Abstract
This article focuses on the eccentric Sombor index, a variant of the degree-based Sombor index in which vertex degrees are replaced by their eccentricities. Several bounds for this index are established in terms of other known topological indices. Additionally, the practical utility of the eccentric Sombor index is demonstrated through its application in predicting physicochemical properties of polycyclic aromatic compounds. Our analysis reveals that the index shows a strong linear correlation with a coefficient of determination \(R^2>0.9\), with key properties such as molecular weight, boiling point, molar refractivity, polarizability, molar volume, and flash point. Furthermore, a comparative study indicates that the eccentric Sombor index offers greater predictive accuracy than the traditional degree-based Sombor index.
Similar content being viewed by others
Introduction
In the modern era of explosive growth in nanomaterials, crystalline materials, and drugs, exhaustive lab experiments to explore chemical behaviors burden pharmaceutical scientists. In this context, the computation of various topological indices has emerged as an effective approach for gaining valuable insights into the medicinal properties of a wide range of compounds and drugs1,2. Such indices enable the extraction of meaningful chemical and medical information while considerably reducing the need for costly and time-consuming experimental procedures3,4.
Chemical graph theory has gained significant popularity among researchers due to its broad applicability in mathematical chemistry. A molecular graph G(V, E) is a simple graph with n vertices and m edges. In the molecular graph G, each vertex \(v_i \in V(G)\) represents a non-hydrogen atom and each edge \((v_i, v_j) \in E(G)\) represents a covalent bond connecting those atoms. The molecular graphs of hydrocarbons, which are specifically made up of just carbon and hydrogen atoms, indicate the carbon skeleton of the molecule. The degree \(d_u\) of a vertex u is the number of its neighboring vertices. The distance between two vertices \(v_i\) and \(v_j\) in a molecular graph is denoted by \(d(v_i,v_j)\), which is the length of the shortest path connecting them. The eccentricity of a vertex \(v_i\) is the distance from the farthest vertex \(v_j\), that is \(\xi (v_i)=max\{d(v_i,v_j): v_i,v_j\in V(G)\}\). The radius and diameter are the two parameters measured from eccentricity defined as \(R(G)=min\{\xi (v_i): v_j\in V(G)\}\) and \(D(G)=max\{\xi (v_i): v_j\in V(G)\}\), respectively. A graph is self-centered if \(D(G)=R(G)\). For more graph-theoretic terms and notations, readers can refer to5,6.
The topological index is a numerical descriptor derived from a molecular graph, which helps in characterizing and predicting physicochemical properties of a molecule. Topological indices play a significant role in theoretical chemistry, mainly in quantitative structure-property relationships and quantitative structure-activity relationships analysis7. Among the various classes of topological indices, the vertex eccentricity-based topological indices (obtained by replacing the degree of a vertex by its eccentricity) play a crucial role in mathematical chemistry. In 1997, Sharma et al.8 introduced the eccentricity-based graphical indices. Over the past few decades, a great deal of study has been conducted in this field. Few eccentricity-based indices are effectively used for mathematical models of a broad spectrum of biological activities9,10,11. Many researchers12,13,14,15,16,17,18,19,20,21,22,23,24 have studied various eccentricity-based topological indices of molecular graphs and their applications.
The first eccentricity-based index introduced by Sharma et al.8 in the year 1997 is the eccentric connectivity index \(\xi ^c(G)\) and is defined as
Subsequently, in the year 2012, eccentricity-based first and second Zagreb indices, denoted by \(\xi _1^*(G)\) and \(\xi _2^*(G)\), were introduced25, which are given by
Another index used effectively by chemists is the forgotten eccentric index \(F^{\xi }(G)\)26, which is defined as
Recently, in the year 202127, Kulli introduced the eccentric version of the degree-based Sombor index, called the eccentric Sombor index (fourth Sombor index) of a graph G, defined as
Additional information on the eccentric Sombor index can be found in28,29.
In an attempt to explore properties and bounds associated with the eccentric Sombor index, some of the existing results are used, which are stated below.
