Abstract
Muscle-invasive bladder cancer (MIBC) continues to pose a significant health challenge, as conventional neoadjuvant chemotherapy (NAC) has shown limited improvements in efficacy outcomes. Recent clinical trials suggest that combining NAC with immune checkpoint blockade (NAC.NICB) may enhance therapeutic efficacy. This study aimed to explore the short-term therapeutic efficacy and outcomes of NAC.NICB compared to NAC in real-world settings for the treatment of MIBC. A total of 100 patients with MIBC who received either NAC or NAC.NICB were included in the study. The treatment efficacy of the NAC and NAC.NICB groups was evaluated based on pathological complete response (pCR) and the rate of pathological downstaging through post treatment pathological assessment. In the NAC.NICB group, clinical characteristics were compared between patients who achieved pCR and those who did not, using the independent samples t-test or the Mann-Whitney U test. Overall, 71 patients received NAC and 29 patients received NAC.NICB. At baseline, the NAC.NICB group exhibited higher T and N stages compared to the NAC group. However, 48.3% (14/29) of the patients in the NAC.NICB group achieved pCR, which was significantly higher than that observed in the NAC group (18/71, 25.4%; p = 0.034). In addition, the pathological downstaging rate in the NAC.NICB group was higher than that of the NAC group (75.9% vs. 47.9%; p = 0.014). The disease control rate (DCR) in the NAC.NICB group was higher than that observed in the NAC group (96.6% vs. 77.5%; p = 0.020). Higher pretreatment hemoglobin levels (p = 0.018) or lower platelet levels (p = 0.026) in patients undergoing NAC.NICB therapy may serve as a potential predictor for achieving a higher pCR rate. Neoadjuvant chemotherapy combined with immune checkpoint blockade improves pCR and pathological downstaging rates in MIBC, highlighting the benefits of neoadjuvant chemoimmunotherapy for MIBC.
Similar content being viewed by others
Introduction
Bladder cancer is one of the most common malignancies worldwide, particularly among men, ranking as the sixth most common cancer in males. Muscle-invasive bladder cancer (MIBC) represents a particularly aggressive form associated with a high risk of metastasis and mortality1,2. Current treatment guidelines for MIBC recommend receiving neoadjuvant therapy before radical cystectomy (RC)3. Studies have confirmed that platinum-based neoadjuvant chemotherapy (NAC) can effectively downstage tumors and provide survival benefits to patients with bladder cancer4. However, the pathological downstaging rate achieved by current NAC remains limited. Even with standard NAC followed by RC, 40% of patients experience recurrence or death within 3 years5,6,7,8. Therefore, the development of novel neoadjuvant treatment strategies is imperative to enhance pathological response rates and improve overall survival (OS) in these patients. In recent years, immune checkpoint blockade (ICB) has demonstrated remarkable efficacy in advanced urothelial carcinoma (UC). A phase III randomized controlled trial (RCT) found that pembrolizumab, as a second-line therapy, significantly improved OS, leading to its approval for advanced UC, either as a second-line treatment or as a first-line option for patients who are platinum-intolerant9. Encouraging results have emerged from clinical trials investigating neoadjuvant immunotherapy (NICB). Powles et al. demonstrated the efficacy of NICB treatment with atezolizumab, finding that 27 of 88 patients (31%) achieved a pathological complete response (pCR)10.
