Abstract
In nanomedicine, gold nanoparticles (GNPs) are based on metal-based nanomaterials and have attracted considerable attention from researchers for their use in drug delivery, including wound healing. This study examined the use of Gum tragacanth (GT) for producing gold nanoparticles (GNPs) through the green synthesis method (GT-GNPs). The antibacterial activity of GT-GNPs against pathogenic bacterial strains, as well as assess the wound healing potential of GT-GNPs combined with photobiomodulation (PBM) on normal and diabetic wound models (in vitro). In physicochemical characterization results, we found that the synthesized nanoparticles are 10–20 nm with an average of 12 ± 2 nm in size, and smooth surface and confirmed the presence of GT on the GT-GNPs. It also possessed optimal antibacterial activity and was confirmed through flow cytometry studies. Furthermore, we examined the combined effect of GT-GNPs and PBM against normal and diabetic wound models in WS1 (Humal Fibroblast cells), including cell morphology, percentage of wound closure, nuclear, and filamentous (F)-actin morphology. The combined effects of GT-GNPs and PBM effectively achieved progressive wound closure. In summary, this study has demonstrated that treating GT-GNPs has no adverse effects on both normal and diabetic wound healing processes.
Similar content being viewed by others
Introduction
Gold nanoparticles (GNPs) have recently attracted considerable interest in pharmaceutical and medicinal research due to their valuable physicochemical properties. Historically, gold was considered the “Elixir of Life”1,2. Several studies have revealed that GNPs have a wide range of medicinal applications. This includes gene delivery, molecular therapeutics, imaging, drug delivery, cancer diagnosis, and therapeutic applications3,4,5. The gold nanoparticle is an effective carrier for drug delivery due to its excellent biocompatibility and nontoxic properties. Gold nanoparticles also show a high affinity for specific receptors, making them suitable for treating different diseases, including cancer6,7.
GNPs can be synthesized using several methods, but citrate reduction is the most used method. However, green chemistry has been shown to provide substantial advantages for synthesizing GNPs8,9. Various reducing agents can synthesize GNPs, including bacteria, fungi, plant extracts, fruits, and essential oils10,11,12. A major advantage of green synthesis is its low cost and ease which makes it is possible to control the physicochemical parameters, ultimately enabling a toxic-free product13. In the food industry, natural gums, polysaccharides, and starch moieties can be used as additives as they contain several functional groups. It is possible to use several natural gums as reducing and capping agents in the production of nanomaterials and it has been well documented in the literature14,15,16,17,18,19,20,21. Some of them are Gellan gum, Xanthan gum, Arabic gum, Katira gum, Guar gum, and Gum Tragacanth (GT)14,15,16,17,18,19,20,21. Gum tragacanth has been identified as a non-toxic polysaccharide macromolecule, a biocompatible, biodegradable polysaccharide that accelerates the healing of skin wounds22.
The use of plant-based systems in treating illnesses has been documented in the literature23,24. Furthermore, they are non-invasive, inexpensive, and widely available, making them an ideal choice for primary health care. World Health Organization (WHO) statistics indicate that approximately 80% of the world’s population from developing countries still uses traditional medicine23. Modern science currently utilizes natural resources to treat a wide range of diseases, including wound healing. There has been considerable interest in using naturally available material as a source of medicine for the treatment of wounds. Some selected natural products are increasingly used in wound healing applications because of their versatile properties, such as their anti-inflammatory, antioxidant, antibacterial, and procollagen synthesis properties24. According to Chen et al. (2012), topical treatment with gold nanoparticles and antioxidants significantly regulates angiogenesis and anti-inflammatory effects and accelerates diabetic cutaneous wound healing25.
Photobiomodulation (PBM) involves irradiating the affected area with low-power density light, and the positive outcomes of PBM have been well documented in the literature26,27,28. It is well known that PBM stimulates the healing process of diabetic wounds and has been extensively used by researchers in wound healing26,27,28. The use of PBM for treating diabetic wounds has also been demonstrated to be effective29. Laser irradiation of diabetic wounded human skin fibroblast cells increased cell migration, viability, proliferation, and collagen production30. In a recent study, the effects of GNPs and PBM therapy were examined in vivo, by measuring the outcome of 808 nm laser irradiation in a wound rat model31. In our previous study, the combined effects of silver nanoparticles and PBM at 830 nm with 5 J/cm2 demonstrated improved results in diabetic wounded models32.
The purpose of this study was to investigate the need for effective wound-healing therapies, particularly for diabetics who often experience complications during wound healing. To enhance wound healing, novel approaches may be necessary in addition to traditional treatments. In this study, we hypothesize that gum tragacanth-reduced gold nanoparticles (GT-GNPs) may possess properties conducive to wound healing, including antibacterial activity and photobiomodulation (PBM) therapy potential. Gold nanoparticles were designed using Gum Tragacanth due to their excellent biocompatibility, stabilizing properties, and alignment with green chemistry principles. A natural polysaccharide structure provides the functional groups necessary for reducing gold ions and stabilizing nanoparticles, preventing aggregation, and maintaining stability. Furthermore, it is cost-effective and environmentally friendly, making it an ideal choice for large-scale nanoparticle production, providing a sustainable and efficient method for synthesis.
The GT-GNPs are predicted to have significant antibacterial effects against pathogenic strains and demonstrate promising healing properties, particularly in diabetic wound models. We contribute to the knowledge gap by exploring the potential of GT-GNPs as a multifaceted approach to wound healing through the present study. This study addresses the gap in understanding the application of nanomaterials in wound management, particularly in diabetic conditions, by synthesizing and characterizing GT-GNPs, assessing their antibacterial activity, and their efficacy in wound healing using PBM therapy. The study also provides insight into the potential effectiveness of GT-GNPs across different wound-healing environments by comparing their performance in both normal and diabetic wound models. The primary objective of this study is to evaluate the impact of GT-GNPs and PBM on accelerating the healing process of diabetic wounds in comparison to normal wounds.
