Abstract
Orthodontic treatment can enhance occlusal force, yet it may increase the sensitivity of periodontal tissues. To compare the impacts of orthodontic treatment, intervention, and combined treatment (intervention first, then orthodontics) on alveolar bone remodeling, we established a rat model with low occlusal function. Three weeks after combined treatment, the levels of BMP2 and BGP increased by approximately 36% and 78% respectively, and the changes were even more remarkable after 4 weeks. Meanwhile, the expression of CTSK was inhibited, while the expression of OPN and RUNX2 increased. From the second to the third week, the key proteins in the SIRT1/β - catenin pathway underwent changes. At 4 weeks, the expression of SIRT1 and β - catenin increased by around 73% and 60% respectively. This study indicates that the combined treatment alleviates the side effects of orthodontics. Orthodontic force may activate the β-catenin pathway via SIRT1 to balance alveolar bone osteogenesis and osteoclast differentiation. This research provides a targeted treatment strategy and a theoretical basis for treating teeth with low occlusal function. It will contribute to optimizing clinical treatment outcomes, reducing the risk of periodontal tissue damage, and improving the prognosis of orthodontic treatment for patients.
Similar content being viewed by others
Introduction
Occlusion plays a crucial role in the oral and maxillofacial system and is essential for maintaining normal function. Reduced occlusal function can adversely affect the orofacial region and may also impact other organs, leading to negative effects on overall health1,2,3. Factors such as congenital low tooth position, tooth wear, and poor chewing habits can weaken occlusal force and diminish occlusal function4,5,6,7. Furthermore, a lack of appropriate occlusal stimulation can disrupt the balance of the masticatory system, potentially resulting in a range of pathological changes, including root resorption, thinning of the periodontal membrane, and periodontal atrophy8. Several studies have indicated that diminished occlusal function may result in a reduced or even complete loss of chewing ability9. Furthermore, over time, hypofunctional occlusion is often associated with an increased likelihood and accelerated rate of cognitive decline in middle-aged and older populations10. Nevertheless, research focusing on the treatment and underlying mechanisms of hypofunctional occlusion remains limited. Consequently, there is an urgent need to investigate effective treatments for hypofunctional occlusion.
Currently, treatments for low occlusal function include restorative treatment, orthodontic treatment, orthognathic surgery, oral muscle training, and other methods11,12,13. Among them, orthodontic treatment is reported to be suitable for patients with different degrees of severity and represents a widely applied and effective treatment approach in clinical practice14,15. During the orthodontic treatment process, for teeth with low occlusal function, orthodontic forces need to be applied to promote the remodeling of the alveolar bone to realign the misaligned teeth to appropriate positions and establish a normal occlusal force16. The process of orthodontic bone remodeling is tightly regulated by osteoclast-mediated bone resorption and osteoblast-mediated bone formation. In the initial stages of bone remodeling, hematopoietic progenitor cells are recruited and differentiate into osteoclasts. As remodeling progresses, osteoclasts undergo apoptosis, which allows newly recruited osteoblasts to mediate new bone formation. Fine-tuning the balance between osteoclasts and osteoblasts enables precise synchronization of bone resorption and formation, thereby maintaining the structural integrity and homeostasis of bone tissue17. However, tooth movement during orthodontic treatment is considered a risk factor for gum recession. Patients with low occlusal function are more prone to adverse changes such as root resorption, gingival recession, and excessive alveolar bone resorption after orthodontic treatment18,19,20. A previous study involving 298 orthodontic patients from the Second People’s Hospital of Yunnan Province also found that patients with low occlusal function (including infraoccluded teeth and open bite teeth) had a greater risk of gingival recession after orthodontic treatment than patients without infraoccluded teeth and open bite. Moreover, the recession rate was related to factors such as whether orthodontic extraction was performed, the gingival biotype, and the gingival index before orthodontic treatment. Therefore, it is necessary to find an effective method to mitigate adverse changes in the alveolar bone and periodontal tissues of teeth with low occlusal function after orthodontic treatment to reduce the risk of gingival recession after orthodontic treatment16.
Wnt/β-catenin plays a crucial role in the growth and development of various organs and tissues, and it is closely associated with normal bone formation and resorption, as well as tooth formation and development21. The β-catenin protein serves as a key mediator in this pathway. Studies have indicated that β-catenin is the core mediator of mechanical stimulation22. Activating the β-catenin pathway appears to promote bone formation and mitigate harmful bone loss. Active β-catenin in mice at different stages of postnatal life enhances bone formation and reduces bone resorption, thereby facilitating vertebral growth23. Kim et al. further reported that activation of the β-catenin pathway may stimulate osteoblast differentiation and odontoblast formation24. Collectively, these studies underscore the importance of the β-catenin pathway in bone remodeling and suggest its potential role in alveolar bone reconstruction. Silent regulator protein 1 (SIRT1), a member of the nicotinamide adenine dinucleotide-dependent protein deacetylase family, is a key factor in the regulation of bone homeostasis and is involved in the regulation of multiple bone metabolism pathways25. Studies have shown that SIRT1 plays a crucial role in osteogenic differentiation by regulating β-catenin. Feng et al. reported that SIRT1 can deacetylate β-catenin, promoting its accumulation in the nucleus and thus facilitating the transcription of key osteogenic genes. The use of the SIRT1 activator resveratrol can promote the β-catenin-mediated osteogenic differentiation of dental pulp stem cells (DPSCs). Recent evidence has also demonstrated that upregulating the expression of SIRT1 can increase the activity of the β-catenin signaling pathway, thereby promoting the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs)26. However, there is currently no research on whether SIRT1 can promote the differentiation of osteoblasts in alveolar bone through β-catenin during either orthodontic treatment or interventions aimed at restoring occlusal force.