Subsequent clinical trials have focused on combining NAC with immune checkpoint blockade (NAC.NICB) to further enhance efficacy. A phase II study involving gemcitabine with split-dose cisplatin and pembrolizumab in patients with MIBC showed that 22 of 39 patients (56%) experienced pathological downstaging, and 14 of 39 patients (35.9%) achieved pCR11. In a single-arm phase II clinical trial investigating gemcitabine and cisplatin (GC) combined with tislelizumab as neoadjuvant therapy for MIBC, encouraging results were observed, with 29 of 57 patients (50.9%) achieving pCR, and 43 of 57 patients (75.4%) achieving pathologic downstaging12. A clinical trial of GC regimen chemotherapy combined with durvalumab immunotherapy found that NAC.NICB enabled 33% of MIBC patients to achieve pCR, leading to greater survival benefits13. These three clinical trials demonstrated the efficacy of NAC.NICB, with pCR rates ranging from 33 to 50.9%. However, the response rates may vary depending on the specific immunotherapy drugs used. In highly selective clinical trials, patients with more advanced stages are often excluded. Therefore, the efficacy of neoadjuvant therapy in real-world settings, particularly for patients with more advanced stages, requires further investigation. The choice of ICB therapy is typically influenced by patient tolerance and financial considerations.
Therefore, we conducted a real-world study to compare the efficacy of NAC and NAC.NICB in patients with MIBC and to explore potential factors influencing the therapeutic effects of NAC.NICB. Our findings indicate that NAC.NICB significantly improves the pCR rate and pathological downstaging rate compared to NAC, emphasizing the advantages of neoadjuvant chemoimmunotherapy in MIBC.
Methods
Study design, outcomes, and patient selection
This study retrospectively collected data from patients at Peking University First Hospital who received NAC or NAC.NICB for bladder cancer between January 2022 and June 2024. We assessed the patients’ tumor staging according to the Tumor, Node, Metastasis (TNM) Classification system (2017, 8th edition)14. The first research outcome was the pCR rate (pT0N0M0), while the second research outcome was the pathological downstaging rate, defined as pT < T2 and pN = N0 (pTis-T1N0M0). All participants met the following inclusion criteria: (1) confirmed diagnosis of MIBC; (2) completion of at least two cycles of neoadjuvant treatment; and (3) undergoing RC with confirmed pathological staging after neoadjuvant treatment. The exclusion criteria were as follows: (1) pathological diagnosis of cancers other than UC; (2) undergoing maximal transurethral resection of the bladder tumor (TURBT) before neoadjuvant treatment; (3) incomplete perioperative pathological data. Variant histological urothelial carcinoma (VH-UC) was defined according to the 2016 WHO classification, which includes invasive urothelial tumors and infiltrating UC with divergent differentiation, including large nested, microcystic, micropapillary, lymphoepithelioma-like, plasmacytoid/signet ring cell/diffuse, sarcomatoid, giant cell, poorly differentiated, lipid rich, clear cell, tumors of Müllerian type, as well as tumors arising in a bladder diverticulum15. All pathological diagnoses were independently performed by two pathologists.
Neoadjuvant therapy schedule
In the NAC cohort, the treatment regimen consisted of gemcitabine (CHIATAI TIANQING, China) administered intravenously at a dose of 1000 mg/m² on days 1 and 8 and cisplatin (Qilu-pharmaceutical, China) administered intravenously at a dose of 70 mg/m² on day 1, every 3 weeks (Q3W). In the NAC.NICB cohort, 21 patients received tislelizumab (BeiGene Ltd, China) at a dose of 200 mg on day 1, 7 patients received pembrolizumab (Merck Sharp & Dohme, MSD, U.S.) at a dose of 200 mg on day 1, and 1 patient received camrelizumab (Jiangsu Hengrui Pharmaceuticals, China) at a dose of 200 mg on day 1 for immunotherapy, combined with gemcitabine administered intravenously at a dose of 1000 mg/m² on days 1 and 8 and cisplatin at a dose of 70 mg/m² administered intravenously on day 1, every 3 weeks (Q3W).
Clinical data collection
Baseline information before neoadjuvant treatment was collected from patient medical records and included age, gender, smoking status, TNM staging, tumor grade, surgical approach, pathological response, pathological characteristics, and pretreatment laboratory values such as red blood cell count, hemoglobin (Hb), platelet count, neutrophil count, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), albumin, and albumin-to-globulin ratio (ALG). A definitive histopathological diagnosis was established through diagnostic TURBT.