Materials and methods
Materials description
Gum Tragacanth [CAS Number 9000-65-1, Product number - G1128-100G, Molecular Weight − 840000], and Gold(III) chloride hydrate (HAuCl4. xH2O) (CAS Number 27988-77-8, Product number—254156-5G) were purchased from Sigma-Aldrich (Merck). All experiments were conducted using deionized water. We used only analytical-grade chemicals and reagents in our study.
Preparation of GT solution and GT-GNPs
Gum tragacanth solution and GT-GNPs were prepared according to21. Briefly, the GT (0.8 g) solution in 1 L of deionized water and the GT solution was allowed to stir for the next 24 h at room temperature and then filtered using 0.20 μm filters. The final filtered solution was stored in the refrigerator (4 °C) for further studies. Gold solution (0.1 mL, 5 mM) was added to GT solution (1 mL, 5 mg/mL) and stirred at 150 rpm at 65 °C for 4 h. The appearance of colorless to deep purple indicates the synthesis of GT-GNPs. Synthesized GT-GNPs will be further evaluated using different physicochemical characterization.
Physicochemical characterization
The physicochemical characterization of GNPs and GT-GNPs was studied using different methods such as UV spectrophotometry method, zeta potential analysis using the DLS (Dynamic Laser Scattering) method, Surface chemistry study by Fourier transform infrared spectroscopy (FTIR), and X-ray diffraction study (XRD), and morphological evaluation study by high-resolution electron microscope (HRTEM), and the stepwise procedure are followed according to Kumar et al., 2018a33. In HRTEM results, an interactive 3D surface plot image was created using the image analysis software, Image J33.
Antibacterial activity studies
In this study, the antibacterial activity of GT and GT-GNPs was tested against the bacterial strains Pseudomonas aeruginosa ATCC 27,853 (Gram-negative) and Staphylococcus aureus ATCC BAA-1026 (Gram-positive). The antibacterial properties of GT and GT-GNPs was evaluated by flow cytometry-based live/dead cell analysis according to Kumar, et al., 2018b34. In the antibacterial study, GT-GNPs were used at a concentration of 24 µg/mL for all the assays. Ciprofloxacin Antimicrobial Susceptibility discs (CIP 5 mcg - OXOID, CT0425B) and Piperacillin/Tazobactam Antimicrobial Susceptibility discs (TZP 110 mcg - OXOID, CT0725B) used as a positive control for Gram-positive and Gram-negative bacteria, respectively, while a nutrient medium served as a negative control for all the bacterial study.
Cell culture and laser irradiation
Cell culture
Human skin fibroblast cells (WS1, ATCC®, CRL-1502™) were used for the experiments. The cells were cultured in the minimum essential medium (MEM, M7278, Sigma–Aldrich, South Africa) with supplements as reported in the recent study32. The University of Johannesburg’s Faculty of Health Science Research Ethics Committee approved this study’s experiments ethically, with the ethical clearance number REC-01-110-2019. All other chemicals and reagents used in the study were of analytical grade. Deionized water (MilliQ element, 18 Mohm/cm) was used for all the experiments. This study was conducted using two different models and their corresponding groups shown in Table 1.
The diabetic model was developed by continuously growing cells in a medium containing high glucose as described35. A wound was created using a sterile disposable 1 mL pipette, as explained in the literature36. Following the treatment with 24 µg/mL GT-GNPs, the selected groups were incubated for 30 min and irradiated. For this study, non-irradiated cells (0 J/cm2) are used as controls. We used a maximum concentration of 24 µg/mL GT-GNPs in our antibacterial study, and it showed satisfactory levels of bactericidal activity against the studied microorganisms, therefore, we used 24 µg/mL concentration in all the cellular assays. Laser irradiation at a wavelength of 830 nm, and we irradiated for 7 min 16 s to achieve 5 J/cm2. Cells were irradiated in 3.4 cm diameter tissue culture plates containing 1 mL of culture medium, with the lid off, covering a spot size of 9.1 cm². Cellular responses were evaluated by various methods after incubation for 24 and 48 h32.
Cellular responses
Cellular morphology was determined for all groups using an inverted microscope. At different intervals of time (0, 24, and 48 h), images were taken at the same location. The ‘Olympus cellSens Entry’ software was used to measure the distance between two edges of the wound. The luminescent cell viability assay was conducted according to the procedure detailed in our previous study32. The percentage of wound closure was calculated using the formula given in the literature37. Rhodamine Phalloidin (RP) and Hoechst staining were used to determine filamentous actin, and nuclear morphology, respectively, as per the procedure reported in our previous study32.
Statistical analysis
All graphs were created using GraphPad Prism (version 5.01). To ensure reproducibility, the experiments were performed in triplicate. SigmaPlot version 13.0 was used for statistical analysis. Student t-tests and one-way analyses of variance (ANOVA) were used to determine differences between groups and models, respectively.
Results and discussion
Gold nanoparticles have excellent optical properties, which makes them ideal for therapeutic imaging, targeting, and drug delivery. Furthermore, they possess antibacterial properties that make them useful in the treatment of infectious diseases and pathogen-infected wounds6,38. Growth factor-modified GNPs are more conducive and promote wound healing by re-epithelializing wounds in both in vivo and in vitro models39. It has been demonstrated that GNPs accelerate wound healing by influencing collagen, VEGF, cytokines (IL10, IL4), and growth factors (fibroblast growth factor and transforming growth factor). The association between PBM and hyaluronic acid-based GNPs exhibits anti-inflammatory, and antioxidant properties in an epithelial lesion model. The study also confirmed the secretion of cell differentiation growth factors that contribute to the repair process40,41. The PBM procedure involves the use of low-level laser light that penetrates the skin and promotes the regeneration and repair of tissue. Additionally, it can increase circulation, reduce inflammation, and promote the production of cellular energy42,43.
