10 transformed.
Since between-participant technical variability related to the experimentation can lead to measurement error, a two-step standardization method based on regression residuals30 was used to correct, when necessary, cytokine concentrations. The same standardized variables as previously described by Manches et al.20 were used. Briefly, the technical variables considered were: (1) for baseline cytokines: analytical batch, time between sample collection and reception, time between sample reception and analysis; (2) for activated cytokines the same variables were used, together with the duration of the activation, R848 or PHA age at the time of sample activation, and storage duration.
Statistical analysis
Summary statistics (mean [sd] or median [Q1-Q3]) were calculated for air pollutant exposure assessments, cytokine levels, and covariates. A correlation matrix (Pearson’s r) was calculated between the cytokine levels and between the air pollutant concentrations. Univariate and adjusted linear regressions were conducted to estimate the associations between each air pollutant exposure and each cytokine level. Each exposure and log-transformed cytokine variable were divided by the interquartile range (IQR), to facilitate comparison of the beta estimates. Potential confounders were selected from the existing literature31,32,33, and using a directed acyclic graph (see Supplementary Fig. S1 online) and included: age of the women (continuous), BMI before pregnancy (continuous), active or passive smoking (active smoking in the 12 months prior to pregnancy, or active or passive smoking during pregnancy; binary: yes/no), educational level (binary: < master’s degree, ≥ master’s degree), leukocyte count (continuous), gestational age at sampling (continuous), and sampling season (4 categories with winter corresponding to January-March, spring to April-June, summer to July–September, and fall to October-December). To avoid reduction of the sample size due to missing data in cofactors (20 individuals had missing value for at least one covariate), multiple imputations (n = 20 datasets) were performed using Multivariate Imputation by Chained Equations (package mice, R).
In addition, sensitivity analyses were carried out to assess the robustness of the results to: (1) extreme values (after exclusion of 1% lowest and 1% highest exposure and cytokine concentrations), (2) influential values (after exclusion of values with a Cook’s distance exceeding 4/n, with n being the number of participants in the main analysis), (3) the set of confounders, with models excluding the leucocyte counts among cofactors and models including history of asthma and rhinitis which could lie in the causal path between air pollution and cytokine levels, and (4) considering bi-pollutant models, e.g. adjusting for NO2 in the PM2.5 and OP models, and adjusting for PM2.5 or OP in the NO2 models. Following statistical recommendations, interpretation of statistical tests was based on examining effects’ magnitude and their 95% confidence intervals and precise p-values (not whether p-values are above or below 0.05)34,35. All analyses were conducted using the statistical software R (version 4.2).
Results
Population characteristics
The population studied included 270 pregnant women with a median age of 32.1, a median BMI of 21.6 and a high educational level (58% had a diploma equivalent to or higher than a master’s degree) (Table 1). Among these women, 9.5% were active smokers before or during pregnancy. Regarding respiratory health, 16% reported asthma symptoms before or during pregnancy, and 40% reported rhinitis.
Exposure to NO 2 , PM 2.5 and OP
Median (Q1-Q3) exposure to NO2, PM2.5, \({\text{OP}}_{\text{m}}^{\text{DTT}}\), \({\text{OP}}_{\text{v}}^{\text{DTT}}\), \({\text{OP}}_{\text{m}}^{\text{AA}}\) and \({\text{OP}}_{\text{v}}^{\text{AA}}\) were 20.2 (16.2–25.8) µg/m3, 13.8 (9.9–18.5) µg/m3, 0.11 (0.09–0.14) nmol/min/µg, 1.48 (1.06–2.00) nmol/min/m3, 0.12 (0.08–0.16) nmol/min/µg and 1.65 (0.98–2.63) nmol/min/m3, respectively (Table 2). A seasonal trend was observed during the cold season with higher concentrations of all studied pollutants. \({\text{OP}}_{\text{v}}^{\text{AA}}\) presents a strong Pearson correlation coefficient (r > 0.5) with \({\text{OP}}_{\text{v}}^{\text{DTT}}\) and with \({\text{OP}}_{\text{m}}^{\text{AA}}\), whereas \({\text{OP}}_{\text{v}}^{\text{DTT}}\) is strongly correlated with PM2.5; and \({\text{OP}}_{\text{m}}^{\text{AA}}\) with \({\text{OP}}_{\text{m}}^{\text{DTT}}\) (Fig. 2). Moderate Pearson correlation coefficients (0.30 < r < 0.50) were observed between \({\text{OP}}_{\text{v}}^{\text{DTT}}\) and \({\text{OP}}_{\text{m}}^{\text{DTT}}\) or \({\text{OP}}_{\text{m}}^{\text{AA}}\), and between \({\text{OP}}_{\text{v}}^{\text{AA}}\) and PM2.5. Remaining correlations were considered weak (r < 0.3).