Statistical methods
Data analysis was performed using SPSS software (version 26.0, SPSS Inc., Chicago, IL, USA). All quantitative data were expressed as mean ± standard deviation (SD) for normally distributed data or median with a range for the non-normally distributed data. The Kolmogorov-Smirnov test was used to test the normality of clinical data. For categorical variables (e.g., PCR and pathological downstaging rates), the appropriate statistical test (Chi-square or Fisher’s exact test) was selected based on expected frequency criteria. Continuous variables underwent normality assessment using the Kolmogorov-Smirnov test, with the independent samples t-test applied for normally distributed measures (e.g., blood biochemical parameters), while non-normally distributed data were analyzed using the nonparametric Mann-Whitney U test.
Results
Characteristics of the neoadjuvant treatment patient cohort
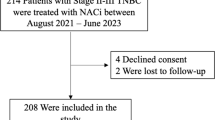
As shown in Fig. 1, our real-world study included a total of 100 patients with MIBC, comprising 71 in the NAC group and 29 in the NAC.NICB group. In the NAC group, 25 out of 71 patients (35.2%) exhibited a higher pathological T stage (> T2). In the NAC.NICB group, 15 out of 29 patients (51.7%) exhibited a higher pathological T stage. While there was no significant difference in the T stage between the two groups, a statistically significant difference was observed in the N stage (p = 0.024). There were no significant differences in age, gender, or smoking history between the two groups (all p > 0.05). The baseline characteristics were generally compared between the NAC and NAC.NICB groups (Table 1). In terms of histopathology, the NAC group included 38 patients with pure UC, accounting for 53.5%, while 33 patients (46.5%) had VH-UC. In the NAC.NICB group, 12 patients (41.4%) had pure UC, and 17 patients (58.6%) had VH-UC. This shows that the proportion of patients with VH-UC in the NAC.NICB group is higher than that in NAC group (58.6% vs 46.5%). However, there was no statistically significant difference between the two groups.
Efficacy analysis of two neoadjuvant treatment regimens in real-world settings
Subsequently, we analyzed the pathological responses of the two neoadjuvant treatment regimens in a real-world setting, with the results summarized in Table 2. All patients underwent thorough postoperative pathological evaluations to accurately determine their pathological stage following neoadjuvant therapy. The two neoadjuvant treatment regimens were compared within the same real-world context. Initially, we assessed the number of neoadjuvant treatment cycles completed by patients in each group. The analysis revealed that a higher proportion of patients in the NAC.NICB group completed all four cycles of neoadjuvant therapy (75.9%) compared to the NAC group (62.0%).
Among 71 patients in the NAC group, 18 patients (25.4%) achieved pCR, while 16 patients (22.5%) experienced partial pathological response (pPR), resulting in a pathological downstaging rate of 47.9% and a DCR of 77.5%. Of the 29 patients in the NAC.NICB group, 14 (48.3%) achieved pCR, and 8 patients (27.6%) had pPR, with a pathological downstaging rate of 75.9% and a DCR of 96.6%. The pCR rate in the NAC.NICB group was significantly higher than in the NAC group (48.3% vs. 25.4%; p = 0.034). The rate of pathological downstaging was notably higher in the NAC.NICB group compared to the NAC group (75.9% vs. 47.9%; p = 0.014). To address the imbalance between the NAC and NAC.NICB groups, we performed propensity score matching (PSM) to adjust for baseline differences (Supplementary Table 1). After PSM, the patient characteristics between the two groups were more comparable. Notably, the pCR rate in the NAC.NICB group after PSM, was significantly higher than that observed in the NAC group (48.3% vs 17.2%; p = 0.024) (Supplementary Table 2). This adjustment strengthens the validity of our findings and provides clearer evidence of NAC.NICB’s effectiveness in improving treatment outcomes.