Lemma 1
The Cauchy–Schwarz inequality30: If \((a_1,a_2,\dots , a_p)\) and \((b_1,b_2,\dots , b_p)\) are real p-vectors then,
Lemma 2
31 If \(a_i,b_i\ge 0\) and \(xb_i \le a_i \le yb_i\) for \(1 \le i \le n\), then
If \(a_i > 0\) for some \(1 \le i \le n\), then the equality holds if and only if \(x = y\) and \(a_i = xb_i\) for every \(1 \le i \le n\).
Lemma 3
The Kober’s inequality32: If \(a_i > 0\) for \(1 \le i \le j\), then
Motivation for the study
The degree-based Sombor index was initially defined as
and used by chemists in QSPR analysis of various compounds33,34. Soon, this index attracted the interest of mathematicians35,36. The Sombor index has gained relevance in network science and has been applied to the modeling of complex dynamical systems in technological, biological, and social contexts37,38. To achieve better precision than the previously available ones, the vertex degrees are replaced by their eccentricities in various degree-based topological indices. The eccentric Sombor index is one such index introduced in 2021. The diverse applications of the Sombor index serve as motivation for us to measure its predictive power for the physicochemical properties of polycyclic aromatic compounds.
In the next section, we give the bounds for the eccentric Sombor index in terms of radius, diameter, and also in terms of other eccentricity-based topological indices. Section 4 deals with an application of the eccentric Sombor index in qualitative structure property analysis of polycyclic aromatic compounds. A statistical technique called regression analysis is used to capture the correlation between the dataset containing the physicochemical properties of compounds and the topological indices.
Bounds for eccentric Sombor index
In this section, some bounds for the eccentric Sombor index in terms of a few significant graph parameters like radius, diameter, are obtained. Further, we established the bounds for the eccentric Sombor index in terms of other graph indices like eccentricity-based Zagreb indices, eccentric connectivity index, forgotten eccentric index, and the multiplicative version of eccentricity-based indices.
Theorem 1
Let G be a graph with diameter D and radius R. Then
where \(\xi ^c(G)\) is the eccentric connectivity index. The equality holds if the graph G is self-centered.
Proof
Let \(m=|E(G)|\). If G is self centered, then \(D=R\) which implies \(SO^{\xi }(G)=\sqrt{2}mD\) and \(\xi ^c(G)=2mD(G)\), attaining the equality in the statement of the theorem.
Let \(R<D\). It true that \(\frac{2DR}{D^2+R^2}\le \frac{2ab}{a^2+b^2}\) for every \(a, b \in [R, D]\) with equality if and only if \(\{a,b\}=\{r, D\}\). Hence, for every \(a, b \in [r, D]\),
Since for any vertex u, it is true that \(\xi (u) \in [R, D]\), we can write
Thus, \(SO^{\xi }(G) \le \frac{\sqrt{D^2+R^2}}{D+R} \xi ^c(G).\) \(\square\)
Theorem 2
Let G be a graph containing m edges having diameter D and radius R. Then
where \(F^\xi (G)\) is the forgotten eccentric index, and the equality holds if the graph G is self-centered.
Proof
From Cauchy-Schwarz inequality 1
To prove lower bound, let \(a_i=\sqrt{\xi (u)^2+\xi (v)^2}\) and \(b_i=1\), then by Lemma 2 we have
\(\square\)
Theorem 3
Let G be a graph having m edges with diameter D and radius R. Then
Equality is attained when G is self-centered.