These observations led us to synthesize GT-GNPs which were characterized by employing various types of physicochemical characterization methodologies (UV spectrophotometry method, DLS method for zeta potential analysis, Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction for surface chemistry, and high-resolution transmission electron microscopy (HRTEM) for morphological evaluation. As part of the investigation into the antibacterial activity of GT-GNPs, pathogenic strains (Gram-negative and Gram-positive) were examined, and antibacterial activities were evaluated using various methods such as flow cytometry-based live/dead cell analysis. Additionally, the wound healing abilities of PBM and GT-GNPs have been assessed in NW and DW cell models using different methods, such as cellular morphology, percentage wound closure, and nuclear and F-actin morphology.
Synthesis of GT-GNPs
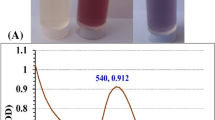
Our study demonstrated a simple, facile, method for the production of GT-GNPs, as reported by Rao, et al., 201721. To be precise, a mixture of gold solution (0.1 mL, 5 mM) and GT solution (1 mL, 5 g/mL) was magnetically stirred, and a deep purple color was observed from an initial colorless stage, indicating the formation of GT-GNPs (Fig. 1a) and the biocompatible GT has acted as a reducing, capping, and stabilizing agent. Naturally available polysaccharides are widely used as a reducing, capping, and stabilizing to produce GNPs owing to their excellent biological activity, including biocompatibility, nontoxicity, and easy modification. The mechanisms of GNPS synthesis from natural polysaccharides are well documented in the literature44. It has been observed that polysaccharide glycosidic bonds are broken via Au (III) and base-mediated mechanisms, reducing free hydroxyl groups and amino groups. The structural parameters of polysaccharides play an important role in the controlled synthesis of polysaccharide-based GNPs. These include the molecular weight, the composition of monosaccharides, the presence of functional groups, and the type of glycosidic bond44.
Physicochemical characterization of GT-GNPs
A UV-vis spectrophotometer study determined the peak band for surface plasmon resonance (SPR) in gold solution, GT solution, and GT-GNPs. According to the UV-vis spectrophotometer results, an SPR band of GT-GNPs (a mixture of gold solution (5 mL, 5 mM) and GT solution (1 mL, 5 g/mL) appeared at 541 nm (indicated in blue color in Fig. 1b). This peak indicates the presence of the characteristic features of GNPs. However, these peak bands were not observed in gold or GT solutions. There is a peak in the UV-VIS spectrum of gold suspension (0.1 mL, 5 mM HAuCl4) at 297 nm (indicated in red color in Fig. 1b), owing to the charge transfer transitions from ligand to metal45. The UV spectrum of GT did not show any potential peak in the studied wavelength range of 200–800 nm (indicated in black color in Fig. 1b). Gold suspension (HauCl4- ions) precursor band was not present in the GT-GNPs spectrum, indicating that most HauCl4- ions were reduced to Au0 as GNPs46. Here, we confirm the presence of GNPs in our results (SPR peak at 541 nm), which are consistent with previous reports. Rao et al. (2017) synthesized GNPs using GT as a reducing agent and observed that the surface plasmon resonance (SPR) band appeared in the wavelength range of 548 nm21. Similarly, Chen et al. (2021) described the presence of multiple SPR bands in the UV range of 520–585 nm using Hedysarum polysaccharides as a reducing agent47.
The zeta potential of GT-GNPs was studied using the dynamic laser scattering method, revealing a surface charge of approximately − 14.1 mV (Fig. 1c). A study has shown that barium sulfate negative zeta potential is more effective at improving skin barrier function than barium sulfate positive zeta potential48. Zeta potential, as a function of the negative surface charge of the material and colloidal stability, is often considered to be the most decisive factor in ensuring the biocompatibility of the material, and it provides an indication of the higher cell viability in biocompatibility and wound healing tests49,50. The DLS experiment showed 198.7 ± 3.7 nm (Fig. 1d) and 0.588 ± 0.03 PdI (Polydispersity Index), and this result is due to the water-absorbable properties inherent in the gum or polysaccharide material, which could lead to larger apparent sizes in DLS measurements compared to our HR-TEM results. The surface chemistry of GT-GNPs was further examined using X-ray crystallography (Fig. 1e). A literature study and JCPDS No.: 4-0784 indicate that four strong Bragg reflections (111), (200), (220), and (311) were observed for GNP peaks at 2Ɵ=38.1°, 44.2°, 64.8°, and 77.3°33,51. The X-ray diffractions of GT (Fig. 1e) showed a completely amorphous nature, whereas GT-GNPs showed Bragg reflections (111) plane at 2Ɵ=38.1°. It has been found that the peak intensity of GT-GNPs has decreased significantly due to their amorphous nature, which may be due to the addition of natural GT polysaccharides onto GT-GNPs.
Synthesis and physicochemical characterization of GT-GNPs. [a] Photographic images of GT-GNPs [i] Initial hours (0 h), and [ii] final hours (4 h at 65 °C, 150 rpm stirring); [b] UV–vis spectrophotometry study of GT, Gold solution, and GT-GNPs, [c] Zeta potential analysis of GT-GNPs, [d] Particle size analysis of GT-GNPs, [e] XRD pattern of GT and GT-GNPs, and [f] FTIR results Gold (blue line), GT (black line) and GT-GNPs (red line).