The DCR in the NAC.NICB group was higher than that observed in the NAC group (96.6% vs. 77.5%; p = 0.020) (Fig. 2A-B). To delineate stage-specific therapeutic efficacy, we conducted a stage-stratified analysis evaluating pathological complete response (pCR) dynamics across tumor progression (Fig. 2C). The analysis revealed superior pCR benefits with NAC combined with ICB therapy compared to NAC alone in both stage II and III MIBC. Notably, in stage III disease, the NAC.NICB group exhibited a significantly higher pCR rate than the NAC group (57.9% vs. 20.7%; p = 0.009).
Comparative efficacy of neoadjuvant NAC.NICB and NAC treatment A-B. Comparative pathological response rates between treatment arms: (A) pCR rates; (B) pathological downstaging success rates C. Stratified pCR analysis demonstrating treatment outcomes in the overall cohort and disease stage subgroups. pCR: pathological complete response.
Comparison of clinical characteristics between patients who achieved pCR and Non-pCR groups
Given the favorable pathological response achieved with NAC.NICB, we focused on this cohort to identify clinical factors associated with pCR (Table 3). In the T2N0M0 subgroup, 3 out of 7 patients (42.8%) achieved pCR, compared to 50% (11 out of 22 patients) in the > T2N0M0 subgroup, with no statistically significant difference (p = 0.742). Our analysis shows that patients who achieved pCR had lower platelet levels compared to those who did not (non-pCR; p = 0.026). Higher pretreatment Hb levels may indicate a higher likelihood of achieving pCR (p = 0.018). No significant differences in clinical stage or pretreatment levels of RBCs, neutrophils, lymphocytes, NLR, PLR, albumin, or ALG were found between patients who achieved pCR and those who did not (non-pCR) in the NAC.NICB group (all p > 0.05).
Discussion
Cisplatin-based NAC for MIBC has been well-established for its efficacy in improving both pathological response rates and overall survival4,16,17. Immune checkpoint blockade (PD-1/PD-L1) is currently approved for first-line treatment of advanced UC in patients who are ineligible for platinum-based therapy, as well as for second-line treatment in advanced UC18,19,20,21. Additionally, VH-UC is often excluded from these trials due to its relative rarity. Therefore, there is a pressing need to further evaluate the effectiveness of neoadjuvant chemoimmunotherapy for MIBC in real-world settings.
We conducted a head-to-head comparison of two neoadjuvant treatment regimens including NAC and NAC.NICB within the same real-world setting. Baseline characteristics were comparable between the two treatment groups. Achieving pCR after neoadjuvant therapy was positively associated with significant improvements in survival outcomes22. In our study, the pCR rate in the NAC.NICB cohort reached 48.3%, significantly higher than the 25.4% pCR rate observed in the NAC cohort (p = 0.034). In the analysis of treatment response across different stages, we found that patients with more advanced stages (Stage III) had a significantly higher pCR rate with NAC.NICB treatment compared to NAC treatment. This marked differential response suggests progressive enhancement of treatment sensitivity with disease advancement, possibly mediated by stage-dependent tumor immunogenicity modulation. Furthermore, these findings provide important clinical implications for treatment stratification based on disease progression. Moreover, in our real-world study, the pCR rate in the NAC.NICB cohort (48.3%) was slightly higher than the rates reported in two clinical trials (36–41%)11,23 and lower than the pCR rate observed in the clinical trial combining tislelizumab and GC chemo-immunotherapy (50.9%)12. This discrepancy may be attributed to the fact that, in our real-world setting, the choice of immunotherapy agents is often individualized, considering the patient’s financial situation and tolerance levels. Therefore, these results suggest that the NAC.NICB combination may provide greater survival benefits for patients.
