Abstract
B-cell lymphoma 2 (Bcl-2) is a crucial regulatory protein involved in the control of apoptosis. Its overexpression in cancer cells facilitates evasion of programmed cell death, contributing to their survival and resistance to chemotherapy. Consequently, Bcl-2 has emerged as a promising drug target in cancer therapy. There is still ongoing research to find potential drug molecules that target Bcl-2 with higher potency, selectivity, and safety profile. This study was carried out by conducting a virtual screening of phytoconstituents from the IMPPAT database that could potentially inhibit the aberrant activity of Bcl-2. We first excluded compounds that did not abide by the Lipinski rule of five based on their physicochemical properties. We also calculated binding affinities, applied PAINS filters, and performed ADMET and PASS analyses, as well as interaction analyses, to identify compounds that were predicted to be safe and effective. Finally, two compounds, Daturilinol and Withametelin B, were selected because of their high binding and selective binding to Bcl-2. We analyzed these compounds in terms of time evolution by employing molecular dynamics simulation (MDS), principal component analysis (PCA), free energy landscape (FEL), and MM/PBSA. Consequently, we suggest that Daturilinol and Withametelin B could be further investigated in vitro and in vivo for therapeutic development against cancer.
Similar content being viewed by others
Introduction
B-cell lymphoma 2 (Bcl-2) is a protein that plays a crucial role in regulating apoptosis1. It was first discovered in the mid-1980s as a result of research on a genetic translocation that was found in follicular lymphoma2. The discovery of Bcl-2 opened up a new field of research into the molecular mechanisms that regulate apoptosis and led to a better understanding of how cancer cells avoid apoptosis3. The Bcl-2 homology (BH) domain is highly conserved among the Bcl-2 family of proteins4 and is a necessary feature for their anti-apoptotic activity. They consist of four BH domains, BH1-BH4, arranged linearly and is a globular protein (predominantly alpha-helical)5. The BH3 domain is the smallest and most conserved of these four domains, and is required for the pro-apoptotic activity of the Bcl-2 family members6.
The main role of Bcl-2 is to control apoptosis, which is a multifaceted process that implies the activation of the proteases called caspases that cleave certain cellular substrates and, thus, cause the death of the cell7. Various intracellular signaling pathways, including the Bcl-2 family of proteins, tightly regulate caspase activation8. Bcl-2 on the other hand works by directly interacting with pro-apoptotic proteins including Bax and Bak9. Apoptosis dysregulation is one of the hallmarks of cancer, and has been mapped to the development of multiple cancer types, of which Bcl-2 has been a main player10. Abnormal expression of Bcl-2 has been reported in various types of cancer including leukemia, lymphoma, breast, prostate, and lung cancer11,12. The overexpression of Bcl-2 is anti-apoptotic and promotes cell survival hence the cells continue to divide without regulation13.
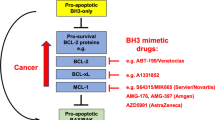
Several efforts have been made to study the involvement of Bcl-2 in cancer and several approaches have been proposed to use it as a therapeutic agent14,15,16. One such strategy uses small-molecule inhibitors, such as Venetoclax to achieve promising outcomes in hematologic malignancies such as chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML) in preclinical studies and clinical trials17,18. Nonetheless, more specific and potent BCL-2 inhibitors are still needed16. Inhibition of Bcl-2 by small molecule inhibitors has been identified as a viable approach to cancer therapy. Natural products, especially plant-derived secondary metabolites, have been identified for centuries as a source of pharmacologically active compounds including anticancer agents19. Natural product-based drug discovery is becoming an emerging hotspot in recent years due to the limitations and challenges of traditional experimental drug discovery approaches, including high cost and low success rate20,21. Natural products libraries are extensively employed for exploring biological activities using sophisticated computational techniques which include Virtual screening22.
Virtual screening is a computational method that employs molecular modeling and simulation strategies to filter and rank the potential drug molecules from large chemical repositories23. It has turned into a useful instrument for drug discovery and design especially to decipher lead compounds for target proteins. Virtual screening may have a large impact on the cost and time taken in the drug discovery process and the likelihood of success in the preclinical and clinical development phases24. The process of computer-aided drug designing includes a screening process in which several chemical databases are used to search for drug-like structures. Other drug-likeness filters assist in the detection of problems such as carcinogenicity, toxicity, and PAINS25. These methods are crucial in the drug discovery process. In the present study, we performed a virtual screening combined with simulation to look for phytoconstituents that can be effective Bcl-2 inhibitors. Several recent studies, including Bcl-2, used structure-based virtual screening to find new inhibitors, illustrating the usefulness of structural methodologies during early-stage drug discovery26,27. Inspired by this, our study takes a step further by incorporating molecular dynamic (MD) simulation approaches to narrow down the list of potential inhibitors. Recent developments in in-silico screening methods, such as virtual screening based on deep learning and enhanced sampling techniques28. We performed a virtual screening of bioactive phytoconstituents as potential strong Bcl-2 inhibitors using simulation-based approaches. We screened a natural product database of 11,708 compounds for potential Bcl-2 binding molecular docking and MDS.