A FTIR spectrum of Gold(III) chloride hydrate, GT and GT-GNPs was recorded to identify the presence of GT functional groups involved in the reduction of Au ions (Fig. 1f). In the FTIR spectrum of Gold (III) chloride hydrate (blue line), a prominent peak is typically observed around 3400 cm− 1, which corresponds to the stretching vibrations of water molecules in the hydrate form. This peak suggests the presence of hydrogen-bonded water molecules associated with the gold chloride complex. Figure 1f shows that the major absorbance peaks of GT were observed at 3338, 2928, 2856, 2175, 1735, 1610, 1419, 1357, 1237, and 1015 cm− 1. In the GT spectrum, a broad band at 3338 cm− 1 is likely to be caused by stretching vibrations of the O-H group. As illustrated in Fig. 1f, the bands between 2928 and 2856 cm− 1 correspond to asymmetric and symmetric stretching vibrations of methylene groups, respectively. The broad band at 2175 cm− 1 is due to the presence of carbonyl substances. The peak at 1735 cm− 1 can be evidenced by carbonyl stretching vibrations in aldehydes, ketones, and carboxylic acids. The peak at 1610 cm− 1 is a characteristic asymmetrical stretching of carboxylate groups. It has been demonstrated that the carboxylate group exhibits a symmetrical stretch at 1419 and 1357 cm− 1. The peaks at 1237 and 1015 cm− 1 are the result of the stretching vibrations between C-O on polyols, and on alcoholic groups, respectively. We observed that GT FTIR measurements are very similar to those reported by Kora and Arunachalam, 201252.
In Fig. 1f, the GT-GNPs nanoparticles showed characteristic absorbance bands at 3197, 2289, 2113, 1609, 1419, and 1124 cm− 1, respectively. According to the GT-GNPs IR spectrum, the peaks at 1609 and 1419 cm− 1, the gold ions are bound to the carboxylate groups of the gum, indicating the bond between the gold ions and the carboxylate groups. In the FTIR spectrum, the GT-GNPs exhibit a shift of carbonyl groups (2175 to 2113 cm− 1). These band shifts indicate that the carboxylate and carbonyl groups of GT played a significant role in the synthesis of gold nanoparticles. The intense band around 1015 cm−1 in the GT spectrum is due to the C–O–C stretching vibration of the glycosidic bond in GT. This band is absent in the GNPs spectrum because the formation of GNPs alters the chemical environment of GT, affecting the functional groups involved in binding with gold. The interaction between GT and GNPs changes the vibrational frequencies of certain bonds, causing the disappearance or shift of specific peaks, including the one at 1015 cm−1. This reflects the incorporation of GT into the nanoparticle matrix during the formation of GNPs.
GT-GNPs were further characterized by HRTEM to determine the size and shape of the nanoparticles. As shown in Fig. 2, a typical HRTEM image of GT-GNPs can be seen. Specifically, Fig. 2a, b, and c show well-dispersed GT-GNPs with an average diameter of 12 ± 2 nm and a mean size within the size range of 10–20 nm. A three-dimensional image (Fig. 2d–f) indicates that the surfaces of all the GT-GNPs were spherical in shape. According to Fig. 2g, the EDX elemental mapping represents the presence of Au in GT-GNPs. These results are highly corroborated with recent findings on natural polysaccharides reduced gold nanoparticles for cellular imaging and targeting therapy33.
Antibacterial activity of GT-GNPs
Live/Dead cell assay
Flow cytometric measurements were conducted on S. aureus (Fig. 3a) and P. aeruginosa (Fig. 3b) after treatment with GT and GT-GNPs at a concentration of 24 µg/mL, and the number of live, injured, and dead cells was determined. The assay uses a combination of dyes of thiazole orange and propidium iodide to distinguish between live and dead cells. Thus, quadrants 1 (Q1), 2 (Q2), 3 (Q3), and 4 (Q4) were used to represent the percentage of unstained, live, injured, and dead cells of both S. aureus and P. aeruginosa cells (Table 2). Figure 3a shows that the percentage of injured cells in GT-GNPs (17.65 ± 1.35) and GT (18.4 ± 0.4) treated S. aureus were much higher than compared to the positive controls (11.25 ± 4.75). However, the percentage of dead cell count was lower (0.1) in quadrant 4 of S. aureus treated with positive, GT, and GT-GNPs.
The percentage of injured cells in GT-GNPs (3.45 ± 1.25) and GT (4.4 ± 1.9) treated P. aeruginosa produced a similar effect compared to the positive controls (4.4 ± 0.9) are shown in Fig. 3b. The percentage of dead cell count was also observed in GT-GNPs (4.2 ± 0.7), GT (6.6 ± 1.1), and positive control (12.7 ± 0.1). Here, the positive control treated cells show more dead cells than GT and GT-GNPs treated cells. Our study demonstrated better antibacterial activity of the synthesized GT-GNPs in terms of causing bacterial injury to S. aureus bacterial cells and similar antibacterial effects in P. aeruginosa bacterial cells compared with the positive control. We also observed that the antibacterial activity of GT is slightly higher than that of GT-GNPs treatment likely due to its inherent antimicrobial properties, which are more pronounced in its natural polysaccharide form compared to the modified interaction with GNPs. We anticipate that the synthesized nanoparticles may induce cellular membrane damage, a commonly reported antimicrobial mechanism associated with gold nanoparticles53.