Pairwise Pearson’s correlations between air pollutants (a). Pairwise Pearson’s correlations between cytokines (imputed, corrected and log-10 transformed) and number of leukocytes (b). The Pearson correlation coefficients are indicated using a scale of size and color only when the p-values were less than 0.05. Abbreviations: PM2.5 particulate matter with diameter ≤ 2.5 μm; NO2 nitrogen dioxide; OP: oxidative potential; DTT: dithiothreitol; AA: ascorbic acid; IL interleukin; IFN interferon; TNF tumor necrosis factor; RANTES regulated on activation, normal T cell expressed and secreted; MCP monocyte chemoattractant protein; PHA phytohemagglutinin; R848 resiquimod
Cytokine levels
Regarding raw concentrations of cytokines, women exhibited median concentrations ranging from 17.8 (IL-8) to 14,026.3 (RANTES) pg/mL at the basal state, from 35.8 (IL-9) to 2664.7 (IL-2) pg/mL after PHA activation, and from 22.5 (IL-12p70) to 44,667.9 (IL-6) after R848 activation (Table 3). Regarding imputed, corrected and log-10 transformed cytokine concentrations, the intra-group (basal, PHA activated, R848 activated) correlations at the basal level displayed a low correlation (−0.1 < Pearson r < 0.2), correlations after PHA activation were moderate-to-strong (r > 0.4) and correlations after R848 activation were weak-to-moderate (0.2 < r < 0.4) (Fig. 2). The inter-group correlation was very weak (r < 0.1), except for IL-8 between basal state and R848-activated measures (r = 0.37), IFN-γ between PHA- and R848- activated measures (r = 0.44) and between PHA-activated IL-2 and R848-activated IL-6, IL-1β, and IL-12p70 (0.22 < r < 0.27). A negative correlation was also observed between IL-10 secretion after PHA activation and IL-8 secretion at basal state and after R848 activation (r = −0.15 and −0.16, respectively).
Associations between personal exposures to air pollutants and immune function parameters
Univariate analyses are presented in Supplementary Tables online. In multivariate analyses (Fig. 3), a 10 µg/m3 (IQR) increase in NO2 exposure was associated with higher PHA-activated IL-10 and lower PHA-activated TNF-α (β [95% CI] = 0.18 [0.03, 0.32], p = 0.02; β [95% CI] = −0.18 [−0.32, −0.02], p = 0.03, respectively). No association was observed with PM2.5 concentration, but increased exposure to PM \({\text{OP}}_{\text{v}}^{\text{AA}}\) (IQR = 1.65 nmol/min/m3) was associated with lower IL-8 measured upon R848 activation (β [95% CI] = −0.17 [−0.33, 0.00], p = 0.05), and a similar trend was observed for basal IL-8 levels (β [95% CI] = −0.18 [−0.41, 0.06], p = 0.14). An IQR-increase in exposure to \({\text{OP}}_{\text{m}}^{AA}\) (IQR = 0.08 nmol/min/µg) was associated with lower R848-activated IL-8 (β [95% CI] = −0.12 [−0.24, 0.00], p = 0.05). Finally, \({\text{OP}}_{\text{m}}^{\text{DTT}}\) was associated with higher PHA-activated IL-17A (β [95% CI] = 0.11 [0.00, 0.22], p = 0.04), and a similar trend of association was observed for \({\text{OP}}_{\text{v}}^{\text{DTT}}\) and \({\text{OP}}_{\text{m}}^{AA}\) (β [95% CI] = 0.08 [−0.04, 0.20], p = 0.18; β [95% CI] = 0.10 [−0.01, 0.22], p = 0.07, respectively) (see Supplementary Tables online).
Adjusted associations between each immunological parameter and each personal exposure to NO2, PM2.5, OPDTT and OPAA during pregnancy. Pollutants and cytokines variables were standardized by their IQR. Beta values and their 95% CI were estimated by multiple linear regression models. Models were adjusted on women age, BMI, active or passive smoking, educational level, white blood cell count, gestational age at sampling, and sampling season. Abbreviations: PM2.5 particulate matter with diameter ≤ 2.5 μm; NO2 nitrogen dioxide; OP oxidative potential; DTT, dithiothreitol; AA, ascorbic acid; CI, confidence interval; IQR, interquartile; BMI, body mass index; Il, interleukin; IFN interferon; TNF tumor necrosis factor; RANTES regulated on activation, normal T cell expressed and secreted; MCP monocyte chemoattractant protein; PHA phytohemagglutinin; R848 resiquimod.