Materials and methods
Computer resources
A wide range of computational tools and resources were utilized to carry out docking-based virtual screening and investigate the interactions between the screened compounds and the target protein. Specifically, AutoDock Vina29 was employed as the primary tool for docking simulations, enabling the identification of potential binding sites and prediction of binding affinities. The visualization of the protein-ligand complexes was performed using PyMOL30, facilitating the analysis and interpretation of the docking results. To further investigate the interactions between the ligands and target proteins, LigPlus was utilized31. This tool enabled the generation of 2D plots, which were used to explore the key interactions between the compounds and the protein. In addition to these core tools, a range of online web servers and databases, including UniProt32, Protein Data Bank (PDB)33, IMPPAT 2.0 (Indian Medicinal Plants Phytochemistry and Therapeutics)34, ADMETlab 2.035, and PASS server36, were employed to extract, assess and investigate the relevant data. The integration of these tools and resources enabled the comprehensive exploration of the ligand-target interactions and the identification of promising leads for further development.
Receptor and library Preparation
To obtain the three-dimensional structure of Bcl-2, the coordinates with PDB ID 6O0K were obtained from the PDB database. Subsequently, the kinase domain was extracted from the whole coordinates using PyMOL, and the resulting structure was refined further using Swiss-PDB Viewer37. Protein preparation was conducted using the MGL AutoDock Tools to ensure an optimized structure for docking studies. Missing residues were modeled using the PyMod-338, while hydrogen atoms were added to restore proper protonation states. Water molecules were removed to eliminate irrelevant solvent interactions that could interfere with docking calculations. The stability of the structure was ensured through energy minimization, which was also performed through Swiss-PDB Viewer. The IMPPAT 2.0 was accessed to download the compounds library in 3D processed form. A total of 17,967 phytochemicals were screened, of which 11,708 compounds adhered to Lipinski’s rule of five and were retained for further analysis. To ensure that the phytochemical compounds library was comprised of biologically active compounds with drug-like properties, we employed the Lipinski rule of five39 to filter the phytochemicals library. Ligand preparation was performed using MGL AutoDock Tools40 to ensure optimal input structures for docking simulations. The ligands were subjected to energy minimization to eliminate any steric clashes and optimize conformations. Additionally, appropriate protonation states were assigned at physiological pH and partial atomic charges were calculated using the Gasteiger method to enhance docking accuracy. These preparatory steps ensured that the ligands were structurally optimized and in a suitable form for docking with the Bcl-2 protein.
Molecular Docking screening
Molecular docking screening has revolutionized the drug discovery process by significantly reducing the time and cost of screening millions of compounds for discovering new therapeutics41. These techniques allow for the efficient screening of large libraries of small molecules against target protein. We employed the AutoDock Vina tool to perform blind docking experiments on a phytochemicals library. The docking search was conducted using a grid box with dimensions (x: 49, y: 48, z: 58), centered at coordinates (x: −5.257, y: 3.401, z: −12.158), and a grid spacing of 1.0 Å. The exhaustiveness was set to 8 to ensure sufficient conformational sampling. All other docking and scoring parameters were maintained at their default settings29. The goal was to identify compounds with the highest binding affinity towards Bcl-2. The outfiles generated by AutoDock Vina were in the form of log files and out-files. These files were further analyzed to determine the binding affinity of the identified compounds towards Bcl-2. We used a range of computational tools to perform the docking analysis, including PyMOL and LigPlus. These tools enabled the visualization and interpretation of the docking results, allowing us to identify potential compounds with the most favorable interactions with Bcl-2.
ADMET prediction
After the docking experiments to select compounds of interest, the ADMET properties of the compounds were predicted by the SwissADME and ADMETlab 2.0 tools. When removing compounds that contain PAINS patterns, we were able to identify those with a better chance of being selective for a given target. In addition, compounds with good ADMET profiles were selected for the next step of assessment. These compounds were considered to have the highest potential for development as drugs because they both complied with drug-likeness and had a high specificity with regard to the protein of interest, Bcl-2. By combining ADMET properties and PAINS filtering, we were able to successfully and rapidly filter the compounds for further analysis and prioritize those with the most promising characteristics42.
PASS analysis
We employed the PASS analysis as a helpful resource to investigate the chemical-biological interactions and evaluate the biological activities of the investigated compounds. The PASS server produces results in the Pa vs. Pi ratio with their activities36. The Pa value represents the likelihood of a compound possessing the related property, where Pa values are greater, suggesting activity. With the help of the PASS server, we got an insight into the biological properties of the compounds, which assisted in the selection of the compounds for further study. This enabled us to select those compounds with the best prospect of demonstrating the requisite biological activity and was useful in the generation of a more productive and efficient drug discovery scheme. Additionally, the anticancer potential of the prioritized compounds was predicted through PDCsmCancer (https://biosig.lab.uq.edu.au/pdcsm_cancer/prediction).
Interaction analysis
PyMOL and LigPlus were used to analyze the binding mode of the identified compounds with Bcl-2. More particularly, we used the conformations of the ligands from the AutoDock Vina docking results. In PyMOL, we built ribbon representations and electrostatic potential maps. We also represented in dotted lines and labeled any hydrogen bonds formed within 3.5Å. Further, we employed LigPlus to generate 2D plots of the compounds and Bcl-2, each of which represented a type of interaction.