Effects of GT-GNPs and PBM treatments on wound healing
Cell viability, cellular morphology, and wound closure
The cell viability study evaluated the biocompatibility of synthesized GT-GNPs at concentrations of 4, 8, 16, and 24 µg/mL using the Cell Titer-Glo® luminescent cell viability assay. These concentrations of GT-GNPs were compared to the control, revealing that concentrations up to 24 µg/mL are safe and no prominent cell death occurs in the studied ranges. Thus, we used a maximum of 24 µg/mL concentration for all our cell culture studies (Fig. 4). We performed statistical comparisons for all groups with the control, as referenced in Fig. 4, and found that the p-value was not significant (4 µg/mL, p = 0.763; 8 µg/mL, p = 0.400; 16 µg/mL, p = 0.336; and 24 µg/mL, p = 0.329). A study was conducted to determine the morphology of cells and the percentage of wound closure. Figures 5a and 6a illustrate the images of NW and DW cell models, respectively. All the models exhibited flat and spindle-shaped WS1 cells at 24 and 48 h. Figures 5b and 6b illustrate the percentages of wound closure for NW and DW models, respectively. Figure 5b illustrates the wound closure (%) of the NW model at 24 h. No statistically significant differences were observed between NW 0 J cells and other groups (NW 0 J NP, p = 0.062; NW 5 J, p = 0.658; and NW 5 J NP, p = 0.064). The wound closure rate (%) at 48 h was significantly higher in NW 0 J cells when compared with NW 5 J (p < 0.01) and NW 5 J NP cells (p < 0.01). There was no statistically significant difference between NW 0 J and NW 0 J NP cells (p = 0.484).
In the diabetic wounded model at 24 h, the percentage of wound closure (Fig. 6b) was significantly higher in DW 0 J cells compared to DW 5 J cells (p < 0.01). There was no significant difference in DW 0 J cells compared to DW 0 J NP cells (p = 0.056) and DW 5 J NP cells (p = 0.192). At 48 h, complete wound closure was observed in DW 5 J NP cells (100%), and it was significantly increased compared to DW 0 J cells (p < 0.001). Wound closure (%) was significantly increased in DW 5 J cells compared to DW 0 J cells (p < 0.05), whereas DW 0 J NP cells significantly decreased compared to DW 0 J cells (p < 0.05). As shown in Figs. 5 and 6, the percentage of wound closure was compared between NW and DW models at 24 h and 48 h.
During the wound closure study at 24 h, results revealed that the percentage of wound closure was significantly higher in DW 0 J cells than in NW 0 J cells (p = 0.021). As a result of all the pairwise multiple comparison procedures (using the Holm-Sidak method), we set the overall significance level at 0.05 in the analysis. However, no statistically significant differences were observed between NW and other groups in DW cells (DW 0 J NP, p = 0.152; DW 5 J, p = 0.197; DW 5 J NP, p = 0.602). The variations in mean values across treatment models are insufficient to dismiss the possibility that the difference arises from random sampling variability. Hence, no statistically significant differences are observed.
During the wound closure study at 48 h, results revealed that the percentage of wound closure was significantly higher in DW 5 J (p < 0.001), DW 5 J NP (p < 0.001), and DW 0 J NP cells (p = 0.018) compared to NW 5 J, NW 5 J NP, and NW 0 J NP cells respectively. The differences in the mean values among the diabetic wounded models are greater than those of normal wounded models. Our analysis established the overall significance level at 0.05 using the Pairwise Multiple Comparison Procedures with the Holm-Sidak method. However, there were no statistically significant differences between NW 0 J and DW 0 J cells (P = 0.949) due to the variations in mean values across treatment models, which are insufficient to dismiss the possibility that the difference arises from random sampling variability. Overall, our study results revealed that both NW and DW cell models showed a progressive percentage of wound closure without any adverse effect of GT-GNPs. The diabetic wound model treated with combined laser and GT-GNPs treatment showed complete wound closure at 48 h.
Cellular morphology, and wound closure study in normal wounded cells. [a] Time-lapse micrographs of NW cells; [b] Percentage of wound closure. Abbreviations: µm, micrometer; NW, normal wounded; NP, nanoparticles; 0 J, non-irradiated cells; 5 J, irradiated cells; h, hour. Student t-test results are shown in the figure and their corresponding significance is shown as **p < 0.01.
Cellular morphology, and wound closure study in diabetic wounded cells. [a] Time-lapse micrographs of DW cells; [b] Percentage of wound closure. Abbreviations: µm, micrometer; DW, diabetic wounded; NP, nanoparticles; 0 J, non-irradiated cells; 5 J, irradiated cells; h, hour. Student t-test results are shown in the figure, and their corresponding significance is shown as *p < 0.05, **p < 0.01 and ***p < 0.001.
Filamentous actin and nuclear morphology analysis
Fluorescence microscopic images of normal wounded cells. [a] 24 h images of NW cells; [b] 48 h images of NW cells. The stain used: Rhodamine Phalloidin (RP) for F-actin fibers (red color); Hoechst stain for nuclear morphology (blue color). Abbreviations: µm, micrometer; NW, normal wounded; NP, nanoparticles; 0 J, non-irradiated cells; 5 J, irradiated cells; h, hour.
As a part of our investigation, we used a fluorescence microscope to determine the F-actin and nuclear morphology of NW and DW models and the results are shown in Figs. 7 and 8, respectively. We found that all groups in NW and DW cells did not undergo any changes in nuclear morphology (Hoechst-stained column, blue color) in both 24 and 48 h. Similarly, the NW and DW cells (RP-stained column, red color) showed the presence of filamentous actin fibers in 24 h (Figs. 7a and 8a) and their fluorescence intensity has been considerably increased in 48 h (Figs. 7b and 8b). This may be caused by the reorganization of cytoskeletal fibres after wounds were treated with PBM and GT-GNPs. As a result of successful cell proliferation, the filamentous actin fibres in both models increased in thickness and density over a period of 48 h. It has been demonstrated that F-actin is closely related to cytoskeleton reorganization during wound healing and the formation of new cell-cell connections54,55. In our previous study, we used silver nanoparticles (AgNPs) to conduct similar experiments for NW and DW models. A similar phenomenon of increased expression of F-actin without a change in nuclear shape was also observed32. Accordingly, these results indicate that the addition of GT-GNPs during PBM did not adversely affect the normal wound-healing process in NW and DW models (in vitro).