On the basis of the above research background, we propose that during the orthodontic treatment of teeth with low occlusal function, SIRT1 promotes alveolar bone remodeling by increasing the activation of the β-catenin signaling pathway. In addition, since interventions for restoring occlusal force cause relatively little damage to periodontal tissues, we speculate that the method of first restoring the occlusal force and then performing orthodontic treatment can combine the advantages of both methods, improving the adverse changes in the alveolar bone and periodontal tissues after the treatment of teeth with low occlusal function. To test this hypothesis, in this study, orthodontic treatment, interventions for restoring occlusal force, and a combination of the two methods were used to treat rats with low occlusal function, preliminarily exploring the effects of these treatment methods on periodontal tissues and their molecular mechanisms and providing new targets and strategies for optimizing clinical treatment plans.
Materials and methods
Animals
Forty-two 8-week-old male Wistar rats were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. Seven rats were housed in each group, and the housing environment met the standards, with the temperature controlled at 23 ± 2 °C, the humidity ranging from 40 to 65%, and a 12-hour light/12-hour dark cycle. All the animals were provided normal standard feed and had free access to water. The animals were divided into the following groups according to different treatment methods: (1) normal occlusal function group (Control); (2) low occlusal function group (Hypo); (3) low occlusal function group with orthodontic treatment (Hypo + Force); (4) normal occlusal function group with orthodontic treatment (Control + Force); (5) low occlusal function group with individual intervention (Hypo + IV); (6) low occlusal function group with intervention followed by orthodontic treatment (Hypo + IV + Force). All animal experiments in this study were approved by the Ethics Committee of Yunnan University (Approval No. YNU20210111) and were carried out in accordance with the ARRIVE Guidelines 2.0 and relevant regulations.
Sample size
The sample size was calculated using the resource equation method27. A multi-group analysis of variance (ANOVA) was carried out. The acceptable range for the degrees of freedom (df) of the error term lies between 10 and 20 (DF = 10–20). Based on the formula n = DF/k + 1, it was inferred that, within the context of this study, each group necessitated 4–6 animals. Taking into account potential unforeseen problems during the experiment, we opted to use 7 animals per group for the current experimental work.
Construction of an animal model of occlusal hypoplasia
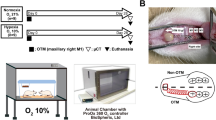
Grouping was performed using a computer-generated random number table. The rats with normal occlusal function were randomly divided into two groups, Group A and Group B. Group A consisted of 14 rats, which were further randomly divided into two subgroups of 7 rats each, namely, the normal occlusal function group (Control group) and the blank orthodontic group (Con + Force group). Group B included 28 rats. The rats in both Group A and Group B were anesthetized via the intraperitoneal injection of pentobarbital sodium (40 mg/kg). After anesthesia, in Group B, the metal crowns and guide plates were bonded to the upper incisors, and the metal crowns were bonded to the lower incisors to disengage the posterior teeth from occlusal contact for 4 weeks. During this period, the animals were fed brittle food to establish an animal model of low posterior tooth occlusal function. The rats in Group A were allowed to recover from anesthesia and then were maintained in a normal environment. Seven rats with low occlusal function were randomly selected as the low occlusal function group (Hypo group) (Fig. 1A). Another seven rats were randomly selected as the low occlusal function orthodontic group (Hypo + Force group) and, together with the blank orthodontic group, underwent orthodontic treatment (Fig. 1B). The specific operation was as follows: after anesthetizing the rats with low occlusal function, an axial groove 0.5–1.0 mm deep was ground at the neck of the left maxillary first molar, the labial surface of the left and right maxillary incisors, and the axial angle at the tooth neck. A nickel‒titanium coil spring and ligature wire were used to apply an orthodontic force of approximately 50 g, a light-curing material was used to reinforce the maxillary incisors, and a ligature wire was used to prevent detachment. The remaining 14 rats with low occlusal function wore personalized orthodontic appliances for occlusal force restoration intervention treatment. The specific operation was as follows: the rats with low occlusal function were randomly numbered. After anesthesia, a suitable position was found using a plastic mold, and then silicone rubber was injected into the plastic mold with a glue gun and left to stand for 4–5 min. After the silicone rubber was completely fixed, the plastic film was removed. A tooth model was subsequently made using die material, and a dental brace was customized according to the model. The corresponding animal was anesthetized according to the number of dental braces, and a dental brace was installed. After the intervention and restoration, the intervention group was further randomly and equally divided into two groups. One group was used as the low occlusal function intervention group (Hypo + IV group) (Fig. 1C), and the other group continued to receive orthodontic treatment as the combined intervention and orthodontic treatment group (Hypo + IV + Force group). At the end of the experiment, all the rats were euthanized via an intraperitoneal injection of 120 mg/kg pentobarbital sodium.
Construction of a rat and orthodontic model of occlusal hypoplasia. (A) Representative image of the occlusal hypofunction rat model. (B) Representative image of a rat subjected to orthodontic treatment for hypofunctional occlusion. (C) Representative image of a rat treated with an individual intervention for hypofunctional occlusion.
Hematoxylin‒eosin (HE) staining
The periodontal tissue was removed and cut into tissue slices (thickness less than 0.5 cm), fixed in fixing solution, modified in size, placed in an embedding box, washed for 30 min, dehydrated with different concentrations of alcohol, cleared in xylene, and embedded in wax. The embedded slices were sliced with a slicer, heated with hot water, placed on a glass slide, and dried. Xylene was used to remove paraffin from the slices, and then alcohol was used for dehydration. The slices were dyed with a hematoxylin aqueous solution for 10 min, separated with acid water and ammonia water for 5 s, washed with running water for 1 h, dehydrated with alcohol, dyed with an eosin dye solution for 2–3 min, dehydrated and made transparent. Mounting medium was added, and the samples were sealed and observed under a microscope.