Molecular dynamics simulations protocol
MD simulation was performed for 200 nanoseconds (ns) to study the atomic-level motions in the protein-ligand complex. For this purpose, we utilized GROMACS 2020, an open-source software program for MD simulation43. To generate the topologies for Daturilinol and Withametelin B, we used the PRODRG server44. The PRODRG server was chosen for ligand topology generation due to its efficiency in parameterizing small molecules for GROMACS. The CHARMM36 force field45 was employed to accurately represent ligand-protein interactions, ensuring reliable molecular dynamics trajectories. To solvate each system, we placed them in a cubic box with a distance of 10 Å from the center to the edges, using the simple point charge (SPC216) water model46. We also added counterions (Na+ and Cl−) to neutralize the simulation system. To eliminate any potential clashes between atoms, we used the steepest descent method followed by the conjugate gradient method to perform energy minimization in the solvated system, taking 1500 steps in total47. The SPC water model was chosen due to its computational efficiency and well-documented accuracy in biomolecular simulations. Next, we conducted a two-step equilibration process under periodic boundary conditions, gradually heating the system from 0 K to 300 K at a constant volume and pressure of 1 atm. The equilibration lasted for 1000 picoseconds (ps). We utilized the built-in tools of GROMACS to analyze the generated MD simulation data and plot it using XMGRACE.
Principal component analysis
PCA is a commonly used mathematical method to reduce the dimensionality of data48. This is achieved by identifying the principal components (PCs), which represent the directions along which the maximal variation in data occurs. Typically, only a few PCs are needed to describe each sample through a limited number of variables. Additionally, PCA can be used to uncover significant movements with high amplitude in MDS. In this study, we applied PCA and the free energy landscape (FEL) approach49 to examine the MD trajectories of Bcl-2 both before and after binding with Daturilinol and Withametelin B. A visual representation of the methodology workflow used in this study is depicted in Fig. 1.
MM-PBSA calculation
The molecular mechanics Poisson-Boltzmann surface area (MM-PBSA) method decomposes the total binding free energy into its components (van der Waals (vdW), electrostatic, polar solvation, and nonpolar solvation energies)50. This decomposition enables a detailed analysis of the binding free energy and the forces driving the protein-ligand interactions. The MM-PBSA method was used to calculate the free energy of binding of Daturilinol and Withametelin B to Bcl-2. This approach allows to obtain a detailed and quantitative prediction of the binding free energy of protein-ligand pairs. For calculating binding free energy of Daturilinol-Bcl-2 and Withametelin B-Bcl-2 complexes the MM-PBSA were calculated with g_mmpbsa module51 embedded in GROMACS. For these calculations, we used trajectory data from a stable section of our simulation window (100 to 110 ns), and the binding free energies of the complexes were estimated by averaging the contributions of the components from the selected trajectory frames.
Results and discussion
Molecular docking screening
We used molecular docking to determine the degree of interaction between the phytochemical compounds from the IMPPAT database and Bcl-2. Docked conformers were generated for each ligand using the split module of the AutoDock Vina software. Then, we screened the docked compounds for their docking score and selected the top 20 hits out of 11,708 phytochemicals, which docked well with Bcl-2. These selected hits showed binding affinities in the range of − 9.5 kcal/mol to − 8.8 kcal/mol to Bcl-2, suggesting significant binding potential. Compounds in addition to the reference co-crystalized Bcl-2 inhibitor Venetoclax, together with their binding affinities with Bcl-2, are listed in Table 1. Bindings energies comparison shows that most screened compounds are competitive binders to Venetoclax and other Bcl-2 inhibitors (such as Obatoclax). The high binding affinities of the selected hits for Bcl-2 indicate that they might serve as useful leads in the structural development of anticancer agents. Thus, the chosen compounds can be considered promising for the development of novel drugs against Bcl-2 and stimulate further research in this direction.
ADMET properties
After conducting molecular docking, we subjected the top 20 hits to ADMET (absorption, distribution, metabolism, excretion, and toxicity) property screening to identify the compounds with favorable drug-like properties. We evaluated the ADMET properties of phytochemical compounds selected from the top 20 hits generated from molecular docking (Supplementary Table S1). ADMET properties are based on the pharmacokinetic parameters of chemical compounds that can influence their efficacy and safety as potential drug candidates52. We selected 8 of the 20 hits that had the most potential ADMET properties to be further tested for their PO activity (Table 2). In addition, the analysis revealed that the screened compounds are not substrates for OCT2, indicating that they are unlikely to be secreted by the kidney. This is particularly relevant for drugs that target other organs. Compared to orally available Bcl-2 inhibitors, the selected compounds demonstrated strong HIA and BBB permeability, indicating their potential as orally bioavailable agents. As demonstrated by the BBB permeability values, the great potential for penetration of CNS not only seems promising for use in the treatment of hematological malignancies, but also hematological malignancies involving the CNS53. Moreover, the desirable pharmacokinetic characteristics associated with the chosen compounds suggest their capacity as orally bioavailable Bcl-2 inhibitors with improved systemic distribution. Mineralocorticoid receptor antagonists sitting in the pitiful medicine cabinet, but many biochemical mechanisms explain their excessive secretion by transmembrane(s), sequestration in cellular organelles, and elimination via organic cation transporter 2 (OCT2) in the renal excretion of drugs54. The non-substrate identity of screened molecules for OCT2 indicates their decreased renal clearance and prolonged systemic circulation which also enhances the bioavailability in the body.
