Abstract
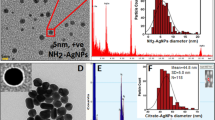
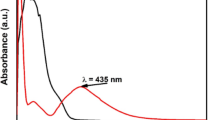
Antibiotic resistance, a growing global challenge, is predicted to cause millions of deaths in the near future. Innovative antibacterial wound dressings loaded with natural substances that have restorative effects can serve as alternatives conventional medicine. This research aims to investigate the antibacterial properties of electrospun nanofibers containing different proportions of chitosan, collagen, and hyaluronic acid-silver nanocomposite. Silver nanoparticles (Ag NPs) were synthesized via a green, solvent‑free method, with hyaluronic acid (HA) obtained from recombinant Corynebacterium glutamicum fermentation serving as the natural reducing agent. with its production optimized using a full factorial design, resulting in a 26% yield increase under conditions of 10 g/L yeast extract, 20 g/L soy protein, and 400 mg/L MgSO₄. The purity of HA obtained from microbial fermentation was measured by Fourier-Transform Infrared spectroscopy. Silver nitrate concentrations of 0.01, 0.1, and 1 M were considered to synthesize nanoparticle precursors. Spectrophotometry and Dynamic Light Scattering analyses showed that 0.1 M AgNO3 produced nanoparticles with an average size of 98.5 nm. Scanning Electron Microscopy revealed that the nanofibers had a coherent and uniform structure. Antibacterial activity was evaluated against Escherichia coli and Staphylococcus aureus. Nanofibers with 1:1:1 and 0.5:1:1 ratios (HA-Ag: collagen: chitosan) inhibited S. aureus growth, producing inhibition zones of 1.4 cm and 1.0 cm, respectively, but showed no effect against E. coli. Cytotoxicity assessment using L929 fibroblast cells through MTT assay indicated cell viabilities of approximately 85% and 70% for the active formulations, suggesting acceptable biocompatibility. Overall, the developed nanocomposite-loaded nanofibers show potential for application against antibiotic-resistant wound infections caused by Gram-positive bacteria.
Similar content being viewed by others
Data availability
The additional data are provided in Supplementary material S1.
Abbreviations
- HA:
-
Hyaluronic acid or Hyaluronan
- HA-Ag:
-
Hyaluronic acid- AgNO3
- AgNPs:
-
AgNO3 Nanoparticles
- CTAB:
-
Cetyltrimethylammonium Bromide
- DLS:
-
Dynamic light scattering analysis
- SEM:
-
Scanning Electron Microscopy
- FTIR:
-
Fourier-transform infrared spectroscopy
References
Bloom, D. E. & Cadarette, D. Infectious disease threats in the twenty-first century: strengthening the global response. Front. Immunol. 10, 549 (2019).
Mohammed, M., Devnarain, N., Elhassan, E. & Govender, T. Exploring the applications of hyaluronic acid-based nanoparticles for diagnosis and treatment of bacterial infections. Wiley Interdisciplinary Reviews: Nanomed. Nanobiotechnol. 14, e1799 (2022).
McArthur, D. B. Emerging infectious diseases. Nurs. Clin. 54, 297–311 (2019).
Vouga, M. & Greub, G. Emerging bacterial pathogens: the past and beyond. Clin. Microbiol. Infect. 22, 12–21 (2016).
Snetkov, P. et al. In-vitro antibacterial activity of curcumin-loaded nanofibers based on hyaluronic acid against multidrug-resistant ESKAPE pathogens. Pharmaceutics 14, 1186 (2022).
Lee, N. Y., Ko, W. C. & Hsueh, P. R. Nanoparticles in the treatment of infections caused by multidrug-resistant organisms. Front. Pharmacol. 10, 1153 (2019).
Canaparo, R. et al. Recent developments in antibacterial therapy: focus on stimuli-responsive drug-delivery systems and therapeutic nanoparticles. Molecules 24, 1991 (2019).
Osmokrovic, A. et al. Current state and advances in antimicrobial strategies for burn wound dressings: from metal-based antimicrobials and natural bioactive agents to future perspectives. Int. J. Mol. Sci. 26, 4381 (2025).
Vo, V. et al. Dermal substitutes for clinical management of severe burn injuries: current and future perspectives. Adv. Ther. 8, 2400455 (2025).
Khosravimelal, S., Chizari, M., Farhadihosseinabadi, B., Moosazadeh Moghaddam, M. & Gholipourmalekabadi, M. Fabrication and characterization of an antibacterial chitosan/silk fibroin electrospun nanofiber loaded with a cationic peptide for wound-dressing application.
Garcia, C. E. G., Martínez, F. A. S., Bossard, F. & Rinaudo, M. Production of chitosan/hyaluronan complex nanofibers. Characterization and physical properties as a function of the composition. Polymers 12 (2020).
Rajeshkumar, S. et al. Synthesis of greener silver nanoparticle-based Chitosan nanocomposites and their potential antimicrobial activity against oral pathogens. 10, 658–665 (2021).