Immunohistochemical assay
Fresh tissues were fixed in 4% paraformaldehyde for more than 24 h, followed by dehydration, embedding, trimming, and sectioning. The sections were transferred to warm water at 40 °C to facilitate the spread of the tissues, retrieved using slides, and then baked in an oven at 60 °C. This was followed by deparaffinization and antigen retrieval. The sections were washed, gently shaken dry, and dried with filter paper to eliminate excess water around the tissue sections. A circle was drawn around the tissue using a histochemical pen, and a drop of 5% BSA sealing solution was added within the circle, which was then incubated at 37 °C for 30 min. After the sealing solution was removed, 100 µL of the primary antibody mixture was added, and the sections were incubated at 4 °C overnight. The primary antibody working solution was subsequently removed, and the slides were washed. Once the sections were patted dry, the corresponding fluorescent secondary antibody was added dropwise within the circle and incubated at 37 °C for 50 min. The secondary antibody solution was then removed, and the sections were washed with PBS three times, with each wash lasting 5 min. Finally, an anti-fluorescence quenching sealing agent containing DAPI was added dropwise, and the sections were sealed with coverslips and subsequently observed under a fluorescence microscope, after which images were captured. Rats with normal occlusal function without any treatment served as the blank control group; rats with normal occlusal function undergoing orthodontic treatment served as the experimental control group.
Western blot
The total protein of the cells was extracted with a protein extraction kit, the protein concentration was measured with a BCA kit, and the sample was loaded at 50 µg/well. After separation on a 4–20% SDS‒PAGE gel (Dakewe, China) for 1 h, the sample was transferred to a PVDF membrane and blocked with PBS containing 5% skim milk for 1 h. The primary antibody (Abcam, UK) and internal reference β-actin (Proteintech, China) antibody of each target protein were added, and each protein was rinsed 3 times with PBST buffer at 4 °C overnight for 5 min each time. Finally, the secondary antibody was added. PBST buffer was used to rinse the samples 3 times, enhanced chemiluminescence (ECL) was developed, β-actin was used as an internal reference, a protein gel imaging system was used for imaging, and ImageJ was used to analyze the gray value of each target protein.
Statistical analysis
Statistical analyses were performed utilizing GraphPad Prism 9.0 software (San Diego, USA). The data are presented as means ± SEM. For time-dependent experiments involving different time points, two-way ANOVA was followed by Šídák’s multiple comparisons test for intergroup comparisons. Each experimental data set included three independent biological replicates. A P value less than 0.05 and less than 0.01 were considered statistically significant and highly significant, respectively.
Results
Combined intervention and orthodontic treatment can alleviate the inflammatory response of alveolar bone tissues during orthodontic treatment
To compare the effectiveness of various orthodontic methods for treating teeth with low occlusal capacity, we developed a rat model of occlusal hypoplasia and subjected it to individualized interventions or orthodontic treatment. We assessed the morphology of pressure side of the alveolar bone tissues at 2, 3, and 4 weeks posttreatment using HE staining. Histological analysis revealed well-aligned osteoblast-nucleated dental tissue fibers in the normal occlusal function group. In contrast, rats in the low-occlusal-function state exhibited infiltration of inflammatory cells 2–3 weeks later. Additionally, disorganized periodontal fiber arrangements and and narrowing of the periodontal ligament were distinctly observed at 4 weeks. There was no significant improvement in adverse tissue changes following treatment with orthodontic forces applied directly to the hypo-occlusal teeth or through interventions alone. However, when a combination of intervention and orthodontic treatment was administered to rats with hypofunctional occlusion, the aforementioned adverse tissue changes were significantly reversed, particularly after 4 weeks of combined treatment (Fig. 2).
Morphological changes in periodontal tissue detected by HE staining. The treatment of rats with occlusal hypofunction was conducted over a period of 2, 3, and 4 weeks, utilizing a combination of orthodontic and individual intervention techniques (scale bar = 10 μm). Hypo indicates occlusal hypofunction; Force indicates applied orthodontic force; IV indicates individual occlusal force intervention; PDL indicates Periodontal Ligament; AB indicates Alveolar Bone; D indicates Dentin.
Inflammatory factors have been proven to induce osteoclast differentiation28. To investigate the mechanism of bone remodeling during dental intervention for low-functioning occlusion or orthodontic treatment, we detected the changes in the expression levels of inflammatory factors on the pressure side of the alveolar bone by Enzyme - Linked Immunosorbent Assay (ELISA). The results showed that after the occlusal function decreased, the levels of pro - inflammatory factors IL − 1β, IL − 6, and TNF - α increased over time, indicating that hypofunctional occlusion promotes the secretion of inflammatory factors by the tissues on the pressure side of the alveolar bone. At week 3, the levels of pro - inflammatory factors IL − 6 and TNF - α in the intervention - only group and the group with orthodontic treatment after intervention were significantly lower than those in the hypofunctional occlusion group, while there was no significant change in the levels of pro - inflammatory factors in the orthodontic treatment group during the same period (Fig. 3A–C). It is worth noting that this difference was more obvious at week 4 compared with week 3.
Expression levels of proinflammatory factors in rat dental tissues treated by different methods. All the data are expressed as the means ± SEMs (two-way ANOVA was followed by Šídák’s multiple comparisons test, ns indicates no significant differences; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
Combined intervention and orthodontic treatment significantly promotes bone formation and inhibits bone resorption
The balance between osteoclast and osteoblast differentiation plays a crucial role in orthodontic treatment. To explore the mechanisms of bone remodeling in dental interventions for hypofunctional occlusion or orthodontic treatments, we used the ELISA to detect the expression levels of bone remodeling - related factors in periodontal tissues. The resulting data showed that, compared with the hypofunctional occlusion group, at week 2, there were no significant differences in the remaining bone remodeling - related factors among each treatment group, except for Bone Gla - Protein (BGP). At week 3, in the orthodontic treatment group and the post - intervention orthodontic group, the expression levels of the bone formation - related factors Bone Morphogenetic Protein 2 (BMP2) and BGP were significantly higher (Fig. 4A,B), while the levels of the bone resorption - related factors Receptor Activator of Nuclear Factor - kappa B Ligand (RANKL) and Matrix Metalloproteinase 9 (MMP9) were significantly lower (Fig. 4C,D). In contrast, in the intervention - only group, there were no significant differences in the levels of bone formation - related factors, and the levels of bone resorption - related factors were significantly decreased. Notably, the differences in these factors were more pronounced at week 4 than at week 3. These findings suggest that for alveolar bone tissues with hypofunctional occlusion, intervention followed by orthodontic treatment may be more effective in promoting bone formation and inhibiting bone resorption compared to intervention - only or orthodontic - only treatments. Additionally, we conducted immunofluorescence analysis on CTSK, an osteoclast marker in the tissues on the pressure side of the alveolar bone. The results showed that within 2 to 4 weeks, reduced occlusal function significantly promoted the time-dependent increase in CTSK expression, indicating the continuous enhancement of bone resorption. After direct orthodontic treatment, CTSK expression continued to show an upward trend. By contrast, after intervention treatment, its expression began to decline. In the combined intervention and orthodontic treatment group, CTSK expression peaked at 3 weeks of treatment and then significantly decreased at 4 weeks (Fig. 4E). This changing trend of CTSK might be related to the complexity during the treatment of teeth with hypofunctional occlusion. However, it is certain that bone resorption was significantly inhibited after 4 weeks of combined treatment.