Overall, results were robust, as displayed by the sensitivity analyses. Only the association between NO2 and PHA activated IL-10 disappeared with the exclusion of extreme and influential values and in the models adjusted on PM or OP. Other findings were either similar, or stronger, as for the NO2-TNF association (see Supplementary Fig. S2 online). The other models excluding the leucocyte counts among cofactors, and including history of asthma and rhinitis, provided the same results as the main models.
Discussion
Comparison with others studies
To our knowledge, this is the first study investigating the association between personal exposures to PM2.5, OP of PM2.5, and NO2, with basal and activated immune function parameters in pregnant women. For PM2.5 and NO2, personal exposure levels are in the range of typical ambient exposure levels, as reported in the ELAPSE project, pooling eight European cohorts36. Our results show that increase in NO2 exposure was associated with higher PHA-activated TNF–α. Similarly, an increase in \({\text{OP}}_{\text{m}}^{\text{AA}}\), as well as in \({\text{OP}}_{\text{v}}^{\text{AA}}\), led to increase in R848-activated IL-8. Finally, a positive association was suggested between PHA-activated IL-17A with the three OP measurements. Interestingly, the study did not find any significant associations with PM2.5, but it did reveal significant associations with the capacity of PM to induce oxidative stress, as measured by OP.
The mechanisms involved in the health impacts of air pollutants include impairment of the innate and adaptative immunity, and the activation of oxidative stress and reactive oxygen species37. Adaptive immunity and oxidative stress closely interact with each other, these two mechanisms being triggered independently but having effects on each other. By investigating the effects of NO2, PM2.5 and OP of PM2.5 exposure on immunological parameters, our study was able to provide a complete picture of the cross-interaction of oxidative stress and adaptative immunity pathways.
The results observed in the current study, showing decreased TNF-α levels upon NO2 exposure, are in line with previous research in non-pregnant adults31. However, positive associations between PM2.5 and TNF-α reported in previous studies32,33,38,39 were not observed in our study. Potential differences in study populations, measurement methodologies and analysis models could play a role in these disparities. More specifically, Chen et al.38 investigated these associations in students and the three other studies (Gong et al.39, Friedman et al.32 and Zhang et al.33) were based on a population of pregnant women but used ambient exposure (not personal exposure assessment), and Gong et al.39 examined cytokine levels in the placenta, which could lead to different results compared to blood.
Very few studies have addressed the effects of OP exposure on immune response in human blood, and when they did, IL-6 levels were mostly investigated14,16. In the RAPTES project16, blood and nasal lavage from 31 healthy student volunteers were retrieved after 5 h of exposure in different ambient settings, with contrasted pollution levels. No effect was observed with any of the analyzed OP tests (AA and Glutathione assays). The observed negative OP-IL-8 association is consistent with some previous findings among non-pregnant individuals that considered PM2.5 exposure from one week31 to three months40 and from both indoor40 and ambient environments31,41,42. Contradictory, positive IL-8-PM2.5 mass and IL-8-NO2 relationships were reported by some experimental studies43,44,45, which may differ from real environmental contexts in human studies.
The results of our study indicate a positive association between OP exposure and IL-17A. To the best of our knowledge, no prior research has specifically examined this relationship. Previous studies used NO2 or PM2.5 exposure to examine effects on IL-17A in non-pregnant participants, and similar positive associations were reported with PM2.546 and NO2 exposure31,41,46. The study conducted by Hu et al.31 addressed these associations by varying the durations of exposure to PM2.5 and NO2, which led to contrasting observations. Specifically, a short-term exposure to NO2 (between 12 and 24 h) was significantly correlated with high levels of IL-17A, whereas prolonged exposure (two weeks) was statistically associated with reduced levels of IL-17A. Our study extends these findings that relied on ambient PM2.5 and NO2 exposure, by using personal exposure assessment, by measuring PM2.5 OP exposure, and by activating the cells.
Our study presents the specificity of analyzing cytokines in pregnant women, who present several variations of immunity compared to non-pregnant women. In a recent study47, at basal state, most inflammatory cytokines among which TNF-α and IL-8 decreased in the second trimester. We observed here at the same period of pregnancy a global trend towards a decrease of these cytokines at basal state and after activation of immune cells, suggesting that there might be a cumulative negative effect of pregnancy and exposure to air pollutants on inflammatory cytokine secretions that could impair maternal health and capacity to respond to pathogens. Moreover, we observed a positive association between NO2 exposure and IL-10 secretion upon activation with PHA, which could participate in the reduction of inflammatory cytokines that was detected here. Indeed, IL-10 has broad regulatory effects on several immune cells, and is involved in normal pregnancy processes of tolerance48. In our experimental settings where whole blood cells are activated by PHA, IL-10 may be produced by regulatory T cells that are also involved in tolerance mechanisms towards the fetus during pregnancy49.