Fluorescence microscopic images of diabetic wounded cells. [a] 24 h images of DW cells; [b] 48 h images of DW cells. The stain used: Rhodamine Phalloidin (RP) for F-actin fibers (red color); Hoechst stain for nuclear morphology (blue color). Abbreviations: µm, micrometer; DW, diabetic wounded; NP, nanoparticles; 0 J, non-irradiated cells; 5 J, irradiated cells; h, hour.
Conclusion
A green synthesis process was used to synthesize GT-GNPs, using natural polysaccharides GT as a reducing, capping, and stabilizing agent. We examined UV-vis spectrophotometer, HRTEM, XRD, and FTIR measurements to confirm the SPR pattern, smooth and spherical morphology, small size, and GT coating on GNPs. The bacterial study results successfully demonstrated the bactericidal activity of GT-GNPs against the studied pathogens. These studies suggest that GT-GNPs may be a potential antibacterial agent for treating wounds infected with pathogens. In the cell culture study, the GT-GNPs alone and combined with PBM had no adverse effects on cellular mechanisms during in vitro wound healing processes in both NW and DW models. We conclude that GT-GNPs and PBM may be useful in wound-healing applications, including diabetic wound healing. However, it is important to acknowledge some limitations of this study. In the present study, in vitro methodologies were used, therefore the in vivo effects of combination therapy on wound healing were not evaluated. Moreover, the study was limited to PBM effect on fibroblast cells (normal and diabetic wound models). Therefore, we recommend that similar studies be conducted with different types of cells and cocultured with keratinocytes and perform bacterial-infected cell culture models. In future investigations, it is important to examine the specific mechanisms by which combination therapy promotes wound healing, including the study of cellular responses and signaling pathways. The effectiveness of combination therapy (incorporating the synthesized GT-GNPs into the biomaterials) can also be enhanced by investigating the effect of various variables, such as dose and frequency of administration. These investigations can provide researchers with a deeper understanding of the potential applications of this treatment through further pathogen-infected in vitro and in vivo studies. Additionally, they may contribute to developing innovative therapeutic strategies for wound healing.
Data availability
The data that support the findings of this study are available from the corresponding author.
References
Arvizo, R., Bhattacharya, R. & Mukherjee, P. Gold nanoparticles: Opportunities and challenges in nanomedicine. Expert Opin. Drug Deliv 7, 753–763. https://doi.org/10.1517/17425241003777010 (2010).
Edwards, P. P. & Thomas, J. M. Gold in a metallic divided State - From Faraday to Present-Day nanoscience. Angew Chem. Int. Ed. 46, 5480–5486. https://doi.org/10.1002/anie.200700428 (2007).
Koushki, K. et al. Gold nanoparticles: Multifaceted roles in the management of autoimmune disorders. Biomolecules 11, 1289. https://doi.org/10.3390/biom11091289 (2021).
Kulkarni, S., Kumar, S. & Acharya, S. Gold nanoparticles in Cancer therapeutics and diagnostics. Cureus 14, e30096. https://doi.org/10.7759/cureus.30096 (2022).
Deng, G., Zha, H., Luo, H. & Zhou, Y. Aptamer-conjugated gold nanoparticles and their diagnostic and therapeutic roles in cancer. Front. Bioeng. Biotechnol. 11, 1118546. https://doi.org/10.3389/fbioe.2023.1118546 (2023).
Kong, F. Y. et al. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules 31, 1445. https://doi.org/10.3390/molecules22091445 (2017).
Shukla, R. et al. Laminin receptor specific therapeutic gold nanoparticles (198AuNP-EGCg) show efficacy in treating prostate cancer. Proc. Natl. Acad. Sci. U S A 109, 12426–12431. https://doi.org/10.1073/pnas.1121174109 (2012).
TiwariP.M., Vig, K., Dennis, V. A. & Singh, S. R. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials (Basel) 1, 31–63. https://doi.org/10.3390/nano1010031 (2011).
Asiya, S. I. et al. Sustainable preparation of gold nanoparticles via green chemistry approach for biogenic applications. Mater. Today Chem. 17, 100327. https://doi.org/10.1016/j.mtchem.2020.100327 (2020).
Nadagouda, M. N. et al. Synthesis of silver and gold nanoparticles using antioxidants from Blackberry, blueberry, pomegranate, and turmeric extracts. ACS Sustainable Chem. Eng. 2, 1717–1723. https://doi.org/10.1021/sc500237k (2014).
Moosavy, M. H. et al. Green synthesis, characterization, and biological evaluation of gold and silver nanoparticles using Mentha spicata essential oil. Sci. Rep. 13, 7230. https://doi.org/10.1038/s41598-023-33632-y (2023).
Pourali, P. et al. Biosynthesis of gold nanoparticles by two bacterial and fungal strains, Bacillus cereus and fusarium oxysporum, and assessment and comparison of their nanotoxicity in vitro by direct and indirect assays. Electron. J. Biotechnol. 29, 86–93. https://doi.org/10.1016/j.ejbt.2017.07.005 (2017).
Kharissova, O. V. et al. The greener synthesis of nanoparticles. Trends Biotechnol. 31, 240–248. https://doi.org/10.1016/j.tibtech.2013.01.003 (2013).
Kulkarni, N. & Muddapur, U. Biosynthesis of metal nanoparticles: A review. J. Nanotechnol. 510246. https://doi.org/10.1155/2014/510246 (2014).