Levels of osteoblast and osteoclast differentiation-related factors in dental tissues were examined via ELISA. (A,B), Expression levels of key factors for osteoblast differentiation in rat dental tissues treated by different methods. (C,D), Expression levels of key factors for osteoclast differentiation in rat dental tissues treated by different methods. E, Expression and fluorescence intensity statistics of CTSK in the tissues of teeth with hypofunctional occlusion after 2, 3 and 4 weeks of treatment. All the data are expressed as the means ± SEMs (two-way ANOVA was followed by Šídák’s multiple comparisons test, ns indicates no significant differences; For Fig. 4A–D, *P < 0.05; **P < 0.01; ***P < 0.001; For Fig. 4E, #P < 0.05 vs. Control; *P < 0.05 vs. Hypo).
SIRT1 significantly inhibits osteoclast differentiation during combined intervention and orthodontic treatment
SIRT1 generally plays an important role in a range of pathophysiological processes by regulating gene transcription. SIRT1 can inhibit IL-6-induced osteoclastogenesis and differentiation and is a potential target for the treatment of ischemic osteonecrosis25,29. To investigate the role of SIRT1/β-catenin signaling during the postintervention orthodontic treatment of teeth, we examined the fluorescent expression of SIRT1/β-catenin in the tissues of teeth with low occlusal capacity. The results indicated that compared with rats having normal occlusal function, the dental tissues of rats with low occlusal competence showed notably reduced SIRT1 and β-catenin expression after 3–4 weeks. For rats with hypofunctional occlusion receiving orthodontic or interventional treatment, the expression patterns of SIRT1 and β-catenin were reversed across all treatment groups, with the most significant changes in the group undergoing intervention followed by orthodontic treatment (Fig. 5A,D). These results indicate that intervening first and then applying orthodontic treatment to hypofunctional occlusion teeth significantly increased SIRT1 and β-catenin expression. This suggests that elevated SIRT1 and β-catenin expression might contribute to the mechanism of intervention combined with orthodontic treatment for teeth.
Tissue immunofluorescence was used to detect the role of SIRT1 in osteoclast differentiation. (A,B), Expression and fluorescence intensity statistics of SIRT1 in the tissues of teeth with hypofunctional occlusion after 2, 3 and 4 weeks of treatment. (C,D), Expression and fluorescence intensity statistics of β-catenin in the tissues of teeth with hypofunctional occlusion after 2, 3 and 4 weeks of treatment. All the data are expressed as the means ± SEMs (two-way ANOVA was followed by Šídák’s multiple comparisons test; #P < 0.05 vs. Control; *P < 0.05 vs. Hypo).
SIRT1 May promote osteogenic‒osteoclastic differentiation in occlusal hypoplasia through the β-catenin pathway
To further investigate the mechanism of action of SIRT1 in osteogenic and osteoblastic differentiation, RT - qPCR and Western blot analyses were performed to detect changes in the expression of key proteins associated with the SIRT1 and β-catenin pathways, as well as proteins related to osteogenic and osteoclastic differentiation in the pressure side of the alveolar bone tissue. The RT - qPCR results revealed no significant changes in the expression of genes related to osteogenic and osteoblastic differentiation after 2 weeks of orthodontic treatment; in contrast, after 3 weeks of treatment, the expression of the osteoblastic differentiation genes NFATC1 and RANK was significantly downregulated, and the expression of the osteogenic differentiation genes OPN and RUNX2 was significantly upregulated in the individual intervention followed by orthodontic treatment group. This gene expression change was more significant after 4 weeks of treatment (Fig. 6A,D).
Expression and quantification changes of key genes involved in osteoblast - osteoclast differentiation in dental tissues after 2–4 weeks of treatment with different orthodontic methods. (A,B), Genes associated with osteogenic differentiation after 2 weeks of orthodontic dental treatment, as detected via RT - qPCR. (C,D), Genes associated with osteoclast differentiation detected by RT - qPCR after 2 weeks of orthodontic treatment.
Changes in the expression of pathway key proteins involved in osteoblast‒osteoclast differentiation in dental tissues after 2–4 weeks of treatment with different orthodontic methods. (A–C), The key proteins OPN and RUNX2 for osteogenesis, the key proteins RANK and NFATC1 for osteoclastic differentiation and formation, and key proteins of the β-catenin signaling pathway (SIRT1, β-catenin, and GSK3β) were detected by Western blotting after 2, 3 and 4 weeks of orthodontic treatment. β-Actin was used as an internal control, the data displayed below the protein bands represent the target protein/β-actin following greyscale analysis of the bands using Image J software (the Western blot results are presented as a cropped representative image; the uncropped original blot can be found in the Supplementary Information section).