Proof
We have \(\sqrt{2}R\le \sqrt{\xi (u)^2+\xi (v)^2} \le \sqrt{2}D\), which implies
\(\square\)
Theorem 4
Let G be a graph with the eccentric connectivity index \(\xi ^c(G)\). Then
Proof
By the root mean square and arithmetic mean inequality, \(\sqrt{\frac{a^2+b^2}{2}} \ge \frac{a+b}{2}\)
On substituting \(a=\xi (u)\) and \(b=\xi (v)\), we get
\(\square\)
The next bound is in terms of the eccentricity version of the first multiplicative hyper Zagreb index, \(HM_{1}(G)=\prod \limits _{u\sim v} \left( deg(u)+deg(v)\right) ^2\). The multiplicative version of Zagreb indices was introduced in 201239, and it is proved that \(HM_{1}(G)\) is minimal when G is a path graph. The eccentricity-based first multiplicative hyper Zagreb index, denoted by \(HM^{\xi }_{1}(G)\) is given by
Theorem 5
Let G be a graph containing m edges with \(F^\xi (G)\), \(\xi _2^*(G)\) and \(HM^{\xi }_{1}(G)\) being the forgotten eccentric index, the second Zagreb eccentricity index and the eccentricity-based first multiplicative hyper Zagreb index, respectively. Then
Proof
Substituting \(a_i=\xi (u)^2+\xi (v)^2\) in Kober’s inequality, we get
Also,
\(\square\)
Structure property analysis of polycyclic aromatic hydrocarbons using the eccentric Sombor index
Polycyclic aromatic hydrocarbons are organic compounds composed of two or more fused benzene rings, in which adjacent rings share a pair of carbon atoms, and contain no heteroatoms or substituent groups. Based on the number of fused rings, polycyclic aromatic hydrocarbons are classified into light polycyclic aromatic hydrocarbons, which contain up to four fused rings, and heavy polycyclic aromatic hydrocarbons, which comprise more than four fused rings. In comparison with light PAHs, heavy PAHs exhibit enhanced stability and significantly higher levels of toxicity40. The structural arrangement of benzene rings in PAHs gives rise to a wide range of physical, chemical, and toxicological properties. These ring systems can adopt various configurations and may occur either with or without substituent groups. Polycyclic aromatic hydrocarbons vary from semi-volatile to high-boiling compounds. Many researchers have studied the QSPR analysis of polycyclic aromatic hydrocarbons41,42,43.
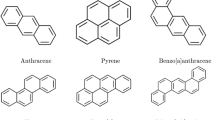
Although polycyclic aromatic hydrocarbons are not typically synthesised on an industrial scale, several of them are used in commercial applications. Their primary uses include acting as intermediates in the production of photographic materials, thermosetting plastics, pharmaceuticals, agricultural chemicals, and various other products. Polycyclic aromatic hydrocarbons may be present as impurities in carbon black dyes. In particular, naphthalene frequently occurs as an impurity originating from low-quality raw materials used as intermediates in the production of textile dye-dispersing agents and can also be detected in finished textile products. Some of the well-known polycyclic aromatic compounds, having a wide variety of applications, are given in Fig. 1. In this article, a predictive analysis of the eccentric Sombor index among polycyclic aromatic compounds is undertaken.
Regression models
Regression analysis is a statistical technique widely used in finance, investment studies, and many other fields to examine the relationship between a dependent variable and one or more independent variables. Among the various regression models, linear regression is the most commonly applied to identify the best-fitting straight line that describes the observed data based on a defined mathematical criterion. Typically, linear regression employs the least-squares method, which determines the optimal fit by minimising the sum of squared deviations between the observed values and those predicted by the model. The coefficient of determination is denoted by \(R^2\), and it measures how effectively a linear model explains the relationship between variables as depicted in a scatter plot. Specifically, \(R^2\) measures the extent to which the model’s predictions correspond to the observed data and ranges from 0 to 1, representing 0% to 100% of the total variation explained by the model.
The regression model is designed to establish and quantify the relationship between eccentric Sombor index with molecular properties, like boiling point BP (\(^{0}C\)), melting point MP (\(^{0}C\)), molecular weight MW(g/mol), molar refractivity MR (\(cm^3\)), flash point FP (\(^{0}C)\), molar volume MV (\(cm^3\)) and polarizability PO (\(10^{-24}cm^3\)). The empirical data (source: PubChem database) containing the properties of various PAHs used for the analysis along with the calculated eccentric Sombor index is given in Table 1.
The scatter plots for the above collection of data are given in Fig. 2. For each of the parameters, a linear regression model is derived, and the coefficient of determination \(R^2\) is given.
Comparisons and discussion
This study effectively predicted 32 of the most abundant polycyclic aromatic compounds using the eccentricity-based Sombor index. We have considered 7 physico-chemical properties of polycyclic aromatic compounds, among which, the molecular weight, polarizability, and flash point yield the highest coefficient of determination. Other properties like molar refractivity and molar volume also have a relatively strong correlation. But melting point and complexity have a weak correlation with eccentric Sombor index. The detailed list is given below.