Apart from the ADME properties, the toxicity and carcinogenicity prediction analysis demonstrated that the chosen phytoconstituents are harmless, indicating that they do not pose a risk of toxicity to the body. The analysis suggested that the ADMET properties of chosen compounds are better than the reference inhibitor Venetoclax. These compounds demonstrated similar ADMET properties and were therefore chosen for further analysis. The results suggest that the selected compounds have the potential to serve as promising drug candidates. Their favorable ADMET properties imply a high likelihood of successful clinical translation and efficacy in humans. Moreover, the absence of toxic patterns further supports the potential of these compounds as safe and effective therapeutic agents.
PASS analysis
To investigate the biological activity of the elucidated compounds and guarantee their efficacy, the PASS analysis was performed for 8 compounds derived from the ADMET analysis. Table 3 represents the biological properties of our interest in the phytochemical compounds and the activity or inactivity score. Here, the biological properties associated with the anticancer activities were chosen along with their Pa values. We then selected potential compounds based on a cut-off of > 0.5. This threshold was applied to prioritize compounds with significant predicted activity, as commonly used in PASS-based screening studies. The results show that IMPHY008964 (Daturilinol) and IMPHY009120 (Withametelin B) have moderate to high potential as antineoplastic, antileukemic, chemopreventive, and apoptosis agonist activities with Pa values varying between 0.957 and 0.554. The PASS prediction of the reference inhibitor Venetoclax displayed its Bcl-2 inhibitory activity, thus indicating the validity of the prediction. Taken together, these results suggest that Daturilinol and Withametelin B could be useful in the treatment of cancer. At the same time, the anticancer potential of both compounds was also confirmed in multiple cancer types through the PDCsmCancer (https://biosig.lab.uq.edu.au/pdcsm_cancer/prediction) program. A literature review was conducted to evaluate previous research on Daturilinol and Withametelin B, organic compounds abundantly present in the leaves of Datura metel. While both compounds have been associated with various pharmacological activities in cancer, inflammation, pain and depression, no studies to date have explored their potential as Bcl-2 inhibitors55. This underscores the novelty of our study and suggests that further in vitro and in vivo validation may reveal their therapeutic potential in targeting Bcl-2-driven cancers.
Interaction analysis
After the ADMET and PASS screening, we identified the two most active phytochemical compounds from the molecular docking and conducted an interaction analysis. The study also included covering all the possible docking conformations of the molecules obtained with the help of docked Daturilinol and Withametelin B to understand the binding patterns with Bcl-2 (Fig. 2). The Bcl-2 binding mode of Daturilinol and Withametelin B is illustrated in Fig. 2A, which indicates that the compounds are well anchored to the binding pocket of Bcl-2. The study identified that both compounds are bound to the same residues of the BH1 domain of Bcl-2 (Fig. 2B). The structural representation also established that Daturilinol and Withametelin B were interacting with the deep binding pocket of Bcl-2 (Fig. 2C). These observations suggest that the selected compounds have a strong binding propensity to Bcl-2 and can interact with the key residues necessary for the protein to function. Therefore, they have the potential to act as effective Bcl-2 inhibitors, thereby contributing to the development of novel therapies for cancer and other diseases.
We conducted further investigation to examine the interactions between Daturilinol and Withametelin B and the binding site residue of Bcl-2. The binding site residue of Bcl-2 is critical for the functional activities of the protein. The analysis revealed that both Daturilinol and Withametelin B share similar interactions when binding to the binding site residue of Bcl-2, as shown in Fig. 3. Daturilinol interacted with Phe104, Phe112, Met115, Val133, Glu136, Leu137, Arg139, Asp140, and Ala149 (Fig. 3A). At the same time, interacted Withametelin B showed interactions with Phe104, Phe112, Met115, Glu136, Leu137, Arg139, Asp140, and Ala149 (Fig. 3B). Both compounds showed similar interactions with several common residues as the reference Bcl-2 inhibitor, Venetoclax (Fig. 3C). Various residues involved in hydrogen bonding and hydrophobic interactions include Asp103, Phe104, Phe112, Met115, Arg139, and Gly145, which are crucial for ligand binding stability. This finding suggests that the compounds have the potential to act as potential inhibitors of Bcl-2, thereby contributing to the development of novel therapeutic agents for cancer and other diseases.
MD simulation analysis
To investigate the dynamic behavior and structural details of the protein-ligand complexes, we employed the MDS method56,57. In this study, we performed 200 ns of all-atom MDS on three systems: Bcl-2-Daturilinol, Bcl-2-Withametelin B, and free Bcl-2. The stability and dynamics of Bcl-2 in association with Daturilinol and Withametelin B were evaluated by examining various parameters. The results of this analysis provide valuable insights into the interaction between Bcl-2 and the selected compounds. The stability and dynamics of Bcl-2 in association with Daturilinol and Withametelin B were evaluated by examining several systematic and structural parameters, which are discussed below.