The results revealed significant changes in the expression of all proteins detected in normal rats with reduced occlusal function. After 2 weeks of intervention or orthodontic treatment, no significant changes were observed in the expression of any proteins, except for β-catenin. After 3 weeks of treatment, SIRT1 and β-catenin protein expression was significantly elevated in the orthodontic treatment group, the individual intervention group, and the combined intervention and orthodontic treatment group, whereas the expression of osteoblast differentiation-associated proteins decreased and the expression of osteoclast differentiation-associated proteins increased, although these changes were not statistically significant. After 4 weeks of treatment, the protein expression levels of SIRT1 and β-catenin were elevated in the treatment group compared with those in the hypo-occlusal group (Fig. 7A,C, S1), whereas the expression levels of the osteoblast differentiation-related proteins NFATC1 and RANK were significantly reduced. Conversely, the expression of the osteogenic differentiation-related proteins OPN and RUNX2 significantly increased, with the most pronounced changes observed in the group receiving combined interventional and orthodontic treatment (Figs. 7A,C, S2). These results suggest that the elevated expression of SIRT1 during the combination of intervention and orthodontic treatment of occlusal hypoplasia may promote osteoblastogenesis and inhibit osteoclast formation by activating the β-catenin pathway.
Discussion
In this study, we established a rat model with low occlusal function and compared the effects of three methods, namely, interventions for restoring occlusal force, orthodontic treatment, and intervention followed by orthodontic treatment, on alveolar bone remodeling. Drawing on the research of Zong et al.30. and the work of the Kakali - led team31, it has been established that the periodontal tissues’ response to mechanical force culminates within a 2–4 week span. During this same 2–4 week period, remarkable dynamic changes take place in both alveolar bone remodeling and periodontal tissue alterations. Consequently, we have selected the 2–4 week time window as the pivotal time point for delving into the molecular mechanisms following the treatment of teeth with compromised occlusal function. Previous studies have shown that a decrease in occlusal function can lead to the recession of periodontal tissues and alveolar bone tissues32, which is consistent with our histological observations. Directly applying orthodontic forces to treat teeth with low occlusal function may trigger the release of inflammatory factors in periodontal tissues, thereby leading to excessive absorption of the alveolar bone. The results of the histological staining and ELISA in this study support this conclusion. No adverse changes were observed in the periodontal tissues of the rats with normal occlusal function. However, in the periodontal tissues of the rats with low occlusal function, the fiber arrangement was disordered, the number of osteoclasts increased, and proinflammatory factors such as IL-1β, IL-6, and TNF-α were secreted. After direct orthodontic treatment for 2–3 weeks, the adverse changes and inflammatory responses in the periodontal tissues did not significantly improve. After 4 weeks, the number of osteoclasts and the secretion of inflammatory factors decreased compared with those in rats with low occlusal function. In contrast, after 2–4 weeks of treatment with the occlusal restoration intervention, no significant increase in inflammatory factors was observed, and the fiber arrangement in the periodontal tissues was relatively improved. After 4 weeks of intervention followed by orthodontic treatment, the morphology of the periodontal tissues was closer to that of the normal occlusal function group, and the levels of inflammatory factors were significantly lower than those in the low occlusal function group. Notably, regardless of orthodontic treatment, intervention measures, or intervention followed by orthodontic treatment, the proinflammatory factors in the periodontal tissues of rats with low occlusal function tended to increase within 2–4 weeks. This may be related to the preexisting inflammatory level in the periodontal tissues of the rats with low occlusal function. Moreover, regardless of the degree of damage, all three methods applied mechanical forces to the teeth, further aggravating the inflammatory level of the periodontal tissues. Studies have shown that mechanical stimulation can promote the secretion of inflammatory factors in the tissues around the alveolar bone, thus potentially affecting alveolar bone remodeling33. Jia et al.34. confirmed that when cyclic stress was applied to human periodontal ligament fibroblasts in an inflammatory state, the levels of the inflammation-related markers TNF-α, IL-1β, IL-6, RANKL, and M-CSF increased significantly. This finding is consistent with our results.
Studies have shown that proinflammatory factors such as TNF-α and IL-1β can inhibit the differentiation of osteoblasts by suppressing the expression of BMP and RUNX2 and can also upregulate the expression of RANKL, thereby activating the differentiation of osteoclasts. In this study, the secretion of proinflammatory factors in the periodontal tissues of rats increased after occlusal function decreased. Therefore, we explored the effects of the abovementioned treatment methods on alveolar bone remodeling in rats with low occlusal function. Bone remodeling is a process in which new bone replaces old bone. Fundamentally, it involves osteoblast-mediated bone formation and osteoclast-mediated bone resorption. An imbalance between the two leads to abnormal bone remodeling35. A reduction in occlusal function can lead to a decrease in bone density, and this situation is reversed when occlusal function is restored, which highlights the importance of normal occlusal function for effective bone remodeling36. In this study, the levels of bone remodeling-related factors in the periodontal tissues of the rats in each group were detected 2–4 weeks after treatment. Compared with those in rats with normal occlusion, the levels of the osteoblast differentiation factors BMP2 and BGP in the periodontal tissues of rats with low occlusion decreased over time, whereas the levels of the osteoclast differentiation factors MMP9 and RANKL increased over time. Orthodontic treatment, occlusal restoration intervention, and intervention followed by orthodontic treatment could reverse the levels of these factors to a certain extent. Among them, the method of intervention followed by orthodontic treatment had the most significant reversing effect, and significant reversals of BMP2 and MMP9 were observed as early as 2 weeks after treatment. The changes in the expression levels of these factors were similar to the trends of the abovementioned proinflammatory factors, indicating that proinflammatory factors may be the cause of the increase in osteoclasts. In addition, the response of the abovementioned factor levels to orthodontic forces applied to rats with low occlusal function was more sensitive than that to occlusal restoration measures, and significant changes in these factors were observed only 3–4 weeks after treatment. In conclusion, our study shows that staged intervention followed by orthodontic treatment can reverse the levels of alveolar bone remodeling-related factors in rats with low occlusal function more quickly by preferentially reducing the inflammatory response in periodontal tissues.