Properties | Regression equation | \(R^2\) |
|---|---|---|
Molecular weight | \(MW = 0.7307~SO^{\xi }(G)+90.099\) | 0.9571 |
Melting point | \(MP = 0.8317 ~SO^{\xi }(G) + 12.087\) | 0.7282 |
Boiling point | \(BP = 1.2171 ~SO^{\xi }(G) + 198.19\) | 0.9159 |
Molar refractivity | \(MR= 0.2775 ~SO^{\xi }(G) + 28.229\) | 0.9370 |
Polarizability | \(PO=0.1100~SO^{\xi }(G)+ 11.195\) | 0.9371 |
Molar volume | \(MV=0.4575~SO^{\xi }(G)+ 95.528\) | 0.9422 |
Flash point | \(FP=0.779~SO^{\xi }(G)+ 54.883\) | 0.9502 |
In44, the authors carried out a similar study and hence determined the impact of the degree-based Sombor index on the physico-chemical properties of polycyclic aromatic compounds. In comparison with the degree-based Sombor index, it was found that the eccentricity-based Sombor index yields higher predictive power. Clearly, we can see that molar volume yields a higher coefficient of determination (\(R^2=0.9422\)) than that of the degree-based Sombor index (0.7786). The comparison between the coefficient of determination values of degree-based and eccentricity-based Sombor index is given in the following Table 2.
Conclusion
The output of the above analysis demonstrates that the eccentric Sombor index shows a fine and adequate linear relationship (\(R^2>0.9)\) with most of the properties. The results obtained in this article indicate that the Sombor index achieves better correlation with physicochemical properties when vertex degrees are replaced by their corresponding eccentricities.
Limitations and scope for future work
Although the QSPR study of eccentric Sombor index exhibits strong predictive ability for several physicochemical properties of polycyclic aromatic hydrocarbons, the present study has certain limitations.
-
The analysis is restricted to polycyclic aromatic hydrocarbons, and therefore, the predictive capability of the eccentric Sombor index for other classes of chemical compounds remains to be explored.
-
The regression analysis employed in this work is limited to linear models, although high coefficients of determination \(R^2>0.9\) were obtained. Other regression analyses, such as logarithmic, exponential, quadratic, etc, can also be adopted.
-
Since the dataset used for validation is finite, analysing a larger and more diverse set of compounds would enable a more reliable and applicable approach across different molecular classes.
Extending the study of the eccentric Sombor index to other classes of compounds beyond polycyclic aromatic hydrocarbons to evaluate its predictive power for a wider range of molecular structures would be a promising direction for future work. Conducting similar studies for several degree-based topological indices by replacing vertex degrees with their corresponding eccentricities may lead to improved correlations and, consequently, contribute more significantly to the QSPR analysis of chemical compounds.
Data availability
All data generated or analysed during this study are included in this article.
References
González-Díaz, S., Vilar, H. & Uriarte, E. Medicinal chemistry and bioinformatics: Current trends in drugs discovery with networks topological indices. Curr. Top. Med. Chem.7, 1025–1039. https://doi.org/10.2174/156802607780906771 (2007).
Gozalbes, J. P., Doucet, R. & Derouin, F. Application of topological descriptors in QSAR and drug design: History and new trends. Curr. Drug Targets Infect. Disord.2, 93–102. https://doi.org/10.2174/1568005024605909 (2002).
Kier, L. B. & Hall, L. H. Molecular Connectivity in Structure-Activity Analysis (John Wiley and Sons, 1986).
Alaeiyan, J., Asadpour, M. & Mojarad, R. A numerical method for MEC polynomial and MEC index of one-pentagonal carbon nanocones. Fuller. Nanotub. Carbon Nanostructures2013, 825–835. https://doi.org/10.1080/1536383X.2011.613546 (2013).
Trinajstić, N. Chemical Graph Theory (CRC Press, 2018).
Ravindra, R. B. Graphs and Matrices (Springer, New York, 2010).
Todeschini, R. & Consonni, V. Handbook of Molecular Descriptors (Weinheim, Wiley-VCH, 2000).
Sharma, V. G. R. & Madan, A. K. Eccentric connectivity index: A novel highly discriminating topological descriptor for structure-property and structure-activity studies. J. Chem. Inf. Comput. Sci.37, 273–282. https://doi.org/10.1021/ci960049h (1997).