Structural dynamics and compactness
In order to evaluate the stability and dynamics of Bcl-2 in association with Daturilinol and Withametelin B, we used the root-mean-square deviation (RMSD), a widely used parameter to assess structural deviations in proteins58. The average RMSD values for Bcl-2, Bcl-2-Daturilinol, and Bcl-2-Withametelin B complexes were 0.34 nm, 0.42 nm, and 0.43 nm, respectively, indicating good stability of the docked complexes without any structural shift throughout the simulation time. As shown in Fig. 4A, the RMSD graph demonstrated that the binding of Daturilinol and Withametelin B with Bcl-2 was equilibrated during the simulation period. Although a slight increase was observed in the RMSD for the Bcl-2 docked complexes, there was no significant shift in the RMSD graph. The RMSD values for all systems remained stable and balanced throughout the 200 ns simulation period. These findings suggest that both compounds, Daturilinol and Withametelin B, can maintain stable interactions with Bcl-2 over a period of time and are, therefore, suitable for further investigation (Fig. 4A). The RMSD plot shows that after an initial fluctuation during the first 10–20 ns, the RMSD values for the Bcl-2-ligand complexes stabilized, indicating that equilibrium was achieved within 50 ns. This stabilization suggests that both Daturilinol and Withametelin B remained bound to Bcl-2 without significant structural perturbations. Annotations in Fig. 4A highlight this transition period and the steady-state phase.
The root means square fluctuation (RMSF) parameter was utilized to investigate the residual vibrations in the protein structures of Bcl-2 before and after ligand binding during MD simulation. The RMSF graph revealed significant stabilization of RMSF fluctuations upon Withametelin B binding, indicating a highly reliable interaction between Withametelin B and Bcl-2 (Fig. 4B). In contrast, a marginal increase in residual vibrations at some regions was observed for Daturilinol, indicating higher dynamics at the loop regions. This finding suggests that the Bcl-2-Withametelin B complex is more stable than the Bcl-2-Daturilinol complex. The RMSF analysis provides a useful tool for studying the residual dynamics of Bcl-2 upon ligand binding, and the results suggest that the Withametelin B complex may be a more reliable candidate for further drug development studies. The RMSF values for both Daturilinol and Withametelin B-bound systems showed a consistent pattern throughout the simulation time, which further supports the stable nature of the ligand-protein complex.
Structural dynamics and compactness of Bcl-2 upon Daturilinol and Withametelin B binding. (A) RMSD plot of Bcl-2 with Daturilinol and Withametelin B. (B) RMSF plot, (C) rGyr plot, and (D) SASA plot of Bcl-2 with Daturilinol and Withametelin B. The results confirm that the Bcl-2-ligand complexes achieved equilibrium within the first 25 ns of simulation.
We utilized the radius of gyration (rGyr) parameter to assess the compactness of the Bcl-2-Daturilinol and Bcl-2-Withametelin B complexes before and after the compounds’ binding (Fig. 4C). The rGyr value provides insights into the tertiary structure of the protein and is related to its stability59. The rGyr values for Bcl-2-Daturilinol and Bcl-2-Withametelin B complexes were studied, and it was found that the rGyr value slightly increased for the Bcl-2-Daturilinol complex, which is consistent with the RMSD and RMSF values. This suggests that Daturilinol binding may result in a slightly more expanded Bcl-2 structure due to the occupancy of some intermolecular space within Bcl-2. However, both complexes were found to be stable and well-folded based on the rGyr analysis.
We further investigated the folding behavior of Bcl-2 in complex with Daturilinol and Withametelin B, using the solvent-accessible surface area (SASA) parameter. The SASA values of all systems were calculated and analyzed throughout the 200 ns MD simulation period. The analysis showed that the SASA values of the Bcl-2-Daturilinol and Bcl-2-Withametelin B complexes did not change significantly compared to the free form of Bcl-2, indicating that the complexes are stable (Fig. 4D). However, there was a slight increase in the SASA value of Bcl-2 in the complex with Withametelin B, suggesting a slightly more open conformation compared to the other systems. Nevertheless, the overall protein folding remained unchanged, indicating that the interaction of Daturilinol with Bcl-2 does not affect the protein stability. These findings are consistent with the RMSD, RMSF, and rGyr analyses, indicating that both complexes are stable and well-folded. Therefore, we can conclude that the SASA analysis supports the stability of the Bcl-2-Daturilinol and Bcl-2-Withametelin B complexes.
Dynamics of hydrogen bonds
The study of hydrogen bonds is crucial for understanding protein structure60. In this study, we analyzed the time evolution of hydrogen bonds in the Bcl-2-Daturilinol and Bcl-2-Withametelin B complexes to examine the consistency of intramolecular bonding. The results indicated no significant changes in the number of hydrogen bonds within Bcl-2 upon binding with Daturilinol and Withametelin B (Fig. 5A). A minor increase in the number of hydrogen bonds was observed, which could be attributed to the increased compactness resulting from ligand binding. The probability distribution function (PDF) analysis of intramolecular hydrogen bonds for all three systems showed good reliability (Fig. 5B). Based on these findings, we conclude that the intramolecular hydrogen bonds in Bcl-2 remained stable.
Furthermore, we investigated the stability of the intermolecular hydrogen bonds formed between Daturilinol and Withametelin B with Bcl-2 by analyzing their time evolution. The analysis revealed that each complex formed an average of 1 intramolecular hydrogen bond throughout the simulation (Fig. 6, upper panel). The PDF analysis showed that both systems exhibited good stability of intramolecular hydrogen bonds, with an average number of hydrogen bonding as 1 and a higher PDF value (Fig. 6, lower panel). This suggests that the Daturilinol and Withametelin B compounds remained stably bound to Bcl-2 throughout the simulation, with little or no disruption of hydrogen bonding. Moreover, the lack of significant movement of Daturilinol and Withametelin B from their initial docking position on Bcl-2, as indicated by the intermolecular hydrogen bonding, further supports the stabilization of the complex structures. Overall, the analysis suggests that the intramolecular and intermolecular hydrogen bonds in Bcl-2-Daturilinol and Bcl-2-Withametelin B complexes remained stable throughout the simulation, indicating reliable binding between the compounds and Bcl-2. Overall, the analysis revealed that Daturilinol and Withametelin B remained stably bound to Bcl-2 throughout the simulation, with minimal deviations, supporting their stable interactions.