Hypoxia and inflammation critically regulate the tumor immune microenvironment (TIME), serving as pivotal determinants of neoadjuvant chemoimmunotherapy efficacy. Tumor-associated hypoxia stabilizes hypoxia-inducible factors (HIFs), driving the recruitment of immunosuppressive cellular components such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) that collectively impair antitumor immune surveillance24,25,26. Paradoxically, this hypoxic state concurrently upregulates immune checkpoint molecules (e.g., PD-L1/CTLA-4), creating actionable therapeutic vulnerabilities that can be exploited through checkpoint inhibitor co-administration to synergize with cytotoxic therapies26,27. Concurrently, tumor-propagated inflammation elevates pro-inflammatory mediators including IL-6 and TNF-α, which exhibit dualistic roles: while fostering protumorigenic signaling cascades and conferring therapy resistance, these cytokines paradoxically enhance immune activation through improved antigen-presenting cell (APC) maturation and effector T-cell trafficking to tumor28. The dynamic interplay between hypoxic gradients and inflammatory milieu establishes a therapeutically pliable TIME landscape, wherein strategic immunomodulation may recalibrate immune equilibrium to potentiate treatment responses.
Pathological downstaging is a crucial indicator for assessing the efficacy of neoadjuvant therapy in MIBC. Current research has confirmed that a higher rate of pathological downstaging following neoadjuvant therapy is associated with better survival outcomes29,30. In our study, the pathological downstaging rate in the NAC.NICB cohort reached 75.9%, significantly higher than the 47.9% observed in the NAC cohort (p = 0.014). This rate also exceeds those reported in other prospective clinical trials for neoadjuvant therapy, where downstaging rates typically range from 45–50%11,13,31. Consequently, our findings indicate that the NAC.NICB cohort exhibited higher rates of both pCR and pathological downstaging compared to the NAC cohort. This suggests that patients receiving NAC.NICB may achieve better survival outcomes, and those with pathological downstaging could have a greater chance of benefitting from bladder-preserving treatments, thereby enhancing their quality of life. Our study’s value lies in validating findings from clinical trials in a real-world setting, particularly by including more advanced-stage patients. Unlike controlled trials, our study reflects a broader patient population, making the results more applicable to clinical practice.
Previous studies have demonstrated that NAC.NICB can offer greater benefits for patients with MIBC. However, to date, there are no definitive biomarkers to predict the efficacy of this treatment. Our analysis revealed that patients who achieved pCR had lower platelet levels or higher Hb compared to non-pCR patients, aligning with findings from previous research. Hematological markers associated with systemic inflammation have been found to correlate with the efficacy of NAC.NICB. One possible mechanism involves platelets promoting tumor progression by enhancing tumor growth, protecting tumor cells from immune system attacks, facilitating metastasis, and stimulating angiogenesis32,33. Platelets are known to significantly contribute to tumorigenesis, not only through direct support of tumor growth but also by shielding tumor cells from immune-mediated destruction and aiding metastatic dissemination. Additionally, platelets can accelerate tumor progression by inducing angiogenesis32. Supporting this notion, research in UC patients has indicated that an interaction between platelet count and tumor PD-L1 expression might regulate tumor progression34. These studies demonstrate the critical role of platelet levels in tumor progression and response to treatment, which supports our findings on the association between hematological markers and the efficacy of NAC.NICB.
On the other hand, previous studies have shown that anemia can impair T-cell responses and contribute to immune suppression in patients with advanced cancers35. Consistent with this, a study identified low hemoglobin levels, an ECOG score of ≥ 1, and liver metastasis as independent prognostic factors associated with shorter survival in metastatic UC patients who experienced platinum-based treatment failure, further supporting our observations36.prognostic indicators for advanced non-small cell lung cancer(NSCLC) patients receiving ICI therapy. Hemoglobin, therefore, represents an accessible and cost-effective biomarker with distinct advantages in clinical measurability compared to other reported biomarkers37 .These findings highlight the crucial role of hemoglobin as a prognostic biomarker in immunotherapy and systemic treatment outcomes, reinforcing our conclusions on its potential predictive value in therapy efficacy. Elevated pretreatment NLR has been associated with poorer prognosis in both localized and metastatic UC38. However, our study did not find a correlation between NLR and response to NAC.NICB for bladder cancer. As suitable predictive biomarkers for NAC.NICB in MIBC were not identified through pretreatment blood markers, it is essential to further develop molecular-based biomarkers.