Lin, Z. et al. Biofunctions of antimicrobial peptide-conjugated alginate/hyaluronic acid/collagen wound dressings promote wound healing of a mixed-bacteria-infected wound. Int. J. Biol. Macromol. 140, 330–342 (2019).
Karthik, V., Arivarasu, L. & Rajeshkumar, S. Hyaluronic acid mediated zinc nanoparticles against oral pathogens and its cytotoxic potential. J. Pharm. Res. Int. 32, 113–117 (2020).
Liang, Z. & Chen, D. Targeting therapy effects of composite hyaluronic acid/chitosan nanosystems containing inclusion complexes. Drug Deliv. 29, 2734–2741 (2022).
Chen, C. H., Cheng, Y. H., Chen, S. H., Chuang, A. D. C. & Chen, J. P. Functional hyaluronic acid-polylactic acid/silver nanoparticles core-sheath nanofiber membranes for prevention of post-operative tendon adhesion. Int. J. Mol. Sci. 22, 8781 (2021).
Lee, H., Park, H., Noh, G. J. & Lee, E. S. pH-responsive hyaluronate-anchored extracellular vesicles to promote tumor-targeted drug delivery. Carbohydr. Polym. 202, 323–333 (2018).
Talebi, M., Ghale, R. A., Asl, R. M. & Tabandeh, F. Advancements in characterization and preclinical applications of hyaluronic acid-based biomaterials for wound healing: a review. Carbohydrate Polym. Technol. Applications, 100706 (2025).
Saadati, F. et al. Advances and principles of hyaluronic acid production, extraction, purification, and its applications: A review. International J. Biol. Macromolecules, 143839 (2025).
Nikuiyan, Z. et al. Reconstruction of a genome-scale metabolic model for Streptococcus zooepidemicus: comparison with Corynebacterium glutamicum to study hyaluronic acid production. PLoS One. 20, e0335509 (2025).
Chang, R. et al. Nanocomposite multifunctional hyaluronic acid hydrogel with photothermal antibacterial and antioxidant properties for infected wound healing. Int. J. Biol. Macromol. 226, 870–884 (2023).
Zamboni, F., Wong, C. K. & Collins, M. N. Hyaluronic acid association with bacterial, fungal and viral infections: can hyaluronic acid be used as an antimicrobial polymer for biomedical and pharmaceutical applications? Bioactive Mater. 19, 458–473 (2023).
Westbrook, A. W., Ren, X., Moo-Young, M. & Chou, C. P. Application of hydrocarbon and perfluorocarbon oxygen vectors to enhance heterologous production of hyaluronic acid in engineered Bacillus subtilis. Biotechnol. Bioeng. 115, 1239–1252 (2018).
Ijaz, M. et al. Dissecting Streptococcus pyogenes interaction with human. Arch. Microbiol. 202, 2023–2032 (2020).
Liu, Y. et al. Springer,. in Advances in Applied Biotechnology 439–452 (2015).
Garlapati, V. K. Comprehensive review on biotechnological production of hyaluronic acid status, Innovation, Market and Applications. (2022).
Hoffmann, J. & Altenbuchner, J. Hyaluronic acid production with Corynebacterium glutamicum: effect of media composition on yield and molecular weight. J. Appl. Microbiol. 117, 663–678 (2014).
Cheng, F., Luozhong, S., Guo, Z., Yu, H. & Stephanopoulos, G. Enhanced biosynthesis of hyaluronic acid using engineered Corynebacterium glutamicum via metabolic pathway regulation. Biotechnol. J. 12, 1700191 (2017).
Tabasi, A. et al. Improved production of food-grade hyaluronic acid in Recombinant Corynebacterium glutamicum by medium optimization and feeding strategy. Applied Food Biotechnology 12, 1–14 .
Michalska-Sionkowska, M., Kaczmarek, B., Walczak, M. & Sionkowska, A. Antimicrobial activity of new materials based on the blends of collagen/chitosan/hyaluronic acid with gentamicin sulfate addition. Mater. Sci. Engineering: C. 86, 103–108 (2018).
Oryan, A. & Sahvieh, S. Effectiveness of Chitosan scaffold in skin, bone and cartilage healing. Int. J. Biol. Macromol. 104, 1003–1011 (2017).
Michalska-Sionkowska, M., Walczak, M. & Sionkowska, A. Antimicrobial activity of collagen material with thymol addition for potential application as wound dressing. Polym. Test. 63, 360–366 (2017).
Hosny, S. et al. A comprehensive review of silver nanoparticles (AgNPs): synthesis strategies, toxicity concerns, biomedical applications, ai-driven advancements, challenges, and future perspectives. Arabian J. Sci. Engineering, 1–48 (2025).
Duman, H. et al. Silver nanoparticles: A comprehensive review of synthesis methods and chemical and physical properties. Nanomaterials 14, 1527 (2024).
Del Olmo, J. A. et al. Hyaluronic acid-based hydrogel coatings on Ti6Al4V implantable biomaterial with multifunctional antibacterial activity. Carbohydr. Polym. 301, 120366 (2023).