We also detected the expression of CTSK, an indicator of osteoclast activity. The expression of CTSK in the periodontal tissues of rats with low occlusal function was significantly greater than that in the periodontal tissues of rats with normal occlusal function. After 2–4 weeks of orthodontic treatment, the expression of CTSK continued to increase. In contrast, after the intervention, the expression of CTSK continuously decreased within 3 weeks, and its expression was significantly lower than that in the low occlusal function group after 4 weeks of treatment. There was no significant difference in the expression of CTSK 2 weeks after the intervention followed by orthodontic treatment; it increased significantly after 3 weeks and decreased to a level close to normal after 4 weeks. As CTSK is a key enzyme in osteoclast differentiation, its expression reflects the activity of osteoclasts. In this study, due to the lack of mechanical force stimulation, the activity of osteoclasts was enhanced in rats with low occlusal function. The orthodontic force failed to reverse the level of CTSK within 4 weeks, possibly because osteoclasts still dominated the periodontal tissues of the rats with low occlusal function in the short term. Treatment with occlusal restoration intervention followed by orthodontic treatment may first promote the differentiation of osteoblasts in periodontal tissues to a certain extent. After orthodontic force was applied, the differentiation of osteoclasts was promoted. Therefore, a significant increase in CTSK scores was observed 3 weeks after treatment. The activity of osteoclasts was inhibited in the 4th week, and osteogenic differentiation was increased, suggesting that treatment with orthodontic intervention may improve patient prognosis by regulating the balance between bone resorption and bone formation.
Studies have shown that the β-catenin pathway is a key pathway involved in the response to mechanical force stimulation. This pathway can promote the expression of downstream target genes such as RUNX2 and osteocalcin, enhance the osteogenic differentiation ability of cementoblasts, and promote the balance of alveolar bone remodeling37,38. SIRT1 is an upstream regulator of this pathway. Previous studies have shown that SIRT1 can affect bone remodeling by activating the β-catenin signaling pathway26. The results of RT-qPCR revealed that the expression of SIRT1 and β-catenin in the periodontal tissues of rats with low occlusal function was significantly downregulated. The occlusal restoration intervention has not reversed its expression level after 4 weeks of treatment. However, orthodontic treatment can significantly reverse it at the 3rd week, and the treatment combining occlusal restoration intervention first followed by orthodontic treatment can significantly reverse it at the 2nd week. The Western blot results further verified these findings.
In the typical Wnt/β-catenin signaling pathway, after the Wnt ligand binds to the receptor, it can regulate the activity of β-catenin through GSK3β, thereby regulating cell proliferation, differentiation, and apoptosis. This study demonstrated that the expression of GSK3β increased significantly over time after occlusal function impairment. Consistent with the findings of Tang et al., the expression level of GDK3β was negatively correlated with the expression of β-catenin39. Orthodontic treatment or occlusal restoration intervention can reverse their expression. In addition, RT‒qPCR and Western blot experiments further verified that the reduction in occlusal function promoted osteoclast differentiation (manifested as the increase in its key transcription factors NFATC1 and RANK) and inhibited osteoblast differentiation (manifested as the decrease in its key transcription factors OPN and RUNX2). The complexity of the bone remodeling process may explain the differences in the protein expression trends observed during the treatment cycles of the different methods in this study40. Notably, both the application of orthodontic forces and the occlusal restoration intervention exerted mechanical force stimulation on teeth with low occlusal function. These mechanical forces may be involved in the process by which SIRT1 upregulates the β-catenin signaling pathway to regulate alveolar bone remodeling in rats with reduced occlusal function41.
The results of our study provide key targets and a theoretical basis for the development and optimization of future clinical treatment strategies for teeth with low occlusal function. After the intervention, the combined application of orthodontic treatment can significantly improve the inflammatory response of the dental tissues in rats with low occlusal function and markedly promote bone formation. Therefore, in future clinical treatments, combined intervention and orthodontic treatment plans should be preferentially considered. In addition, this study indicates that orthodontic forces may promote alveolar bone remodeling by regulating the β-catenin pathway through SIRT1. Therefore, for patients with poor orthodontic treatment outcomes, it may be possible to develop drugs or biological reagents that regulate the activities of the SIRT1 and β-catenin pathways can be developed to regulate the balance of osteoblast and osteoclast differentiation in alveolar bone, thereby reducing the risk of excessive alveolar bone resorption and periodontal tissue damage and improving the prognosis of orthodontic treatment. However, this study has certain limitations. First, only changes within 4 weeks were observed in this study, and it is impossible to determine the long-term effects. Second, this study explored only the effects of the abovementioned treatment methods on the periodontal tissues of rats at the basic research level and the sample size is not large. Third, there are differences between animal models and human clinical situations. The existence of various factors, such as individual differences and the oral environment, may affect the application of these research results in clinical practice. In subsequent studies, we will further verify the impact of the method of intervention followed by orthodontic treatment on the macroscopic state of rat teeth and verify the molecular mechanism by which mechanical force stimulation targets SIRT1 to regulate the β-catenin pathway to promote alveolar bone remodeling at the cellular level to improve the effectiveness and safety of intervention followed by orthodontic treatment in clinical patients.
In summary, this study explored the effects of three treatment methods on the periodontal tissues of rats with low occlusal function and their molecular mechanisms through in vivo experiments. This study revealed that orthodontic treatment alone can induce alveolar bone remodeling in rats with decreased occlusal function, but this process is accompanied by adverse tissue changes. Intervention alone can effectively reduce tissue damage and promote alveolar bone remodeling, but the effect of this method is slow. Intervention followed by orthodontic treatment combines the advantages of both methods, reducing the damage caused by directly applying orthodontic forces to periodontal tissues with low occlusal function, and may be a better treatment method for low occlusal function. Moreover, this study revealed for the first time that mechanical force stimulation may promote the activation of the β-catenin signaling pathway through SIRT1, regulating alveolar bone remodeling in rats with low occlusal function. These findings fill the literature gap regarding the molecular mechanisms involved in the treatment of teeth with low occlusal function, highlighting the innovativeness and significance of this study.
Data availability
Available on request from the corresponding author.