Our study addresses two key limitations of existing clinical trials. Firstly, in highly selective clinical trials, patients with poor general health are often excluded, which may affect the assessment of treatment outcomes. Real-world studies help overcome this limitation by including a broader patient population. We further analyzed patients at different stages and found that in stage III patients, the difference in pCR rates between the NAC.NICB and NAC groups was more pronounced than that in stage II. This provides new evidence supporting the greater therapeutic benefit of combination therapy in more advanced-stage patients. Secondly, clinical trials typically evaluate only a single ICI, whereas real-world treatment decisions are often influenced by patients’ financial status and drug accessibility, leading to the use of different ICIs. This suggests that various ICIs can be widely applied in neoadjuvant treatment for bladder cancer. Future large-scale, head-to-head studies are warranted to further compare the efficacy of different ICIs in treatment response.
Our study also has several limitations. First, one limitation is the small sample size, particularly patients receiving the novel NAC.NICB treatment, which is relatively rare application in clinical practice. Large-scale multicenter studies are needed to validate these findings. Second, another limitation is the lack of long-term clinical outcomes, due to the short follow-up period of the NAC.NICB cohort. Extended follow-up will be implemented in subsequent research to evaluate this novel treatment’s survival benefits. Third, additional transcriptomic data should be gathered, as integrating multiomics analyses could provide deeper insights into the mechanisms affecting prognosis. Addressing these limitations in future research will strengthen the robustness of our findings and contribute to a better understanding of the underlying biological processes.
Conclusion
This study demonstrates that combining NAC with immunotherapy significantly enhances the pCR rate and pathological downstaging in patients with MIBC compared to NAC alone. Given the superior efficacy of NAC.NICB, it holds promise as a first-line neoadjuvant therapy for MIBC, providing greater benefits to patients.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Theodorescu, D., Li, Z. & Li, X. Sex differences in bladder cancer: emerging data and call to action. Nat. Rev. Urol. 19, 447–449. https://doi.org/10.1038/s41585-022-00591-4 (2022).
Han, B. et al. Cancer incidence and mortality in China, 2022. J. Natl. Cancer Cent. 4, 47–53. https://doi.org/10.1016/j.jncc.2024.01.006 (2024).
Alfred Witjes, J. et al. European association of urology guidelines on Muscle-invasive and metastatic bladder cancer: summary of the 2023 guidelines. Eur. Urol. 85, 17–31. https://doi.org/10.1016/j.eururo.2023.08.016 (2024).
Griffiths, G., Hall, R., Sylvester, R., Raghavan, D. & Parmar, M. K. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J. Clin. Oncol. 29, 2171–2177. https://doi.org/10.1200/jco.2010.32.3139 (2011).
Patel, H. D. et al. Four versus 3 cycles of neoadjuvant chemotherapy for Muscle-Invasive bladder cancer: implications for pathological response and survival. J. Urol. 207, 77–85. https://doi.org/10.1097/ju.0000000000002189 (2022).
Raj, G. V. et al. Contemporary use of perioperative cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. Cancer 117, 276–282. https://doi.org/10.1002/cncr.25429 (2011).
Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. European urology. 48, 202–205; discussion 205–206, (2005). https://doi.org/10.1016/j.eururo.2005.04.006
Boeri, L. et al. Delaying radical cystectomy after neoadjuvant chemotherapy for Muscle-invasive bladder Cancer is associated with adverse survival outcomes. Eur. Urol. Oncol. 2, 390–396. https://doi.org/10.1016/j.euo.2018.09.004 (2019).