Wu, C. C. & Chen, D. H. Facile green synthesis of gold nanoparticles with gum Arabic as a stabilizing agent and reducing agent. Gold. Bull. 43, 234–240. https://doi.org/10.1007/BF03214993 (2010).
Dhar, S., Reddy, E. M., Shiras, A., Pokharkar, V. & Prasad, B. L. Natural gum reduced/stabilized gold nanoparticles for drug delivery formulations. Chemistry 14, 10244–10250. https://doi.org/10.1002/chem.200801093 (2008).
Pooja, D., Panyaram, S., Kulhari, H., Rachamalla, S. S. & Sistla, R. Xanthan gum stabilized gold nanoparticles: Characterization, biocompatibility, stability and cytotoxicity. Carbohydr. Polym. 110, 1–9. https://doi.org/10.1016/j.carbpol.2014.03.041 (2014).
Wu, C. C. & Chen, D. H. Spontaneous synthesis of gold nanoparticles on gum arabic-modified iron oxide nanoparticles as a magnetically recoverable nanocatalyst. Nanoscale Res. Lett. 7, 317. https://doi.org/10.1186/1556-276X-7-317 (2012).
Maity, S., Sen, I. K. & Islam, S. S. Green synthesis of gold nanoparticles using gum polysaccharide of Cochlospermum religiosum (katira gum) and study of catalytic activity. Phys. E 45, 130–134. https://doi.org/10.1016/j.physe.2012.07.020/ (2012).
Pandey, S., Goswami, G. K. & Nanda, K. K. Green synthesis of polysaccharide/gold nanoparticle nanocomposite: An efficient ammonia sensor. Carbohydr. Polym. 94, 229–234. https://doi.org/10.1016/j.carbpol.2013.01.009 (2013).
Rao, K. et al. Gum tragacanth stabilized green gold nanoparticles as cargos for naringin loading: A morphological investigation through AFM. Carbohydr. Polym. 174, 243–252. https://doi.org/10.1016/j.carbpol.2017.06.071 (2017).
Fayazzadeh, E. et al. Acceleration of skin wound healing with tragacanth (Astragalus) preparation: An experimental pilot study in rats. Acta Med. Iran. 52, 3–8 (2014).
WHO Traditional Medicine. Strategy 2002–2005. Geneva, WHO,2002 (document reference WHO/EDM/TRM/2002.1).
Ibrahim, N. I. et al. Wound healing properties of selected natural products. Int. J. Environ. Res. Public. Health 15, 2360. https://doi.org/10.3390/ijerph15112360 (2018).
Chen, S. A. et al. Topical treatment with anti-oxidants and Au nanoparticles promote healing of diabetic wound through receptor for advance glycation end-products. Eur. J. Pharm. Sci. 47, 875–883. https://doi.org/10.1016/j.ejps.2012.08.018 (2012).
Woodruff, L. D. et al. The efficacy of laser therapy in wound repair: A meta-analysis of the literature, photomed. Laser Surg. 22, 241–247. https://doi.org/10.1089/1549541041438623/ (2004).
Dawood, M. S. & Salman, S. D. Low level diode laser accelerates wound healing. Lasers Med. Sci. 28, 941–945. https://doi.org/10.1007/s10103-012-1182-4 (2013).
Silveira, P. C., Silva, L. A., Freitas, T. P., Latini, A. & Pinho, R. A. Effects of low-power laser irradiation (LPLI) at different wavelengths and doses on oxidative stress and fibrogenesis parameters in an animal model of wound healing. Lasers Med. Sci. 26, 125–131. https://doi.org/10.1007/s10103-010-0839-0/ (2011).
Houreld, N. Healing effects of photobiomodulation on diabetic wounds. Appl. Sci. 9, 5114. https://doi.org/10.3390/app9235114/ (2019).
Ayuk, S. M., Houreld, N. N. & Abrahamse, H. Collagen production in diabetic wounded fibroblasts in response to low-intensity laser irradiation at 660 Nm. Diabetes Technol. Ther. 14, 1110–1117. https://doi.org/10.1089/dia.2012.0125 (2012).
Lau, P. et al. Influence of gold nanoparticles on wound healing treatment in rat model: Photobiomodulation therapy. Lasers Surg. Med. 49, 380–386. https://doi.org/10.1002/lsm.22614 (2017).
Dhilip Kumar, N. N., Houreld, H. & Abrahamse Selective laser efficiency of Green-Synthesized silver nanoparticles by Aloe arborescens and its wound healing activities in normal wounded and diabetic wounded fibroblast cells: In vitro studies. Int. J. Nanomed. 15, 6855–6870. https://doi.org/10.2147/IJN.S257204 (2020).
Kumar, S. S. D. et al. Cellular imaging and folate receptor targeting delivery of gum Kondagogu capped gold nanoparticles in cancer cells. Int. J. Biol. Macromol. 109, 220–230. https://doi.org/10.1016/j.ijbiomac.2017.12.069 (2018a).
Kumar, S. S. D., Houreld, N. N., Kroukamp, E. M. & Abrahamse, H. Cellular imaging and bactericidal mechanism of green-synthesized silver nanoparticles against human pathogenic bacteria. J. Photochem. Photobiol B Biol. 178, 159–169. https://doi.org/10.1016/j.jphotobiol.2017.11.001 (2018b).
N.Houreld, H. & Abrahamse Low-intensity laser irradiation stimulated wound healing in diabetic wounded fibroblast cells (WS1). Diabetes Technol. Ther. 12, 971–978. https://doi.org/10.1089/dia.2010.0039 (2010).
N.Houreld, H. & Abrahamse Laser light influences cellular viability and proliferation in diabetic-wounded fibroblast cells in a dose- and wavelength-dependent manner. Lasers Med. Sci. 23, 11–18. https://doi.org/10.1007/s10103-007-0445-y (2008).