Dureja, S., Gupta, H. & Madan, A. K. Predicting anti-hiv-1 activity of 6-arylbenzonitriles: Computational approach using superaugmented eccentric connectivity topochemical indices. J. Chem. Inf. Comput. Sci.26, 1020–1029. https://doi.org/10.1016/j.jmgm.2007.08.008 (2008).
Gupta, M., Singh, S. & Madan, A. K. Eccentric distance sum: A novel graph invariant for predicting biological and physical properties. J. Math. Anal. Appl.275, 386–401. https://doi.org/10.1016/S0022-247X(02)00373-6 (2002).
Padmapriya, P. & Mathad, V. Eccentricity-based topological indices of some graphs. TWMS J. App. Eng. Math.10, 1084–1095 (2020).
Kizilirmak, G. O. The eccentricity-based topological indices. Acta Univ. Sapientia. Informat.15, 294–305. https://doi.org/10.2478/ausi-2023-0018 (2023).
Jebreen, H., Iqbal, K. & Barham, A. Study of eccentricity-based topological indices for benzenoid structure. S. Afr. J. Chem. Eng.45, 221–227. https://doi.org/10.1016/j.sajce.2023.05.010 (2023).
Farooq, R. & Malik, M. A. On some eccentricity-based topological indices of nanostar dendrimers. Optoelectron. Adv. Mat.9, 842–849 (2015).
Farahani, M. R. Eccentricity version of atom bond connectivity index of benzenoid family \(ABC_5(H_k)\). World Appl. Sci. J.21, 1260–1265. https://doi.org/10.17344/acsi.2016.2378 (2013).
Imran, M. et al. Eccentricity-based topological indices of an oxide network. Mathematics6, 126–139. https://doi.org/10.3390/math6070126 (2018).
Imran, M., Baig, A. Q., Azhar, M. R., Farahani, M. R. & Zhang, X. Eccentricity-based geometric-arithmetic and atom-bond connectivity indices of copper oxide Cuo[372]. Int. J. Pure Appl. Math.17, 481–502. https://doi.org/10.12732/ijpam.v117i3.12 (2018).
Gao, M. R., Farahani, W. & Jamil, M. K. The eccentricity version of Atom-Bond Connectivity index of linear polycene parallelogram benzenoid \({ABC_5}(p(n, n))\). Acta Chim. Slov.63, 376–379. https://doi.org/10.17344/acsi.2016.2378 (2016).
Khaji, S., Hanif, B. & Bhat, K. A. Eccentricity-based topological indices of chain graphs. IAENG Int. J. Appl. Math.55, 1235–1241 (2025).
Khaji, S., Hanif, B. & Bhat, K. A. Spectral properties of eccentricity sum matrix of graphs. Commun. Comb. Optim. https://doi.org/10.22049/cco.2025.30199.2355 (2025).
Min, M., Ishaq, K. S. & Nazeer, W. On eccentricity-based topological indices and polynomials of phosphorus-containing dendrimers. Symmetry10, 237–247. https://doi.org/10.3390/sym10070237 (2018).
Xiujun, M. K., Siddiqui, Z. & Baig, A. Q. Computing eccentricity-based topological indices of octagonal grid \({O}_{nm}\). Mathematics6, 153–167. https://doi.org/10.3390/math6090153 (2018).
Rashid, F. & Malik, A. On some eccentricity-based topological indices of nanostar dendrimers. Optoelectron. Adv. Mater. Rapid Commun.9, 842–849 (2015).
Hanif, S. & Bhat, A. K. The total eccentricity index of some benzanoid systems. Int. J. Appl. Math.38, 513–521. https://doi.org/10.12732/ijam.v38i4.5 (2025).
De, N. On multiplicative Zagreb eccentricity indices. South Asian J. Math.2, 570–577 (2012).
Gao, W., Siddiqui, M. & Farahani, M. R. Forgotten topological index of chemical structure in drugs. Saudi Pharm. J.24, 258–264. https://doi.org/10.1016/j.jsps.2016.04.012 (2016).
Kulli, V. R. Different versions of Sombor index of some chemical structures. Int. J. Eng. Sci. Res. Tech.10, 23–32 (2021).