Overall, our results on the impact of air pollution exposure on the inflammatory function of the immune system may provide insights into the mechanisms underlying the adverse health effects of air pollution. In particular, IL-17A secretion is strongly linked to severe forms of asthma50,51, hypertension during pregnancy52 and changes in birth weight53. T lymphocytes, producers of IL-17A, are also pathogenic cellular components of autoimmune diseases, such as multiple sclerosis or psoriasis52. Circulating IL-17A decreases during pregnancy47, and our results suggest that air pollution could interact synergistically with this regulation of Th17 cells, with potential consequences on maternal health. In addition, TNF-α has also been identified as playing a role in the inflammatory response in allergies, which are related to air pollution54.
Strengths and limitations
One of the primary strengths of this study lies in its meticulous exposure measurements. Personal exposure measurements provided accurate assessment, tailored to the individual, in contrast to conventional assessments based on ambient exposure data obtained from monitoring stations or exposure models. The potential effect of a peak exposure over a short period of the week is diluted in our study, as we used particle samples integrated over 7 days. Consequently, the associations reported in our study may be underestimated. However, pregnant women are inclined to spend a greater portion of their time indoors, where air pollutants sources and chemical components differ from the ambient environment; personal exposure measurements are therefore all the more important. Another asset is that the study focuses not only on cytokines circulating at basal level, but also on cytokine levels measured after activation of innate and T cells. Consequently, the results obtained closely approximate real immune cell functionality, reflecting the actual immune system response to aggression in the context of potential damages induced by air pollution exposure. Analyses on cytokine levels after activation could potentially reduce confusion bias compared to analyses considering cytokine basal levels since participants’ cells were activated using the same procedure. The main asset of this work is probably the use of OP measurements, that aims to account for the detrimental impacts of PM2.5 through the oxidative stress pathway, and this led to clearer associations compared to the mass concentration metric. Furthermore, since the main sources contributing to PM and to the OP of PM were already reported in the Grenoble area55, it is particularly relevant to examine both factors in the current Grenoble-based study.
Although the study is based on an a priori hypothesis, the number of associations tested is relatively high and we have not applied a formal correction for multiple comparisons. It should therefore be recognized that some of the associations identified could result from chance finding and should therefore be interpreted with caution. The relatively small sample size of this study limits the statistical power and the possibility to robustly investigate differences in the effects of air pollution in the immune function by the asthma/rhinitis status. A larger population would potentially lead to more robust conclusions. However, this is counterbalanced by the accuracy of the measurements through the use of personal samplers, that significantly decreases measurement error. A further constraint of this study relates to the recruited population in SEPAGES, which does not reflect the overall diversity of the general population. This study is geographically restricted to the Grenoble region with a specific semi-continental climate and orography that lead to important thermal-inversions in the winter season, thereby increasing ground concentrations of pollutants. This drawback is compensated by the fact that such concentrations are nevertheless quite common in Europe, and concern a large share of the population. In addition, the participants included had higher levels of education and were more often non-smoker, as compared to pregnant women in France and some potential confounders were not taken into account, e.g. gas or electric cooking, heating system. Nevertheless, analyses in this homogeneous population are less prone to confounding biases related to social environment. Lastly, the conducted study specifically focuses on the independent effects of each pollutant on each included cytokine. The bi-pollutant models provided additional support to the observed association. Potential synergistic effects between pollutants would require a larger sample size to be robustly tested. This might be considered as simplistic, because interaction and cumulative effect of various air pollutants are expected, and cytokines do not operate in isolation within the immune system but are involved in a complex network of regulations and interactions of the body. However, studying the isolated effects provides a solid grasp of the underlying mechanisms, even though all intricacies of the system are not captured.
Conclusion
In conclusion, our study provides significant insights into the impact of exposure to air pollutants, including oxidative potential of PM, on immune function among pregnant women. This crucial data can be instrumental in creating strategies to reduce the oxidative potential of PM2.5 and thus to mitigate the adverse health effects of air pollution.
Data availability
All data generated or analyzed during this study are available upon reasonable request from the corresponding author.
References
Fuller, R. et al. Pollution and health: A progress update. Lancet Planet. Health 6(6), e535–e547. https://doi.org/10.1016/S2542-5196(22)00090-0 (2022).
Bernstein, J. A. et al. Health effects of air pollution. J. Allergy Clin. Immunol. 114(5), 1116–1123. https://doi.org/10.1016/j.jaci.2004.08.030 (2004).
Li, N., Hao, M., Phalen, R. F., Hinds, W. C. & Nel, A. E. Particulate air pollutants and asthma: A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin. Immunol. 109(3), 250–265. https://doi.org/10.1016/j.clim.2003.08.006 (2003).
Mudway, I. S., Kelly, F. J. & Holgate, S. T. Oxidative stress in air pollution research. Free Radic. Biol. Med. 151, 2–6. https://doi.org/10.1016/j.freeradbiomed.2020.04.031 (2020).