Fox, L. T. et al. In vitro wound healing and cytotoxic activity of the gel and whole-leaf materials from selected aloe species. J. Ethnopharmacol. 200, 1–7. https://doi.org/10.1016/j.jep.2017.02.017 (2017).
Okkeh, M. et al. Gold nanoparticles: can they be the next magic bullet for Multidrug-Resistant bacteria?? Nanomaterials (Basel) 11(312). https://doi.org/10.3390/nano11020312.A (2021).
Pan, M. et al. Topical application of keratinocyte growth factor conjugated gold nanoparticles accelerate wound healing. Nanomedicine 14, 1619–1628. https://doi.org/10.1016/j.nano.2018.04.007 (2018).
Kim, J. E. et al. Accelerated healing of cutaneous wounds using phytochemically stabilized gold nanoparticle deposited hydrocolloid membranes. Biomater. Sci. 3, 509–519. https://doi.org/10.1039/C4BM00390J (2015).
Mendes, C. et al. Effects of the association between photobiomodulation and hyaluronic acid linked gold nanoparticles in wound healing. ACS Biomater. Sci. Eng. 6, 5132–5144. https://doi.org/10.1021/acsbiomaterials.0c00294 (2020).
Felician, M. C. P., Belotto, R., Tardivo, J. P., Baptista, M. S. & Martins, W. K. Photobiomodulation: Cellular, molecular, and clinical aspects. J. Photochem. Photobiol. 17, 100197. https://doi.org/10.1016/j.jpap.2023.100197 (2023).
Dompe, C. et al. Photobiomodulation-Underlying mechanism and clinical applications. J. Clin. Med. 9, 1724. https://doi.org/10.3390/jcm9061724 (2020).
Liu, H., Zhang, M., Meng, F., Su, C. & Li, J. Polysaccharide-based gold nanomaterials: synthesis mechanism, polysaccharide structure-effect, and anticancer activity. Carbohydr. Polym. 321, 121284. https://doi.org/10.1016/j.carbol.2023.121284 (2023).
Liang, X., Wang, Z. & Liu, C. Size-Controlled synthesis of colloidal gold nanoparticles at room temperature under the influence of glow discharge. Nanoscale Res. Lett. 5, 124. https://doi.org/10.1007/s11671-009-9453-0 (2010).
Lopes, L. C. et al. Gold nanoparticles capped with polysaccharides extracted from pineapple gum: Evaluation of their hemocompatibility and electrochemical sensing properties. Talanta 223, 121634. https://doi.org/10.1016/j.talanta.2020.121634 (2021).
Chen, X. et al. Hedysarum polysaccharides mediated green synthesis of gold nanoparticles and study of its characteristic, andlytical merit, catalytic activity. Mater. Res. Bull. 133, 111070. https://doi.org/10.1016/j.materresbul.2020.111070 (2021).
Fuziwara, S. et al. Barium sulphate with a negative zeta potential accelerates skin permeability barrier recovery and prevents epidermal hyperplasia induced by barrier disruption. Br. J. Dermatol. 151, 557–564. https://doi.org/10.1111/j.1365-2133.2004.06085.x (2004).
García-Villén, F. et al. Wound healing activity of nanoclay/spring water hydrogels. Pharmaceutics 12, 467. https://doi.org/10.3390/pharmaceutics12050467 (2020).
Rimondini, L. et al. How do wettability, zeta potential and hydroxylation degree affect the biological response of biomaterials? Mater. Sci. Eng. C Mater. Biol. Appl. 74, 542–555. https://doi.org/10.1016/j.msec.2016.12.107 (2017).
Elasmawi, I. S. & Elsayed, N. H. The role of gold nanoparticles in the structural and electrical properties of Cs/PVP blend. Polym. Bull. 77, 949–962. https://doi.org/10.1007/s00289-019-02786-z (2020).
Kora, A. J. & Arunachalam, J. Green fabrication of silver nanoparticles by gum tragacanth (Astragalus gummifer): A dual functional reductant and stabilizer. J. Nanomat. 869765. https://doi.org/10.1155/2012/869765 (2012).
Li, X. et al. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano. 8, 9689–11024. https://doi.org/10.1021/nn5042625 (2014).
Abreu-Blanco, M. T., Watts, J. J., Verboon, J. M. & Parkhurst, S. M. Cytoskeleton responses in wound repair. Cell. Mol. Life Sci. 69, 2469–2483. https://doi.org/10.1007/s00018-012-0928-2 (2012).
Muller, S. et al. HIF stabilization inhibits renal epithelial cell migration and is associated with cytoskeletal alterations. Sci. Rep. 8, 9497. https://doi.org/10.1038/s41598-018-27918-9 (2018).
Acknowledgements
The authors would like to thank the Water and Health Research Centre, University of Johannesburg, South Africa for providing a laboratory facility to perform bacteriological work. This work is based on the research supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa (Grant No 98337), as well as grants received from the University of Johannesburg (URC), the National Research Foundation (NRF), and the CSIR (Council for Scientific and Industrial Research)—NLC (National Laser Centre) Laser Rental Pool Programme.
Author information
Authors and Affiliations
Contributions
SS Dhilip Kumar: Conceptualization, Methodology, Data curation, Formal analysis, Writing-original draft, Writing—review & editing, Visualization, Software. NN Houreld: Supervision, Writing—review & editing, Project administration. H Abrahamse: Funding Acquisition, Resources. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dhilip Kumar, S.S., Nadene Houreld, N. & Abrahamse, H. Influence of biopolymer based gold nanoparticles and photobiomodulation in in vitro wound healing. Sci Rep 15, 15793 (2025). https://doi.org/10.1038/s41598-025-99400-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99400-2