PCA and fels analysis
PCA is a useful tool for examining the conformational sampling of proteins and protein-ligand complexes49. We utilized PCA to investigate the collective motions in the Bcl-2, Bcl-2-Daturilinol, and Bcl-2-Withametelin B complexes during MDS. We projected the conformational sampling of these complexes onto two essential subspace vectors, Eigenvectors (EVs) 1 and 2, and the results are shown in Fig. 7. We overlaid the projections of Bcl-2 conformations on these two vectors with those of Bcl-2-Daturilinol and Bcl-2-Withametelin B complexes. Interestingly, we observed that the bound complexes occupy a similar conformational space as the free protein, with the clusters of Bcl-2-Daturilinol and Bcl-2-Withametelin B projections. However, we found that the Bcl-2-Withametelin B complex showed a more restricted and less flexible conformational space compared to the Bcl-2-Daturilinol complex, indicating that it is more stable. The results suggest that the essential dynamics approach using PCA is a useful tool for investigating the conformational sampling of protein-ligand complexes and can provide valuable insights into their stability and flexibility (Fig. 7).
Furthermore, FEL analysis was carried out to investigate the protein folding mechanism of Bcl-2, Bcl-2-Daturilinol, and Bcl-2-Withametelin B systems. The FELs, shown in Fig. 8, allow us to examine the global minima of these systems. The deeper blue shades in the FELs represent the lower energy protein conformations that are closer to the native states. The FEL plot for the free state of Bcl-2 indicated that it has 3 global minima confined within 3–4 basins (Fig. 8A). The binding of Bcl-2 with Daturilinol and Withametelin B slightly disturbed the size and position of the phases. Overall, when Bcl-2 bound with Daturilinol and Withametelin B, the FEL plots indicated that it adopted different states with multiple basins but ultimately reached the global minimum (Fig. 8B-C). Taken together, the promising binding stability and favorable ADMET profiles of Daturilinol and Withametelin B suggest their potential as lead compounds for further development into anti-cancer therapeutics. Future experimental validation and optimization could facilitate their clinical translation as targeted Bcl-2 inhibitors.
MM-PBSA analysis
The binding free energy using the MM-PBSA method was calculated to estimate the influential potential of Bcl-2 binding with Daturilinol and Withametelin B. Analysis was performed in a stable part of the MDS trajectory from 100 to 110 ns. Daturilinol and Withametelin B (− 28.21 ± 4.18 kJ/mol and 32.22 ± 7.10 kJ/mol, respectively) were also found to bind to Bcl-2 with high affinities according to the results. These scores indicate that Daturilinol, Withametelin B and Bcl-2 interact strongly and favorably. Interestingly, the binding affinities of both compounds to Bcl-2 from MM-PBSA results strongly confirm our previous docking results. These findings confirm the conclusions drawn from molecular docking and other simulation analyses. Taken together, our computational workflow follows a rigorous multi-tiered approach, integrating molecular docking, MDS, and binding free energy calculations to enhance the reliability of virtual screening results. Previous studies have demonstrated the predictive power of such in silico methods, with many computationally identified inhibitors later confirmed through experimental validation61,62. Overall, this study identifies Daturilinol and Withametelin B as promising Bcl-2 binding partners for further investigation in cancer therapy development.
Conclusions
The modern world is faced with various complex diseases such as cancer, for which there is a need to develop new and efficient treatments. Among the other approaches, targeting Bcl-2 has been considered as a perspective for developing anticancer therapies, and the purpose of this work was to investigate the ability of natural compounds to target Bcl-2. By incorporating computational analysis, we found two phytoconstituents, namely Daturilinol and Withametelin B, that showed druglike properties and have a high affinity for Bcl-2. From the docking, pharmacokinetics, and MD simulations, it can be concluded that Daturilinol and Withametelin B have a strong and stable interaction with Bcl-2. The generated FELs and PCA projections also suggest that Daturilinol and Withametelin B bind to Bcl-2 without causing any significant conformational changes. More research is required on these compounds in an experimental context to investigate the possibility of using them as the basis for future anticancer therapies targeting Bcl-2. These results’ implications may serve as a basis for the development of novel and efficient drugs against cancer.
Data availability
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
References
Davids, M. S. & Letai, A. Targeting the B-cell lymphoma/leukemia 2 family in cancer. J. Clin. Oncol. 30, 3127 (2012).
Tomita, N. BCL2 and MYC dual-hit lymphoma/leukemia. J. Clin. Experimental Hematopathology. 51, 7–12 (2011).
Delbridge, A. R., Grabow, S., Strasser, A. & Vaux, D. L. Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies. Nat. Rev. Cancer. 16, 99–109 (2016).
Levine, B., Sinha, S. C. & Kroemer, G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy 4, 600–606 (2008).
Gross, A., McDonnell, J. M. & Korsmeyer, S. J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13, 1899–1911 (1999).