Binder, C. J., Papac-Milicevic, N. & Witztum, J. L. Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol. https://doi.org/10.1038/nri.2016.63 (2016).
Kelly, F. J. & Fussell, J. C. Linking ambient particulate matter pollution effects with oxidative biology and immune responses. Ann. N. Y. Acad. Sci. 1340(1), 84–94. https://doi.org/10.1111/nyas.12720 (2015).
Lim, Y.-H. et al. Inflammatory markers and lung function in relation to indoor and ambient air pollution. Int. J. Hyg. Environ. Health 241, 113944. https://doi.org/10.1016/j.ijheh.2022.113944 (2022).
Xu, Z. et al. Association between gaseous air pollutants and biomarkers of systemic inflammation: A systematic review and meta-analysis. Environ. Pollut. 292, 118336. https://doi.org/10.1016/j.envpol.2021.118336 (2022).
Glencross, D. A., Ho, T.-R., Camiña, N., Hawrylowicz, C. M. & Pfeffer, P. E. Air pollution and its effects on the immune system. Free Radical Biol. Med. 151, 56–68. https://doi.org/10.1016/j.freeradbiomed.2020.01.179 (2020).
Zhao, C.-N. et al. Emerging role of air pollution in autoimmune diseases. Autoimmun. Rev. 18(6), 607–614. https://doi.org/10.1016/j.autrev.2018.12.010 (2019).
Leni, Z. et al. Oxidative stress-induced inflammation in susceptible airways by anthropogenic aerosol. PLOS ONE 15(11), e0233425. https://doi.org/10.1371/journal.pone.0233425 (2020).
Liu, Q. et al. Oxidative potential and inflammatory impacts of source apportioned ambient air pollution in Beijing. Environ. Sci. Technol. 48(21), 12920–12929. https://doi.org/10.1021/es5029876 (2014).
Delfino, R. J. et al. Associations of primary and secondary organic aerosols with airway and systemic inflammation in an elderly panel cohort. Epidemiology 21(6), 892–902. https://doi.org/10.1097/EDE.0b013e3181f20e6c (2010).
Liu, L. et al. Metals and oxidative potential in urban particulate matter influence systemic inflammatory and neural biomarkers: A controlled exposure study. Environ. Int. 121, 1331–1340. https://doi.org/10.1016/j.envint.2018.10.055 (2018).
Janssen, N. A. H. et al. Associations between three specific a-cellular measures of the oxidative potential of particulate matter and markers of acute airway and nasal inflammation in healthy volunteers. Occup Environ. Med. 72(1), 49–56. https://doi.org/10.1136/oemed-2014-102303 (2015).
Steenhof, M. et al. Acute nasal pro-inflammatory response to air pollution depends on characteristics other than particle mass concentration or oxidative potential: The RAPTES project. Occup. Environ. Med. 70(5), 341–348. https://doi.org/10.1136/oemed-2012-100993 (2013).
Mor, G. & Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 63(6), 425–433. https://doi.org/10.1111/j.1600-0897.2010.00836.x (2010).
Baïz, N. et al. Maternal exposure to air pollution before and during pregnancy related to changes in newborn’s cord blood lymphocyte subpopulations. The EDEN study cohort. BMC Pregn. Childbirth 11(1), 87. https://doi.org/10.1186/1471-2393-11-87 (2011).
García-Serna, A. M. et al. Air pollution from traffic during pregnancy impairs newborn’s cord blood immune cells: The NELA cohort. Environ. Res. 198, 110468. https://doi.org/10.1016/j.envres.2020.110468 (2021).
Manches, O. et al. Maternal imprinting and determinants of neonates’ immune function in the SEPAGES mother-child cohort. Front. Immunol. https://doi.org/10.3389/fimmu.2023.1136749 (2023).
Lyon-Caen, S. et al. Deciphering the impact of early-life exposures to highly variable environmental factors on foetal and child health: Design of SEPAGES couple-child cohort. IJERPH 16(20), 3888. https://doi.org/10.3390/ijerph16203888 (2019).
Borlaza, L. J. S. et al. Personal exposure to PM2.5 oxidative potential and its association to birth outcomes. J. Expo. Sci. Environ. Epidemiol. https://doi.org/10.1038/s41370-022-00487-w (2022).
Lepeule, J. et al. Pre-natal exposure to NO2 and PM2.5 and newborn lung function: An approach based on repeated personal exposure measurements. Environ. Res. 226, 115656. https://doi.org/10.1016/j.envres.2023.115656 (2023).
Marsal, A. et al. Prenatal exposure to PM2.5 oxidative potential and lung function in infants and preschool- age children: A prospective study. Environ. Health Perspect. 131(1), 017004. https://doi.org/10.1289/EHP11155 (2023).