Abbreviations
- BMP2:
-
Bone morphogenetic protein 2
- BGP:
-
Bone gla protein/osteocalcin
- RANKL:
-
Receptor activator of nuclear factor κ-B ligand
- MMP9:
-
Matrix metalloproteinase 9
- SIRT1:
-
Silent information regulator 1
- OPN:
-
Osteopontin
- RUNX2:
-
Runt-related transcription factor 2
- CTSK:
-
Cathepsin K
- NFATC1:
-
Nuclear factor of activated T cells cytoplasmic 1
- GSK3β:
-
Glycogen synthase kinase 3β
References
Yi, Q. et al. Comparison of dynamic mechanical properties of dentin between deciduous and permanent teeth. Connect. Tissue Res. 62, 402–410. https://doi.org/10.1080/03008207.2020.1758684 (2021).
Sollenius, O. et al. Three-dimensional evaluation of forced unilateral posterior crossbite correction in the mixed dentition: A randomized controlled trial. Eur. J. Orthod. 42, 415–425. https://doi.org/10.1093/ejo/cjz054 (2020).
Bukhari, A., Kennedy, D., Hannam, A., Aleksejūnienė, J. & Yen, E. Dimensional changes in the palate associated with slow maxillary expansion for early treatment of posterior crossbite. Angle Orthod. 88, 390–396. https://doi.org/10.2319/082317-571.1 (2018).
Khayat, N. et al. The prevalence of temporomandibular disorders and dental attrition levels in patients with posterior crossbite and/or deep bite: A preliminary prospective study. Pain Res. Manag. 2021, 8827895. https://doi.org/10.1155/2021/8827895 (2021).
Ebadian, B., Abbasi, M. & Nazarifar, A. M. Frequency distribution of temporomandibular disorders according to occlusal factors: A cross-sectional study. Dent. Res. J. 17, 186–192 (2020).
Song, Y. L. & Yap, A. U. Impact of pain-related temporomandibular disorders on jaw functional limitation, psychological distress and quality of life in postoperative class III East Asian patients. Clin. Oral Invest. 24, 953–961. https://doi.org/10.1007/s00784-019-02994-x (2020).
Zhou, C. et al. Expert consensus on pediatric orthodontic therapies of malocclusions in children. Int. J. Oral Sci. https://doi.org/10.1038/s41368-024-00299-8 (2024).
Wang, J., Zhang, R., Zhang, Z., Geng, C. & Zhang, Y. Micro-computed tomography evaluation of the effects of orthodontic force on immature maxillary first molars and alveolar bone mineral density of Sprague-Dawley rats. Korean J. Orthod. 53, 205–216. https://doi.org/10.4041/kjod22.209 (2023).
Fan, J. & Caton, J. G. Occlusal trauma and excessive occlusal forces: Narrative review, case definitions, and diagnostic considerations. J. Clin. Periodontol. 45 (Suppl 20), S199–S206. https://doi.org/10.1111/jcpe.12949 (2018).
Takahashi, T., Hatta, K. & Ikebe, K. Risk factors of cognitive impairment: Impact of decline in oral function. Jpn. Dent. Sci. Rev. 59, 203–208. https://doi.org/10.1016/j.jdsr.2023.06.006 (2023).
Dimitroulis, G. Management of temporomandibular joint disorders: A Surgeon’s perspective. Aust. Dent. J. 63 (Suppl 1), S79–s90. https://doi.org/10.1111/adj.12593 (2018).
Albagieh, H. et al. Occlusal splints-types and effectiveness in temporomandibular disorder management. Saudi Dent. J. 35, 70–79. https://doi.org/10.1016/j.sdentj.2022.12.013 (2023).
Jung, H. J., Hwangbo, N. K., Park, Y. & Ahn, H. J. Effects of gum chewing training on occlusal force, masseter muscle thickness and mandibular shape: A randomised controlled clinical trial. J. Rehabil. 51, 2529–2536. https://doi.org/10.1111/joor.13830 (2024).
Papageorgiou, S. N., Giannakopoulou, T., Eliades, T. & Vandevska-Radunovic, V. Occlusal outcome of orthodontic treatment: A systematic review with meta-analyses of randomized trials. Eur. J. Orthod. https://doi.org/10.1093/ejo/cjae060 (2024).
Papadimitriou, A., Mousoulea, S., Gkantidis, N. & Kloukos, D. Clinical effectiveness of Invisalign® orthodontic treatment: A systematic review. Prog. Orthodont. https://doi.org/10.1186/s40510-018-0235-z (2018).
Li, Y., Zhan, Q., Bao, M., Yi, J. & Li, Y. Biomechanical and biological responses of periodontium in orthodontic tooth movement: Up-date in a new decade. Int. J. Oral Sci. https://doi.org/10.1038/s41368-021-00125-5 (2021).
Li, Y., Ling, J. & Jiang, Q. Inflammasomes in alveolar bone loss. Front. Immunol. 12, 691013. https://doi.org/10.3389/fimmu.2021.691013 (2021).
Galler, K. M., Grätz, E. M., Widbiller, M., Buchalla, W. & Knüttel, H. Pathophysiological mechanisms of root resorption after dental trauma: A systematic scoping review. BMC Oral Health. 21, 163. https://doi.org/10.1186/s12903-021-01510-6 (2021).
Hatrom, A. A. et al. Pulp volume changes after piezocision-assisted tooth movement: A randomized clinical trial. BMC Oral Health https://doi.org/10.1186/s12903-020-01382-2 (2021).
Wei, D., Zhang, L., Li, W. & Jia, Y. Quantitative comparison of cephalogram and cone-beam computed tomography in the evaluation of alveolar bone thickness of maxillary incisors. Turkish J. Orthod. 33, 85–91. https://doi.org/10.5152/TurkJOrthod.2020.19097 (2020).
Arroyo, R. et al. Carboxy-terminal cementum protein 1-derived peptide 4 (cemp1-p4) promotes mineralization through wnt/β-catenin signaling in human oral mucosa stem cells. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21041307 (2020).