Balar, A. V. et al. Efficacy and safety of pembrolizumab in metastatic urothelial carcinoma: results from KEYNOTE-045 and KEYNOTE-052 after up to 5 years of follow-up. Annals Oncology: Official J. Eur. Soc. Med. Oncol. 34, 289–299. https://doi.org/10.1016/j.annonc.2022.11.012 (2023).
Powles, T. et al. Clinical efficacy and biomarker analysis of neoadjuvant Atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat. Med. 25, 1706–1714. https://doi.org/10.1038/s41591-019-0628-7 (2019).
Rose, T. L. et al. Phase II study of gemcitabine and Split-Dose cisplatin plus pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with Muscle-Invasive bladder Cancer. J. Clin. Oncol. 39, 3140–3148. https://doi.org/10.1200/jco.21.01003 (2021).
Li, K. et al. Neoadjuvant gemcitabine-cisplatin plus Tislelizumab in persons with resectable muscle-invasive bladder cancer: a multicenter, single-arm, phase 2 trial. Nat. cancer. https://doi.org/10.1038/s43018-024-00822-0 (2024).
Cathomas, R. et al. Perioperative chemoimmunotherapy with durvalumab for Muscle-Invasive urothelial carcinoma: primary analysis of the Single-Arm phase II trial SAKK 06/17. J. Clin. Oncol. 41, 5131–5139. https://doi.org/10.1200/jco.23.00363 (2023).
Brierley, J. D. & Wittekind, G. M. C. TNM classification of malignant tumors. UICC International Union Against Cancer. 8th edn. Available online: https://www.uicc.org/what-we-do/sharing-knowledge/tnm/publications-and-resources (accessed on.
Humphrey, P. A., Moch, H., Cubilla, A. L., Ulbright, T. M. & Reuter, V. E. The 2016 WHO classification of tumours of the urinary system and male genital Organs-Part B: prostate and bladder tumours. Eur. Urol. 70, 106–119. https://doi.org/10.1016/j.eururo.2016.02.028 (2016).
Patel, V. G., Oh, W. K. & Galsky, M. D. Treatment of muscle-invasive and advanced bladder cancer in 2020. Cancer J. Clin. 70, 404–423. https://doi.org/10.3322/caac.21631 (2020).
Dyrskjøt, L. et al. Bladder cancer. Nat. Reviews Disease Primers. 9, 58. https://doi.org/10.1038/s41572-023-00468-9 (2023).
Sharma, P. et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 18, 312–322. https://doi.org/10.1016/s1470-2045(17)30065-7 (2017).
Patel, M. R. et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 19, 51–64. https://doi.org/10.1016/s1470-2045(17)30900-2 (2018).
Bellmunt, J. et al. Pembrolizumab as Second-Line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 376, 1015–1026. https://doi.org/10.1056/NEJMoa1613683 (2017).
Balar, A. V. et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 18, 1483–1492. https://doi.org/10.1016/s1470-2045(17)30616-2 (2017).
Ravi, P. et al. Optimal pathological response after neoadjuvant chemotherapy for muscle-invasive bladder cancer: results from a global, multicentre collaboration. BJU Int. 128, 607–614. https://doi.org/10.1111/bju.15434 (2021).
Funt, S. A. et al. Neoadjuvant Atezolizumab with gemcitabine and cisplatin in patients with Muscle-Invasive bladder cancer: A multicenter, Single-Arm, phase II trial. J. Clin. Oncol. 40, 1312–1322. https://doi.org/10.1200/jco.21.01485 (2022).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242. https://doi.org/10.1038/nature11986 (2013).
Nagy, M. Z. et al. Effector T cells under hypoxia have an altered transcriptome similar to tumor-stressed T cells found in non-responsive melanoma patients. J. Immunother. Cancer. 13 https://doi.org/10.1136/jitc-2024-010153 (2025).
Shi, S., Ou, X., Liu, C., Wen, H. & Ke, J. Research progress of HIF-1a on immunotherapy outcomes in immune vascular microenvironment. Front. Immunol. 16, 1549276. https://doi.org/10.3389/fimmu.2025.1549276 (2025).