T. Hafkenscheid et al., Review of the application of diffusive samplers in the European Union for the monitoring of nitrogen dioxide in ambient air. European Commission (2009).
Calas, A. et al. The importance of simulated lung fluid (SLF) extractions for a more relevant evaluation of the oxidative potential of particulate matter. Sci. Rep. https://doi.org/10.1038/s41598-017-11979-3 (2017).
Calas, A. et al. Comparison between five acellular oxidative potential measurement assays performed with detailed chemistry on PM10 samples from the city of Chamonix (France). Atmos. Chem. Phys. 18(11), 7863–7875. https://doi.org/10.5194/acp-18-7863-2018 (2018).
Helsel, D. R. Less than obvious—statistical treatment of data below the detection limit. Environ. Sci. Technol. 24(12), 1766–1774. https://doi.org/10.1021/es00082a001 (1990).
Lubin, J. H. et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ. Health Perspect. 112(17), 1691–1696. https://doi.org/10.1289/ehp.7199 (2004).
Mortamais, M. et al. Correcting for the influence of sampling conditions on biomarkers of exposure to phenols and phthalates: A 2-step standardization method based on regression residuals. Environ. Health 11(1), 29. https://doi.org/10.1186/1476-069X-11-29 (2012).
Hu, X. et al. Inflammatory and oxidative stress responses of healthy adults to changes in personal air pollutant exposure. Environ. Pollut. 263, 114503. https://doi.org/10.1016/j.envpol.2020.114503 (2020).
Friedman, C. et al. Exposure to ambient air pollution during pregnancy and inflammatory biomarkers in maternal and umbilical cord blood: The healthy start study. Environ. Res. 197, 111165. https://doi.org/10.1016/j.envres.2021.111165 (2021).
Zhang, B. et al. Ambient PM2.5 exposures and systemic inflammation in women with early pregnancy. Sci. Total Environ. 829, 154564. https://doi.org/10.1016/j.scitotenv.2022.154564 (2022).
Greenland, S. et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur. J. Epidemiol. 31(4), 337–350. https://doi.org/10.1007/s10654-016-0149-3 (2016).
Greenland, S. & Poole, C. Living with P values: Resurrecting a Bayesian perspective on frequentist statistics. Epidemiology 24(1), 62. https://doi.org/10.1097/EDE.0b013e3182785741 (2013).
Strak, M. et al. Long term exposure to low level air pollution and mortality in eight European cohorts within the ELAPSE project: Pooled analysis. BMJ 374, n1904. https://doi.org/10.1136/bmj.n1904 (2021).
Leikauf, G. D., Kim, S.-H. & Jang, A.-S. Mechanisms of ultrafine particle-induced respiratory health effects. Exp. Mol. Med. https://doi.org/10.1038/s12276-020-0394-0 (2020).
Chen, R. et al. Fine particulate air pollution and the expression of microRNAs and circulating cytokines relevant to inflammation, coagulation, and vasoconstriction. Environ. Health Perspect. 126(1), 017007. https://doi.org/10.1289/EHP1447 (2018).
Gong, C. et al. Ambient fine particulate matter exposures and human early placental inflammation. Environ. Pollut. 315, 120446. https://doi.org/10.1016/j.envpol.2022.120446 (2022).
Audi, C. et al. Serum cytokine levels related to exposure to volatile organic compounds and PM2.5 in dwellings and workplaces in French farmers—a mechanism to explain nonsmoking COPD. COPD 12, 1363–1374. https://doi.org/10.2147/COPD.S117866 (2017).
Fiorito, G. et al. Oxidative stress and inflammation mediate the effect of air pollution on cardio- and cerebrovascular disease: A prospective study in nonsmokers: Effect of air pollution on cardio- and cerebrovascular disease. Environ. Mol. Mutagen. 59(3), 234–246. https://doi.org/10.1002/em.22153 (2018).
Parenteau, A. M. et al. Associations of air pollution with peripheral inflammation and cardiac autonomic physiology in children. New Drctns Chld Adlscnt 2022(181–182), 125–154. https://doi.org/10.1002/cad.20474 (2022).
Cachon, B. F. et al. Proinflammatory effects and oxidative stress within human bronchial epithelial cells exposed to atmospheric particulate matter (PM2.5 and PM>2.5) collected from Cotonou, Benin. Environ. Pollut. 185, 340–351. https://doi.org/10.1016/j.envpol.2013.10.026 (2014).
Jeong, S.-C., Cho, Y., Song, M.-K., Lee, E. & Ryu, J.-C. Epidermal growth factor receptor (EGFR)-MAPK-nuclear factor(NF)-κB-IL8: A possible mechanism of particulate matter(PM) 2.5-induced lung toxicity: JEONG et al. Environ. Toxicol. 32(5), 1628–1636. https://doi.org/10.1002/tox.22390 (2017).