Lu, X., Yang, J., Zhao, S. & Liu, S. Advances of Wnt signalling pathway in dental development and potential clinical application. Organogenesis 15, 101–110. https://doi.org/10.1080/15476278.2019.1656996 (2019).
Chen, K. et al. Osteocytic HIF-1α pathway manipulates bone Micro-structure and remodeling via regulating osteocyte terminal differentiation. Front. Cell. Dev. Biol. 9, 721561. https://doi.org/10.3389/fcell.2021.721561 (2021).
Tokavanich, N., Wein, M. N., English, J. D., Ono, N. & Ono, W. The role of Wnt signaling in postnatal tooth root development. Front. Dent. Med. https://doi.org/10.3389/fdmed.2021.769134 (2021).
Chen, Y. et al. SIRT1, a promising regulator of bone homeostasis. Life Sci. 269, 119041. https://doi.org/10.1016/j.lfs.2021.119041 (2021).
Zhang, W. et al. Reversing the imbalance in bone homeostasis via sustained release of SIRT-1 agonist to promote bone healing under osteoporotic condition. Bioact. Mater. 19, 429–443. https://doi.org/10.1016/j.bioactmat.2022.04.017 (2023).
Ghorbanpour, S., Pourhajibagher, M., Noroozian, M., Ghaffari, H. & Bahador, A. Photoactivation of curcumin doped poly-lactic-co-glycolic acid nanoparticles in rat model with fixed orthodontic appliances. TheScientificWorldJournal 2022 3613345. https://doi.org/10.1155/2022/3613345 (2022).
Fan, Z., Pathak, J. L. & Ge, L. The potential role of RP105 in regulation of inflammation and osteoclastogenesis during inflammatory diseases. Front. Cell. Dev. Biol.. 9, 713254. https://doi.org/10.3389/fcell.2021.713254 (2021).
Kim, K. M. et al. Interferon Β protects against avascular osteonecrosis through Interleukin 6 Inhibition and silent information regulator transcript-1 upregulation. Oncotarget 9, 3562–3575. https://doi.org/10.18632/oncotarget.23337 (2018).
Zong, C., Van Dessel, J., Vande Velde, G., Willems, G. & Cadenas de Llano-Pérula, M. Dynamic changes in tooth displacement and bone morphometry induced by orthodontic force. Sci. Rep. 12, 13672. https://doi.org/10.1038/s41598-022-17412-8 (2022).
Kakali, L. et al. Fluctuation of bone turnover markers’ levels in samples of gingival crevicular fluid after orthodontic stimulus: A systematic review. Syst. Rev. https://doi.org/10.1186/s13643-021-01860-w (2022).
Damanaki, A. et al. Influence of occlusal hypofunction on alveolar bone healing in rats. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24119744 (2023).
He, D. et al. Mechanical load-induced H(2)S production by periodontal ligament stem cells activates M1 macrophages to promote bone remodeling and tooth movement via STAT1. Stem Cell. Res. Ther. 11, 112. https://doi.org/10.1186/s13287-020-01607-9 (2020).
Jia, R. et al. Cyclic compression emerged dual effects on the osteogenic and osteoclastic status of LPS-induced inflammatory human periodontal ligament cells according to loading force. BMC Oral Health https://doi.org/10.1186/s12903-019-0987-y (2020).
Hong, S. Y. et al. Alveolar bone remodeling during maxillary incisor intrusion and Retraction. Prog. Orthodont. https://doi.org/10.1186/s40510-019-0300-2 (2019).
Yotsuya, M., Iriarte-Diaz, J. & D, A. R. Temporomandibular joint hypofunction secondary to unilateral partial discectomy attenuates degeneration in murine mandibular condylar cartilage. Bull. Tokyo Dent. Coll. 61, 9–19. https://doi.org/10.2209/tdcpublication.2019-0008 (2020).
吴铭 J. 中. 调控骨髓间充质干细胞成骨分化的Wnt/β-catenin信号通路及相关因素. 25, 7 (2021).
Shao, X., Hu, Z., Su, H., Wang, Y. & Lin, Y. Effects of tension on mitochondrial autophagy and osteogenic differentiation of periodontal ligament stem cells. Cell Prolif. 57, e13561. https://doi.org/10.1111/cpr.13561 (2024).
Tang, J., Qing, M. F., Li, M. & Gao, Z. Dexamethasone inhibits BMP7-induced osteogenic differentiation in rat dental follicle cells via the PI3K/AKT/GSK-3β/β-catenin pathway. Int. J. Med. Sci. 17, 2663–2672. https://doi.org/10.7150/ijms.44231 (2020).
Feng, X. & McDonald, J. M. Disorders of bone remodeling. Annu. Rev. Pathol. 6, 121–145. https://doi.org/10.1146/annurev-pathol-011110-130203 (2011).
Bordukalo-Nikšić, T., Kufner, V. & Vukičević, S. The role of BMPs in the regulation of osteoclasts resorption and bone remodeling: From experimental models to clinical applications. Front. Immunol. 13, 869422. https://doi.org/10.3389/fimmu.2022.869422 (2022).
Funding
This study was supported by a joint special top-level project of Kunming Medical University, Department of Science and Technology of Yunnan Province (202201AY070001-269).
Author information
Authors and Affiliations
Contributions
Juanjuan Ji was responsible for the conceptualization, methodology, and writing of the manuscript. Zhi Zhou contributed to the conceptualization, data collation, and formal analysis. Yaling Zhu and Rui Wang were responsible for the software development and validation. Yali Liu oversaw the conceptualization, access to funding, methodology, project management, resources, supervision, and the review and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All animal experiments conducted in this study received ethical approval from the Ethics Committee of Kunming Medical University.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ji, J., Zhou, Z., Zhu, Y. et al. Orthodontic treatment after occlusal intervention balances osteoblast and osteoclast differentiation via SIRT1 beta catenin signaling in rats with hypofunctional occlusion. Sci Rep 15, 13872 (2025). https://doi.org/10.1038/s41598-025-98800-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98800-8