Aouacheria, A., de Laval, V. R., Combet, C. & Hardwick, J. M. Evolution of Bcl-2 homology motifs: homology versus homoplasy. Trends Cell Biol. 23, 103–111 (2013).
Nuñez, G., Benedict, M. A., Hu, Y. & Inohara, N. Caspases: the proteases of the apoptotic pathway. Oncogene 17, 3237–3245 (1998).
Ola, M. S., Nawaz, M. & Ahsan, H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol. Cell. Biochem. 351, 41–58 (2011).
Brunelle, J. K. & Letai, A. Control of mitochondrial apoptosis by the Bcl-2 family. J. Cell Sci. 122, 437–441 (2009).
Packham, G. & Stevenson, F. K. Bodyguards and assassins: Bcl-2 family proteins and apoptosis control in chronic lymphocytic leukaemia. Immunology 114, 441–449 (2005).
Placzek, W. et al. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell Death Dis. 1, e40 (2010).
Correia, C. et al. Emerging Understanding of Bcl-2 biology: implications for neoplastic progression and treatment. Biochim. Biophys. Acta Mol. Cell. Res. 1853, 1658–1671 (2015).
Ghobrial, I. M., Witzig, T. E. & Adjei, A. A. Targeting apoptosis pathways in cancer therapy. Cancer J. Clin. 55, 178–194 (2005).
Kang, M. H. & Reynolds, C. P. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin. Cancer Res. 15, 1126–1132 (2009).
Lu, Q. L., Abel, P., Foster, C. S. & Lalani, E. N. bcl-2: role in epithelial differentiation and oncogenesis. Hum. Pathol. 27, 102–110 (1996).
Vogler, M., Dinsdale, D., Dyer, M. J. & Cohen, G. M. Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell. Death Differ. 16, 360–367 (2009).
Li, Z., He, S. & Look, A. T. The MCL1-specific inhibitor S63845 acts synergistically with venetoclax/ABT-199 to induce apoptosis in T-cell acute lymphoblastic leukemia cells. Leukemia 33, 262–266 (2019).
Konopleva, M. & Letai, A. BCL-2 Inhibition in AML: an unexpected bonus? Blood J. Am. Soc. Hematol. 132, 1007–1012 (2018).
Atanasov, A. G., Zotchev, S. B., Dirsch, V. M. & Supuran, C. T. Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discovery. 20, 200–216 (2021).
Huang, B. & Zhang, Y. Teaching an old dog new tricks: drug discovery by repositioning natural products and their derivatives. Drug Discovery Today. 1, 1 (2022).
Singh, R., Manna, S., Nandanwar, H. & Purohit, R. Bioactives from medicinal herb against bedaquiline resistant tuberculosis: removing the dark clouds from the horizon. Microb. Infect. 26, 105279 (2024).
Schneider, G. Virtual screening: an endless staircase? Nat. Rev. Drug Discovery. 9, 273–276 (2010).
Mohammad, T., Mathur, Y., Hassan, M. I. & InstaDock A single-click graphical user interface for molecular docking-based virtual high-throughput screening. Brief. Bioinform. 22, 279 (2021).
Li, Q. & Shah, S. Structure-based virtual screening. Protein Bioinf. 1, 111–124 (2017).
Jia, C. Y., Li, J. Y., Hao, G. F. & Yang G.-F. A drug-likeness toolbox facilitates ADMET study in drug discovery. Drug Discovery Today. 25, 248–258 (2020).
Gupta, A. & Purohit, R. Identification of potent BRD4-BD1 inhibitors using classical and steered molecular dynamics based free energy analysis. J. Cell. Biochem. 125, e30532 (2024).
Sharma, B., Bhattacherjee, D., Zyryanov, G. V. & Purohit, R. An insight from computational approach to explore novel, high-affinity phosphodiesterase 10A inhibitors for neurological disorders. J. Biomol. Struct. Dynamics. 41, 9424–9436 (2023).
Singh, R., Bhardwaj, V. K., Sharma, J., Das, P. & Purohit, R. Identification of selective cyclin-dependent kinase 2 inhibitor from the library of pyrrolone-fused benzosuberene compounds: an in Silico exploration. J. Biomol. Struct. Dynamics. 40, 7693–7701 (2022).
Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of Docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2010).
DeLano, W. L. Pymol: An open-source molecular graphics tool. CCP4 Newsl. Protein Crystallogr. 40, 82–92 (2002).
Laskowski, R. A. & Swindells, M. B. (ACS Publications, 2011).
Consortium, U. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 47, D506–D515 (2019).
Berman, H. M. et al. The protein data bank. Nucleic Acids Res. 28, 235–242 (2000).
Vivek-Ananth, R., Mohanraj, K., Sahoo, A. K. & Samal, A. IMPPAT 2.0: an enhanced and expanded phytochemical atlas of Indian medicinal plants. ACS Omega. 8, 8827–8845 (2023).
Xiong, G. et al. ADMETlab 2.0: an integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 49, W5–W14 (2021).
Lagunin, A., Stepanchikova, A., Filimonov, D. & Poroikov, V. PASS: prediction of activity spectra for biologically active substances. Bioinformatics 16, 747–748 (2000).
Guex, N. & Peitsch, M. C. SWISS-MODEL and the Swiss‐Pdb viewer: an environment for comparative protein modeling. Electrophoresis 18, 2714–2723 (1997).