Longhin, E., Holme, J. A., Gualtieri, M., Camatini, M. & Øvrevik, J. Milan winter fine particulate matter (wPM2.5) induces IL-6 and IL-8 synthesis in human bronchial BEAS-2B cells, but specifically impairs IL-8 release. Toxicol. Vitro 52, 365–373. https://doi.org/10.1016/j.tiv.2018.07.016 (2018).
Gao, N. et al. Lung function and systemic inflammation associated with short-term air pollution exposure in chronic obstructive pulmonary disease patients in Beijing, China. Environ. Health 19(1), 12. https://doi.org/10.1186/s12940-020-0568-1 (2020).
Jarmund, A. H. et al. Cytokine patterns in maternal serum from first trimester to term and beyond. Front. Immunol. https://doi.org/10.3389/fimmu.2021.752660 (2021).
Thaxton, J. E. & Sharma, S. REVIEW ARTICLE: Interleukin-10: A multi-faceted agent of pregnancy. Am. J. Reprod. Immunol. 63(6), 482–491. https://doi.org/10.1111/j.1600-0897.2010.00810.x (2010).
Tsuda, S., Nakashima, A., Shima, T. & Saito, S. New paradigm in the role of regulatory T cells during pregnancy. Front Immunol 10, 573. https://doi.org/10.3389/fimmu.2019.00573 (2019).
Brandt, E. B. et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J. Allergy Clin. Immunol. 132(5), 1194-1204.e2. https://doi.org/10.1016/j.jaci.2013.06.048 (2013).
Weng, C.-M. et al. Diesel exhaust particles up-regulate interleukin-17A expression via ROS/NF-κB in airway epithelium. Biochem. Pharmacol. 151, 1–8. https://doi.org/10.1016/j.bcp.2018.02.028 (2018).
Dhillion, P. et al. IL-17-mediated oxidative stress is an important stimulator of AT1-AA and hypertension during pregnancy. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 303(4), R353–R358. https://doi.org/10.1152/ajpregu.00051.2012 (2012).
Laine, J. E. et al. Prenatal exposure to multiple air pollutants, mediating molecular mechanisms, and shifts in birthweight. Environ. Sci. Technol. 54(22), 14502–14513. https://doi.org/10.1021/acs.est.0c02657 (2020).
Melén, E. et al. Interactions between Glutathione S- Transferase P1, tumor necrosis factor, and traffic-related air pollution for development of childhood allergic disease. Environ. Health Perspect. 116(8), 1077–1084. https://doi.org/10.1289/ehp.11117 (2008).
Borlaza, L. J. S. et al. Disparities in particulate matter (PM10) origins and oxidative potential at a city-scale (Grenoble, France)—Part II: Sources of PM10 oxidative potential using multiple linear regression analysis and the predictive applicability of multilayer perceptron neural network analysis. Atmos. Chem. Phys. https://doi.org/10.5194/acp-2021-57 (2021).
Acknowledgements
We thank the Sepages study group: E. Eyriey, A. Licinia, A. Vellement (Groupe Hospitalier Mutualiste, Grenoble), S. Bayat, P. Hoffmann, E. Hullo, C. Llerena (Grenoble Alpes University Hospital, La Tronche), X. Morin (Clinique des Cèdres, Echirolles), A. Morlot (Clinique Belledonne, Saint-Martin d’Hères), J. Lepeule, S. Lyon-Caen, C. Philippat, I. Pin,and J. Quentin, V. Siroux, R. Slama (Grenoble Alpes University, Inserm, CNRS, IAB). Many thanks to Dr I.Pin who was the Sepages PI from 2012 until 2022. We thank also Mrs. A. Benlakhryfa, Mrs. L. Borges, Mr. Y. Gioria, clinical research assistants; Mrs. J. Giraud, Mrs. M. Marceau, Mrs. M-P. Martin, nurses; Mrs. E. Charvet, Mrs A. Putod, midwives; Mrs. M. Graca, Mrs. K.Gridel, Mrs. C. Pelini, Mrs M.Barbagallo fieldworkers; Mrs. A.Bossant, K. Guichardet, J-T Iltis A. Levanic, C.Martel, E. Quinteiro, S.Raffin neuropsychologists; the staff from Grenoble Center for Clinical Investigation (CIC): Prof. J.