Chen, Z., Han, F., Du, Y., Shi, H. & Zhou, W. Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal. Transduct. Target. Therapy. 8, 70. https://doi.org/10.1038/s41392-023-01332-8 (2023).
Xie, Y. et al. Inflammation in cancer: therapeutic opportunities from new insights. Mol. Cancer. 24 https://doi.org/10.1186/s12943-025-02243-8 (2025).
Grossman, H. B. et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 349, 859–866. https://doi.org/10.1056/NEJMoa022148 (2003).
Martini, A. et al. Tumor downstaging as an intermediate endpoint to assess the activity of neoadjuvant systemic therapy in patients with muscle-invasive bladder cancer. Cancer 125, 3155–3163. https://doi.org/10.1002/cncr.32169 (2019).
Hoimes, C. J. et al. A phase Ib/II study of neoadjuvant pembrolizumab (pembro) and chemotherapy for locally advanced urothelial cancer (UC). Ann. Oncol. 29, viii726. https://doi.org/10.1093/annonc/mdy424.039 (2018).
Yu, L. et al. Bidirectional interaction between Cancer cells and platelets provides potential strategies for Cancer therapies. Front. Oncol. 11 https://doi.org/10.3389/fonc.2021.764119 (2021).
Can, C. et al. Pretreatment parameters obtained from peripheral blood sample predicts invasiveness of bladder carcinoma. Urol. Int. 89, 468–472. https://doi.org/10.1159/000343278 (2012).
Miyama, Y. et al. The prognostic value of PD-L1 expression in upper tract urothelial carcinoma varies according to platelet count. Cancer Med. 7, 4330–4338. https://doi.org/10.1002/cam4.1686 (2018).
Zhao, L. et al. Late-stage tumors induce anemia and immunosuppressive extramedullary erythroid progenitor cells. Nat. Med. 24, 1536–1544. https://doi.org/10.1038/s41591-018-0205-5 (2018).
Bellmunt, J. et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J. Clin. Oncol. 28, 1850–1855. https://doi.org/10.1200/jco.2009.25.4599 (2010).
Zhang, Z. et al. Pretreatment hemoglobin level as a predictor to evaluate the efficacy of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer. Therapeutic Adv. Med. Oncol. 12, 1758835920970049. https://doi.org/10.1177/1758835920970049 (2020).
Wu, S. et al. Pretreatment Neutrophil-Lymphocyte ratio as a predictor in bladder Cancer and metastatic or unresectable urothelial carcinoma patients: a pooled analysis of comparative studies. Cell. Physiol. Biochemistry: Int. J. Experimental Cell. Physiol. Biochem. Pharmacol. 46, 1352–1364. https://doi.org/10.1159/000489152 (2018).
Acknowledgements
We extend our gratitude to all our colleagues on the research team for their valuable contributions.
Funding
This work was supported by Beijing Medical Award Fund (Grant number: YXJL-2020-0785-0655).
Author information
Authors and Affiliations
Contributions
X.J.D., C.L., and X.H.L. conducted the study, performed the statistical analysis, and prepared the manuscript draft. S.K.W. and W.Y. were involved in the study design. Y.S., Z.G.W., and Y.P.W. contributed to data collection. All authors contributed to the writing of the article and approved the final submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the ethics committee of the Peking University First Hospital (Number: 2020–303) and conducted in accordance with the guidelines of the ethics committee and the 1964 Declaration of Helsinki and its later amendments. Due to the retrospective nature of the study, Peking University First Hospital ethics committee waived the need of obtaining informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ding, X., Liu, C., Li, X. et al. Real-world comparison of neoadjuvant chemoimmunotherapy and chemotherapy in muscle-invasive bladder cancer. Sci Rep 15, 17588 (2025). https://doi.org/10.1038/s41598-025-99889-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-99889-7