Janson, G. & Paiardini, A. PyMod 3: a complete suite for structural bioinformatics in PyMOL. Bioinformatics 37, 1471–1472 (2021).
Lipinski, C. A. Lead-and drug-like compounds: the rule-of-five revolution. Drug Discovery Today: Technol. 1, 337–341 (2004).
Huey, R., Morris, G. M. & Forli, S. Using AutoDock 4 and AutoDock Vina with autodocktools: a tutorial. Scripps Res. Inst. Mol. Graphics Lab. 10550, 1000 (2012).
Meng, X. Y., Zhang, H. X., Mezei, M. & Cui, M. Molecular docking: a powerful approach for structure-based drug discovery. Curr. Comput.-Aided Drug Design. 7, 146–157 (2011).
Ferreira, L. L. & Andricopulo, A. D. ADMET modeling approaches in drug discovery. Drug Discovery Today. 24, 1157–1165 (2019).
Van Der Spoel, D. et al. GROMACS: fast, flexible, and free. J. Comput. Chem. 26, 1701–1718 (2005).
Schüttelkopf, A. W. & Van Aalten, D. M. PRODRG: a tool for high-throughput crystallography of protein–ligand complexes. Acta Crystallogr. Sect. D Biol. Crystallogr. 60, 1355–1363 (2004).
Huang, J. & MacKerell, A. D. Jr CHARMM36 all-atom additive protein force field: validation based on comparison to NMR data. J. Comput. Chem. 34, 2135–2145 (2013).
Wu, Y., Tepper, H. L. & Voth, G. A. Flexible simple point-charge water model with improved liquid-state properties. J. Chem. Phys. 124, 024503 (2006).
Haug, E., Arora, J. & Matsui, K. A steepest-descent method for optimization of mechanical systems. J. Optim. Theory Appl. 19, 401–424 (1976).
David, C. C. & Jacobs, D. J. Protein Dynamics 193–226 (Springer, 2014).
Papaleo, E., Mereghetti, P., Fantucci, P., Grandori, R. & De Gioia, L. Free-energy landscape, principal component analysis, and structural clustering to identify representative conformations from molecular dynamics simulations: the myoglobin case. J. Mol. Graph. Model. 27, 889–899 (2009).
Genheden, S. & Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 10, 449–461 (2015).
Kumari, R., Kumar, R., Consortium, O. S. D. D. & Lynn, A. g_mmpbsa A GROMACS tool for high-throughput MM-PBSA calculations. J. Chem. Inf. Model. 54, 1951–1962 (2014).
Hodgson, J. ADMET—turning chemicals into drugs. Nat. Biotechnol. 19, 722–726 (2001).
Deeken, J. F. & Löscher, W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin. Cancer Res. 13, 1663–1674 (2007).
Rico-Mesa, J. S., White, A., Ahmadian-Tehrani, A. & Anderson, A. S. Mineralocorticoid receptor antagonists: a comprehensive review of finerenone. Curr. Cardiol. Rep. 22, 1–11 (2020).
Baig, M. W. et al. Withametelin: A biologically active withanolide in cancer, inflammation, pain and depression. Saudi Pharm. J. 28, 1526–1537 (2020).
Karplus, M. & McCammon, J. A. Molecular dynamics simulations of biomolecules. Nat. Struct. Biol. 9, 646–652 (2002).
Raza, A. et al. Fructosylation of human insulin causes ages formation, structural perturbations and morphological changes: an in Silico and multispectroscopic study. J. Biomol. Struct. Dynamics. 1, 1–13 (2022).
Pitera, J. W. Expected distributions of root-mean-square positional deviations in proteins. J. Phys. Chem. B. 118, 6526–6530 (2014).
Lobanov, M. Y., Bogatyreva, N. & Galzitskaya, O. Radius of gyration as an indicator of protein structure compactness. Mol. Biol. 42, 623–628 (2008).
Weiss, M. S., Brandl, M., Sühnel, J., Pal, D. & Hilgenfeld, R. More hydrogen bonds for the (structural) biologist. Trends Biochem. Sci. 26, 521–523 (2001).
Cichonska, A. et al. Computational-experimental approach to drug-target interaction mapping: a case study on kinase inhibitors. PLoS Comput. Biol. 13, e1005678 (2017).
Glaab, E., Manoharan, G. B. & Abankwa, D. Pharmacophore model for SARS-CoV-2 3CLpro small-molecule inhibitors and in vitro experimental validation of computationally screened inhibitors. J. Chem. Inf. Model. 61, 4082–4096 (2021).
Acknowledgements
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Large Research Project under grant number RGP 2/389/46 RGP2/389/46.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.G.A. and S.W.; methodology, S.W.; software, M.Y.A and S.W.; validation, M.Y.A., S.W and M.A; formal analysis, S.W.; investigation, S.W.; resources, M.A; data curation, S.W.; writing—original draft preparation, S.W. and A.G.A.; writing—review and editing, S.W. and M.Y.A.; visualization, M.A.; supervision, M.A.; project administration, M.Y.A; funding acquisition, S.W and M.Y.A; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Alkhathami, A.G., Alshahrani, M.Y., Asiri, M. et al. Phytochemicals Daturilinol and Withametelin B identified as novel Bcl2 inhibitors via virtual screening and molecular simulations. Sci Rep 15, 33029 (2025). https://doi.org/10.1038/s41598-025-99901-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99901-0