-L. Cracowski, Dr. C. Cracowski, Dr. E. Hodaj, Mrs. D. Abry, Mr. N. Gonnet and Mrs. A. Tournier. A warm thank you also Dr. M. Althuser, Mr. S. Althuser, Dr. F. Camus-Chauvet, Mr. P. Dusonchet, Mrs. S. Dusonchet, Dr. L. Emery, Mrs. P.Fabbrizio, Prof. P. Hoffmann, Dr. D. Marchal André, Dr. X. Morin, Dr. E.Opoix, Dr. L. Pacteau, Dr. P. Rivoire, Mrs. A. Royannais, Dr. C.Tomasella, Dr. T. Tomasella, Dr. D. Tournadre, Mr. P. Viossat, Mrs. E.Volpi, Mrs. S. Rey, Dr. E. Warembourg and clinicians from Grenoble University Hospital for their support in the recruitment of the study volunteers. We also thank Mrs. A. Buchet, Mrs. SF. Caraby, Dr. J-N.Canonica, Mrs. J. Dujourdil, Dr. E. Eyriey, Prof. P. Hoffmann, Mrs. M. Jeannin, Mrs. A. Licina, Dr. X. Morin, Mrs. A. Nicolas, and all midwives from the four maternity wards of Grenoble urban areas. SEPAGES data are stored thanks to Inserm RE-CO-NAI platform funded by Commissariat Général à l’Investissement, with the implication of Sophie de Visme (INSERM DSI). Many thanks to Dr. M.A. Charles, RE-CO-NAI coordinator, for her support. The authors thank all the numerous people (who could not be listed exhaustively here) from the different laboratories (IGE and Air-O-Sol analytical platform) and from the Grenoble University Hospital who performed mother recruitment and child follow-ups. We thank all the staff and students in the INSERM Institute for Advanced Biosciences (especially André Arnoux, Corélie Salque, Pierre Puppinck, Inès Amine), and in EFS R&D laboratory (especially Caroline Aspord, Jean-Paul Molens, Céline Coppard, Marina Baudot, Morgane Guyonnet) for their help, and to EFS staff in support departments. Finally, and importantly, we would like to express our sincere thanks to participants of the SEPAGES study.
Funding
This work was supported in part by the Agence de la Transition Ecologique—ADEME and by the Agence Nationale de Sécurité Sanitaire de l’Alimentation, de l’Environnement et du Travail—ANSES, both supporting A.M’s PhD grant and by ACME project ANR-15-IDEX-02 supporting L.F internship compensation. OP measurements were funded by a University Grenoble Alpes grant CDP IDEX UGA MOBILAIR (ANR-15-IDEX-02) and by the French Research Agency—ANR (GetOPstandOP ANR-19- CE34-0002). The immunological biomarkers were funded by SHALCOH project ANR-14-CE21-0007. This work was possible thanks to the financial support of EFS-Auvergne-Rhone-Alpes (LC, OM, KU, YM). The SEPAGES cohort was supported by the European Research Council (N°311765-E-DOHaD), the European Community’s Seventh Framework Program (FP7/2007-206—N°308333-892 HELIX), the European Union’s Horizon 2020 research and innovation program (N° 874583 ATHLETE Project, N°825712 OBERON Project), the French Research Agency—ANR (PAPER project ANR-12-PDOC-0029-01, ANR-15-IDEX-02 and ANR-15-IDEX5, GUMME project ANR-18-CE36-005, ETAPE project ANR-18-CE36-0005—EDeN project ANR-19-CE36-0003-01—MEMORI project ANR 21-CE34-0022, ORANDANI project ANR-22-CE36-0018), the French Agency for Food, Environmental and Occupational Health & Safety—ANSES (CNAP project EST-2016-121, PENDORE project EST-2016-121, HyPAxE project EST-2019/1/039, PENDALIRE project EST-2022-169), the Plan Cancer (Canc’Air project), the French Cancer Research Foundation Association de Recherche sur le Cancer—ARC, the French Endowment Fund AGIR for chronic diseases—APMC (projects PRENAPAR, LCI-FOT, DysCard), the French Endowment Fund for Respiratory Health, the French Fund—Fondation de France (CLIMATHES—00081169, SEPAGES 5—00099903, ELEMENTUM—00124527).
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of the research, scientific discussion, and provided final approval for publication. AM and LF drafted the manuscript, analyzed and interpreted the data. IA helped with the statistical analyses. LC, VS, and GU contributed to the conception and design of the research, helped interpret the data, and revised the manuscript for important intellectual content. SLC, AB, RE, KS, JL, JQ, RC were involved in the data acquisition and/or curation. CP, SB, SLC, RS were responsible for the general project administration and research investigation process. LC, JLJ, RS, GU, VS acquired the financial support.
Corresponding author
Ethics declarations
Competing interest
The authors have no competing interests as defined by Nature Research, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marsal, A., Frau, L., Chaperot, L. et al. Personal exposure to air pollutants and immune system biomarkers in pregnant women. Sci Rep 15, 17672 (2025). https://doi.org/10.1038/s41598-025-98712-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98712-7