Chaluvaraju, B., Harish, N. & Nandee Kumar, C. An elliptic-eccentric Sombor index of graphs and its chemical applicabilities. J. Hyperstruct.13, 222–236. https://doi.org/10.22098/jhs.2024.15446.1027 (2024).
Tang, Z., Yunping, Z. & Deng, H. Elliptic Sombor index of trees and unicyclic graphs. Electron. J. Math.7, 9–34. https://doi.org/10.47443/ejm.2024.009 (2024).
Bhatia, R. & Davis, C. A Cauchy-Schwarz inequality for operators with applications. Linear Algebra Appl.223, 119–129. https://doi.org/10.1016/0024-3795(94)00344-D (1995).
Martínez-Pérez, A., Rodrííguez, J. M. & Sigarreta, J. M. CMMSE: A new approximation to the Geometric-Arithmetic index. J. Math. Chem.56, 1865–1883. https://doi.org/10.1007/s10910-017-0811-3 (2018).
Hermann, K. On the Arithmetic and Geometric means and on Holder’s inequality. Proc. Am. Math. Soc.9, 452–459. https://doi.org/10.2307/2033003 (1958).
Alikhani, S. & Ghanbari, N. Sombor index of polymers. MATCH Commun. Math. Comput. Chem.86, 715–728. https://doi.org/10.48550/arXiv.2103.13663 (2021).
Amin, S. R. & Virk, A. U. Analysis of dendrimer generation by Sombor indices. Hindawi J. Chem.11, 9930645. https://doi.org/10.1155/2021/9930645 (2021).
Das, K. C. & Shang, Y. On Sombor index. Symmetry13, 140–152. https://doi.org/10.3390/sym13010140 (2021).
Horoldagva, B. & Xu, C. On Sombor index of graphs. MATCH Commun. Math. Comput. Chem.86, 793–713. https://doi.org/10.48550/arXiv.2305.04554 (2021).
Shang, Y. Sombor index and degree-related properties of simplicial networks. Appl. Math. Comput.419, 126881. https://doi.org/10.1016/j.amc.2021.126881 (2022).
Gutman, I. Spectrum and energy of the Sombor matrix. Mil. Tech. Cour.69, 551–561. https://doi.org/10.5937/vojtehg69-31995 (2021).
Eliasia, A., Iranmanesh, M. & Ivan, G. Multiplicative versions of first Zagreb index. MATCH Commun. Math. Comput. Chem.68, 217–230. https://doi.org/10.1007/s40010-023-00868-2 (2012).
Kuppusamy, S., Thavamani, P., Megharaj, M. & Naidu, R. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by novel bacterial consortia tolerant to diverse physical settings - assessments in liquid- and slurry-phase systems. Int. Biodeterior. Biodegrad.108, 149–157. https://doi.org/10.1016/j.ibiod.2015.12.013 (2016).
Malik, M. A., Binyamin, M. Y. & Hayat, S. Correlation ability of degree-based topological indices for physicochemical properties of polycyclic aromatic hydrocarbons with applications. Polycycl. Aromat. Comp.42, 6267–6281. https://doi.org/10.1080/10406638.2021.1977349 (2021).
Sarkar, P., De, N. & Pal, A. On some topological indices and their importance in chemical sciences: A comparative study. Eur. Phys. J. Plus137, 195–207. https://doi.org/10.1140/epjp/s13360-022-02431-1 (2022).
Arockiaraj, M. et al. Novel molecular hybrid Geometric-Harmonic-Zagreb degree based descriptors and their efficacy in QSPR studies of polycyclic aromatic hydrocarbons. SAR QSAR Environ. Res.34, 569–589. https://doi.org/10.1080/1062936X.2023.2239149 (2023).
Kirana, B., Shanmukha, M. C. & Usha, A. Comparative study of Sombor index and its various versions using regression models for top priority polycyclic aromatic hydrocarbons. Sci. Rep.14, 19841. https://doi.org/10.1038/s41598-024-69442-z (2024).
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal
Author information
Authors and Affiliations
Contributions
All authors contributed equally .
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khaji, B., Hanif, S. & Arathi Bhat, K. Eccentric sombor index of graphs and its role in the structure-property relationship analysis of polycyclic aromatic compounds. Sci Rep 16, 6282 (2026). https://doi.org/10.1038/s41598-026-36192-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-026-36192-z