Villamizar-Sarmiento, M. G. et al. Ionic nanocomplexes of hyaluronic acid and polyarginine to form solid materials: A green methodology to obtain sponges with biomedical potential. Nanomaterials 9, 944 (2019).
Abdel-Mohsen, A. et al. Electrospinning of hyaluronan/polyvinyl alcohol in presence of in-situ silver nanoparticles: Preparation and characterization. Int. J. Biol. Macromol. 139, 730–739 (2019).
Karami, M. et al. Preparation, purification, and characterization of low-molecular-weight hyaluronic acid. Biotechnol. Lett. 43, 133–142 (2021).
Miguel, S. P. et al. An overview of electrospun membranes loaded with bioactive molecules for improving the wound healing process. Eur. J. Pharm. Biopharm. 139, 1–22 (2019).
Bitter, T. A modified uronic acid carbazole reaction. Anal. Biochem. 4, 330–334 (1962).
Cesaretti, M., Luppi, E., Maccari, F. & Volpi, N. A 96-well assay for uronic acid carbazole reaction. Carbohydr. Polym. 54, 59–61 (2003).
Song, J. M., Im, J. H., Kang, J. H. & Kang D.-J. A simple method for hyaluronic acid quantification in culture broth. Carbohydr. Polym. 78, 633–634 (2009).
Bouin, A. S. & Wierer, M. Quality standards of the European pharmacopoeia. J. Ethnopharmacol. 158, 454–457 (2014).
Li, C. et al. Silver nanoparticle/chitosan oligosaccharide/poly (vinyl alcohol) nanofibers as wound dressings: a preclinical study. International J. Nanomedicine, 4131–4145 (2013).
Kanimozhi, K., Basha, S. K., Kumari, V. S., Kaviyarasu, K. & Maaza, M. In vitro cytocompatibility of chitosan/PVA/methylcellulose–Nanocellulose nanocomposites scaffolds using L929 fibroblast cells. Appl. Surf. Sci. 449, 574–583 (2018).
Um, I. C., Fang, D., Hsiao, B. S., Okamoto, A. & Chu, B. Electro-spinning and electro-blowing of hyaluronic acid. Biomacromolecules 5, 1428–1436 (2004).
Ward, P. D., Thibeault, S. L. & Gray, S. D. Hyaluronic acid: its role in voice. J. Voice. 16, 303–309 (2002).
Boldock, E. et al. Human skin commensals augment Staphylococcus aureus pathogenesis. Nat. Microbiol. 3, 881–890 (2018).
Giovane, R., Pernia, L., Faught, W., Cumagen, P. & Comer, J. M. Polymicrobial wound infection caused by Lelliottia amnigena, Staphylococcus aureus, and Corynebacterium following a lawnmower accident. Cureus 17 (2025).
Villanueva, D. M. et al. Escherichia coli ST1193 O75 H5: A rare cause of native valve endocarditis with multifocal emboli to brain and spleen. IDCases 37, e02052 (2024).
Azam, A. et al. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. International J. Nanomedicine, 6003–6009 (2012).
Fazal, A., Ara, S., Ishaq, M. T. & Sughra, K. Green fabrication of copper oxide nanoparticles: a comparative antibacterial study against gram-positive and gram-negative bacteria. Arab. J. Sci. Eng. 47, 523–533 (2022).
Alavi, M. & Varma, R. S. Antibacterial and wound healing activities of silver nanoparticles embedded in cellulose compared to other polysaccharides and protein polymers. Cellulose 28, 8295–8311 (2021).
Bernardo, M. P., Pasquini, D. & Mattoso, L. H. Enhanced antibacterial activity of wound dressings based on alginate/hydroxyapatite modified with copper and Naproxen. J. Mater. Res. 39, 762–773 (2024).
Slavin, Y. N., Asnis, J., Hńfeli, U. O. & Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 15, 1–20 (2017).
Pelgrift, R. Y. & Friedman, A. J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 65, 1803–1815 (2013).
Funding
This work was finantially supported by the National Institute of Genetic Engineering and Biotechnology (NIGEB project No. 643) and the Iran National Science Foundation (INSF project No. 4024643).
Author information
Authors and Affiliations
Contributions
Mahdieh Nadali Hazaveh: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing - original draft, Writing - review & editing. Saeed Salehi: Formal analysis, Investigation, Methodology, Visualization, Writing - original draft, Writing - review & editing. Marjan Talebi: Investigation, Methodology, Visualization, Writing - review & editing. Rouzbeh Almasi Ghale: Methodology, Investigation, Visualization, Writing - review & editing. Hamid Zilouei: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Writing - review & editing. Fatemeh Tabandeh: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing - review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nadali Hazaveh, M., Salehi, S., Talebi, M. et al. Engineered biosynthesis of hyaluronic acid in Corynebacterium glutamicum and green synthesis of HA-silver nanocomposites for advanced antimicrobial wound dressings. Sci Rep (2026). https://doi.org/10.1038/s41598-026-39148-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-39148-5